A.M. Wessels1, E.R. Siemers1, P. Yu1, S.W. Andersen1, K.C. Holdridge1, J.R. Sims1, K. Sundell1, Y. Stern2, D.M. Rentz3, B. Dubois4, R.W. Jones5, J. Cummings6, P.S. Aisen7
1. Eli Lilly and Company, Indianapolis, IN, USA; 2. Department of Neurology, Columbia University Medical Center, New York, NY, USA; 3. Departments of Neurology, Brigham and Women’s Hospital, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA; 4. Centre des Maladies Cognitives et Comportementales (IM2A), Institut du Cerveau et de la Moelle épinière (ICM), UMR-S975, Université Pierre et Marie Curie- Paris6, AP-HP, Hôpital de la Salpêtrière, Paris, France; 5. RICE (The Research Institute for the Care of Older People), Royal United Hospital, Bath, UK; 6. Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, Nevada, USA; 7. University of Southern California, CA, USA
Corresponding Author: Alette M Wessels, Eli Lilly and Company, Lilly Corporate Center, Indianapolis, IN 46285, USA, Email: wesselsal@lilly.com, Telephone: +1 (317) 276-9502, Fax: +1 (317) 276-5791
J Prev Alz Dis 2015;2(4):227-241
Published online October 7, 2015, http://dx.doi.org/10.14283/jpad.2015.82
Abstract
It is generally recognized that more sensitive instruments for the earliest stages of Alzheimer’s disease (AD) are needed. The integrated Alzheimer’s Disease Rating Scale (iADRS) combines scores from 2 widely accepted measures, the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog) and the Alzheimer’s Disease Cooperative Study – instrumental Activities of Daily Living (ADCS-iADL). Disease progression and treatment differences as measured by the iADRS were analyzed using data from solanezumab EXPEDITION, EXPEDITION2, and EXPEDITION-EXT Studies; semagacestat IDENTITY Study; and donepezil ADCS – mild cognitive impairment (ADCS-MCI) Study. Psychometric properties of the iADRS were established through principal component analysis (PCA) and estimation of contributions of subscores and individual item scores to the iADRS total score. The iADRS performed better than most composites and scales in detecting disease progression and comparably or better than individual scales in detecting treatment differences. PCA demonstrated the iADRS can be divided into two principal components primarily representing cognitive items and instrumental ADLs. Dynamic ranges of the subscales were similar across all studies, reflecting approximately equal contributions from both subscales to the iADRS total score. In item analyses, every item contributed to the total score, with varying strength of contributions by item and across data sets. The iADRS demonstrated acceptable psychometric properties and was effective in capturing disease progression from MCI through moderate AD and treatment effects across the early disease spectrum. These findings suggest the iADRS can be used in studies of mixed populations, ensuring sensitivity to treatment effects as subjects progress during studies of putative disease-modifying agents.
Key words: iADRS, Alzheimer’s disease, clinical trials, outcome measure.
Introduction
Clinical trials for new therapies of Alzheimer’s disease (AD) are enrolling patients earlier in the disease continuum to maintain optimal function and intervene before pathological changes are severe. The need for more sensitive and responsive instruments for early stages of AD is increasingly recognized. Because development and validation of new scales de novo is a long process, recent efforts have focused on developing composites from existing scales. Strategies that have been applied toward that end include theory-driven and data-mining approaches. Theory-driven composite development consists of construction of an instrument to include neuropsychological tests measuring domains known to be impaired at a particular disease stage of interest, for example, the Alzheimer’s Disease Cooperative Study (ADCS)-Preclinical Alzheimer Cognitive Composite (ADCS-PACC), a cognitive composite being used in the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4) study of preclinical AD (1, 2). A data-mining approach applies mathematical calculations to existing items within a scale or scales to identify the most sensitive items and applies weighting and adding/subtracting items to improve performance. These approaches may also be combined. A common data-mining strategy for composite outcome measures developed specifically for mild cognitive impairment (MCI) or early AD has been to eliminate the items from the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog) (3, 4) that appear less sensitive to disease progression and combine those with items from measures (with or without weighting of individual items) from other instruments of cognition and/or function, with the goal of improving sensitivity to detect change and reducing variability (5-10).
The objective of our work was to identify a composite scale that would appropriately measure the most important domains of AD (i.e., cognition and function) that could be used to monitor disease progression in observational studies and show treatment effects in placebo-controlled clinical trials.
Adopting a theory-driven approach as described above, the starting point for this work was to test the concept of a composite that combines cognition (with a particular focus on episodic memory, executive function, and global cognitive abilities) and function (activities of daily living) through the evaluation of existing scales. Various composites constructed using several different scales were first evaluated for their ability to detect disease progression in data sets including MCI and mild AD patients. These analyses demonstrated that assessing cognitive and functional items in a single composite scale was more sensitive to detecting disease progression than testing the domains separately. In both MCI and mild AD populations, the best performing composite was the combination of the ADAS-Cog13 with the Functional Activities Questionnaire (FAQ) (11). The next stage of analysis was to determine whether this construct was sensitive in detection of treatment effects. The treatment trials available for these analyses included the ADCS-ADL scale, rather than the FAQ, as the functional measure, and specified the ADAS-Cog14 as the primary cognitive outcome measure for the mild AD population; thus, the construct was represented as the ADAS-Cog14 combined with the ADCS-instrumental Activities of Daily Living (iADL). This process resulted in and supported the use of a simple combination of the ADAS-Cog14 and the ADCS-iADL scales, which we termed the integrated Alzheimer’s Disease Rating Scale (iADRS). Herein, we describe the analyses conducted to develop the iADRS, to assess the ability of the iADRS to detect disease progression and treatment effects, and to describe its psychometric properties.
Methods
The iADRS is calculated as a linear combination of total scores of the two individual components, the ADAS-Cog14 (score range 0 to 90) and the instrumental items of the ADCS-ADL (ADCS-iADL; score range 0 to 56). Because higher scores on the ADAS-Cog14 reflect worse performance, whereas higher scores on the ADCS-iADL reflect better performance, the ADAS-Cog score is multiplied by (-1) in the calculation of the integrated scale. To anchor the ADAS-Cog at 0, a constant (90) is added. The iADRS score is then computed as the sum of the transformed ADAS-Cog14 and the ADCS-iADL, as shown in the formula below:
iADRS score = [-1(ADAS-Cog14)+90]+iADL
The iADRS score ranges from 0 to 146 with lower scores indicating worse performance.
Data Sets
The data sets described below were used in the development or assessment of the iADRS.
Alzheimer’s Disease Neuroimaging Initiative
The Alzheimer’s Disease Neuroimaging Initiative (ADNI) Study is a longitudinal observational study of biomarkers in subjects from North America with MCI and mild AD as well as normal controls (12). From a snapshot of the data made in May 2013 (ADNI1) patients with mild AD (N=181) and late MCI (N=380) were included in the current dataset. The ADCS-ADL scale was not measured in this longitudinal study; thus, it was not available for analysis in this dataset. The key cognitive and functional scales collected in ADNI were the ADAS-Cog13 and the FAQ.
Solanezumab EXPEDITION and EXPEDITION2 Studies Pooled Mild Database
Solanezumab EXPEDITION and EXPEDITION2 Studies (NCT00905372 and NCT00904683, respectively) were identically designed Phase 3, 18-month, placebo-controlled studies investigating solanezumab treatment in patients with mild-to-moderate AD. No treatment benefit was detected in the overall populations. Secondary analyses demonstrated a treatment effect of solanezumab in the mild AD pooled population from the 2 studies, but not in the moderate AD population (13, 14). Patients from both studies with mild AD (Mini–Mental State Examination [MMSE] from 20 to 26; n=1322) and moderate AD (MMSE from 16 to 19; n=723) at baseline were included in the iADRS analyses. The FAQ scale was not measured in these studies; thus, it was not available for analysis in this dataset. The key cognitive and functional scales collected in EXPEDITION and EXPEDITION2 were the ADAS-Cog14 and the ADCS-ADL.
Solanezumab EXPEDITION-EXT Study
The EXPEDITION-EXT Study (NCT01127633) is an ongoing open-label extension study offered to all patients who completed the EXPEDITION and EXPEDITION2 Studies (15). All patients with mild AD at baseline in EXPEDITION and EXPEDITION2 (N=1322) were included in the iADRS analyses. The key cognitive and functional scales collected in EXPEDITION-EXT were the ADAS-Cog14 and the ADCS-ADL.
Semagacestat IDENTITY Study
The semagacestat IDENTITY Study (NCT00594568) was a Phase 3, parallel, placebo-controlled, dose/response, delayed-start 88-week study in patients with mild-to-moderate AD to assess the effect of semagacestat, a γ-secretase inhibitor, on AD progression (16). The study was terminated before completion after the data safety monitoring board observed cognitive worsening and safety concerns. All patients with mild AD in the placebo and 140-mg dose groups were included in the iADRS analysis data set (n=632). For these analyses, Week 76 was used as the endpoint. The FAQ scale was not measured in this study; thus, it was not available for analysis in this dataset. The key cognitive and functional scales collected in IDENTITY were the ADAS-Cog14 and the ADCS-ADL.
Donepezil ADCS-MCI Study
The ADCS-MCI Study was a multicenter, randomized, double-blind, placebo-controlled study comparing development of AD among MCI patients treated with vitamin E, donepezil, or placebo for 3 years (17). No effect of vitamin E was demonstrated. Secondary analyses demonstrated a beneficial effect of donepezil, particularly among subjects with the presence of one or more apolipoprotein (APOE) ε4 alleles “APOE ε4 carriers.” The data set used for iADRS analyses included APOE ε4 carriers (used as a surrogate for amyloid positivity to increase the probability AD pathology was present in this MCI population and that AD progression would be observed) treated with donepezil or placebo through 36 months of follow-up (n=789). The FAQ scale was not measured in this study; thus, it was not available for analysis in this dataset. The key cognitive and functional scales collected in ADCS-MCI were the ADAS-Cog14 and the ADCS-ADL-MCI, which is a modified version of the ADCS-ADL for an MCI population. To best match the ADCS-ADL-MCI scale to the ADCS-iADL in iADRS analyses, one item that measured a basic ADL was removed from the ADCS-MCI scale, resulting in a 17-item instrumental-only scale with a score range of 0 to 49.
Statistical Methods
Disease Progression
The ability of the iADRS to detect disease progression was compared with other newly created composites and existing scales. The composites included tests measuring episodic memory (such as the Auditory Verbal Learning Test), timed executive function (Digit Symbol Substitution), global cognition (MMSE), and activities of daily living (FAQ).
In comparing the ability of composites and existing scales (ADAS-Cog11, ADAS Cog13, MMSE, Clinical Dementia Rating Scale [CDR], FAQ, and other scales included in the composites [listed in Figure 1]) to demonstrate disease progression, bootstrapped data sets were generated based on ADNI and solanezumab EXPEDITION and EXPEDITION2 Study data. Bootstrapping, a general resampling procedure for estimating the distributions of statistics based on independent observations, was used to sample the original dataset with replacement to generate 500 new data sets of the same size as the original data set. Mixed-model repeated measures (MMRM) analyses were conducted to estimate the least squares (LS) mean change from baseline (and standard deviation [SD]) up to 18 months (EXPEDITION data) and 24 months (ADNI data) in each bootstrapped dataset. The MMRM model used to estimate disease progression included the fixed, categorical effect of visit (or time point, in months); study (for solanezumab, EXPEDITION or EXPEDITION2); and the continuous, fixed covariates of baseline score and baseline score-visit interaction. Generally, the within-subject errors were modelled using an unstructured covariance matrix. The Kenward-Roger approximation (18) was used to estimate denominator degrees of freedom.
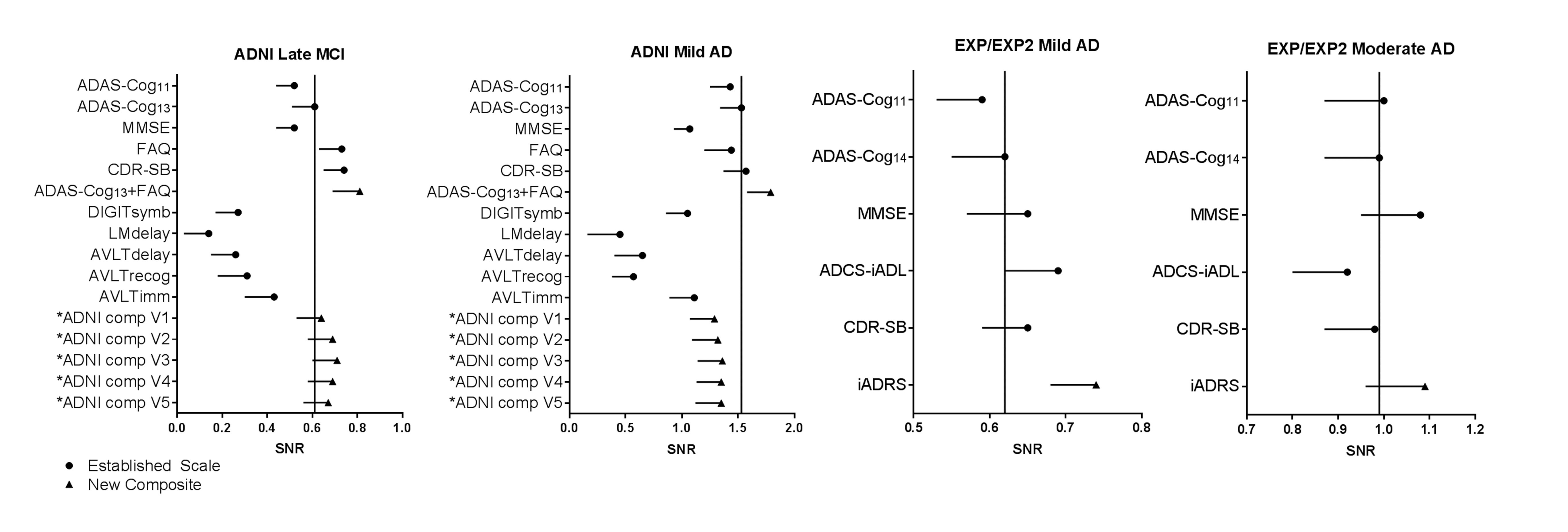
Figure 1. Signal-to-noise ratios (median and 2.5th percentiles) for mean change in outcome measures analyzed in the ADNI late MCI population (n=380), the ADNI mild AD population (n=181), and the solanezumab EXPEDITION and EXPEDITION2 Studies placebo mild AD (n=652), and moderate AD (n=334) populations
Abbreviations: ADAS-Cog = Alzheimer’s Disease Assessment Scale-Cognitive subscale (11-, 13-, or 14-item version); ADCS-iADL = Alzheimer’s Disease Consortium Study-Activities of Daily Living, instrumental items; ADNI = Alzheimer’s Disease Neuroimaging Initiative; AVLTdelay = Auditory Verbal Learning Test Delayed Recall; AVLTimmed = Auditory Verbal Learning Test Immediate Recall; AVLTrecog = Auditory Verbal Learning Test Recognition; CDR-SB = Clinical Dementia Rating scale – Sum of Boxes; comp=composite; DIGITsymb = Digit Symbol Substitution; FAQ = Functional Activities Questionnaire; iADRS = integrated Alzheimer’s Disease Rating Scale; LMdelay = Logical Memory Delayed Recall; MCI-mild cognitive impairment; MMSE = Mini-Mental Status Examination; SNR = signal-to-noise ratios; V = version. * The different versions of the ADNI composites evaluated (designated as ADNI comp V1 through V5) represent measures of executive function (Digit Symbol Substitution), global cognition (MMSE), function (FAQ total score), and memory (ADNI comp V1: ADAS-Cog delayed word recall and logical memory delay; ADNI comp V2: logical memory delay and AVLT delayed recall; ADNI comp V3: logical memory delay, AVLT delayed recall, and AVLT immediate recall; ADNI comp V4: logical memory delay and AVLT immediate recall; and ADNI comp V5: logical memory delay, AVLT immediate recall, and retrieval efficiency [recognition minus delayed word recall]). The vertical lines mark the median of the ADAS-Cog13 (ADNI) or ADAS-Cog14 (EXPEDITION and EXPEDITION2) to aid in visual comparison among the scales.
Because various scales have differences in total points and point differences relative to decline, direct comparisons of the LS mean change or LS mean change difference of the scales is not informative; thus, signal-to-noise ratios (SNRs) were calculated as follows for each scale:
Change over time SNR=(LS mean change)/LS mean change SD)
The 95% confidence interval (CI) of each estimated SNR was determined by the 2.5th and 97.5th percentiles of these 500 SNRs. A higher SNR represents greater change detected by the scale, corrected for variability. The SNRs and 95% CIs for all scales can be directly compared.
Therapeutic Sensitivity
To assess detection of treatment differences by the iADRS and the ADAS-Cog and ADCS-iADL separately in the solanezumab and semagacestat mild AD data sets, the change from baseline for each scale was analyzed using MMRM; the change from baseline score on the iADRS at each scheduled postbaseline visit (during the treatment period) was the dependent variable. For solanezumab EXPEDITION and EXPEDITION2 Studies, the model for the fixed effects included baseline score, study, treatment, study-by-treatment interaction, visit, treatment-by-visit interaction, baseline AD standard-of-care use, and baseline age. For the semagacestat IDENTITY Study, the model for the fixed effects included baseline score, pooled investigator, treatment, visit, treatment-by-visit interaction, baseline AD standard-of-care use, and baseline age. Visit was considered a categorical variable with values equal to the visit numbers at which the scales were assessed. An unstructured covariance matrix was used to model the within-subject variance-covariance errors. The Kenward-Roger approximation was used to estimate the denominator degrees of freedom.
For solanezumab EXPEDITION, EXPEDITION2, and EXPEDITION-EXT Studies, a delayed-start analysis was also conducted, using a single MMRM analysis model including all available data from all randomized patients from the beginning of the placebo-controlled period through the end of the delayed-start period (rather than only patients who participated in the delayed-start period) (15). The following 3 hypotheses were tested:
1. The difference in mean change from baseline on iADRS between treatments at the end of the placebo-controlled period (Δ1) is significant.
2. The difference in mean change from baseline on the iADRS between treatments at the end of the delayed-start period (Δ2) is significant.
3. The lower limit of the 90% CI for the noninferiority test statistic Δ2 – 0.5Δ1 is greater than 0. This test is to measure whether at least 50% of the treatment benefit at the end of the placebo-controlled period is retained at a particular time after the start of the delayed-start period.
To assess detection of treatment differences by the ADAS-Cog and ADCS-ADL-MCI separately and the iADRS calculated using the ADAS-Cog and the ADCS-ADL-MCI (modified iADRS) in the donepezil ADCS-MCI Study data set, the change from baseline for each scale was analyzed using MMRM; the change from baseline score on the modified iADRS at each scheduled postbaseline visit (during the treatment period) was the dependent variable. The model for the fixed effects included terms for baseline score, treatment, visit, treatment-by-visit interaction, and baseline age. Visit was considered a categorical variable with values equal to the visit numbers at which the scales were assessed. An unstructured covariance matrix was used to model the within-subject variance-covariance errors. The Kenward-Roger approximation was used to estimate the denominator degrees of freedom.
Psychometric Analyses
The psychometric properties of the iADRS were established through principal component analysis (PCA), estimation of the contributions of the two subscores to the iADRS total score, and estimation of the contributions of individual item scores to the iADRS total score.
The PCA was performed using baseline and change from baseline data from placebo-treated completer patients from the pooled mild AD population in the solanezumab EXPEDITION and EXPEDITION2 Studies and the donepezil ADCS-MCI Study. Rotation was not performed to ensure that the resulting principal components explained the largest amount of the variability observed in the dataset. The data were not standardized for baseline score and standard deviation. An item loading ≥0.4 or ≤-0.4 indicates significant contribution of that variable to the principal component (19).
The relative contributions of the ADAS-Cog14 and ADCS-iADL scores to the iADRS total score were calculated using baseline and change from baseline data from mild and moderate AD placebo-treated patients in the solanezumab EXPEDITION and EXPEDITION2 Studies, the semagacestat IDENTITY Study, and the donepezil ADCS-MCI APOE ε4 carrier data set. The observed dynamic ranges at baseline and change from baseline at 18 months were defined as baseline mean score and LS mean change from baseline plus/minus one SD for each scale in the solanezumab and semagacestat mild AD data sets and the donepezil ADCS-MCI APOE ε4 carrier data set. In addition, dynamic ranges were calculated in the subsets of mild AD patients from the solanezumab and semagacestat data sets with baseline CDR scores of 0.5 to evaluate the iADRS in patients with milder stages of AD.
To assess the contribution of each item of the iADRS to its total score at baseline and its change from baseline to endpoint score, the performance of each individual item of the ADAS-Cog14 and ADCS-iADL scales was analyzed using pooled placebo-treated patient data from the solanezumab EXPEDITION and EXPEDITION2 Studies and from the semagacestat IDENTITY Study. First, it was determined for each item whether the population baseline score reflected a score at the ceiling of the scale (maximum score) or the floor of the scale (minimum score). Because each item has a different score range, item scores were normalized and expressed as a percentage of maximum possible score; the percentage was calculated as mean raw baseline score divided by the maximum point value for that item. Because a higher score on the ADAS-Cog14 indicates greater cognitive impairment, values for this scale were transformed such that lower scores would indicate greater cognitive impairment by subtracting from 100. Thus, for both scales, 0% would indicate the maximum impairment (floor effect), while 100% would indicate no impairment (ceiling effect) on that item. Given the nature of AD progression, it is expected that patients will decline over time; thus it is desired that no items show a floor effect at baseline as that would indicate no room for worsening over time. Next, to determine how much patients declined within the remaining space of the scale (for example, if baseline performance was 50%, then 50% of the scale remains to decline), the LS mean change score at endpoint was divided by the total possible point value for that item minus the baseline mean to correct for the remaining space at baseline.
To examine the ability of individual items to detect a treatment effect in data from the mild AD population in the solanezumab EXPEDITION and EXPEDITION2 Studies and the semagacestat IDENTITY Study, the LS mean difference between the placebo and solanezumab/semagacestat treatment groups was divided by the maximum point value for that item to determine the normalized LS mean difference to allow for direct comparison between items.
Results
Disease Progression
In both MCI and mild AD ADNI populations, the scale most sensitive to disease progression was the ADAS-Cog13+FAQ (Figure 1). The ADAS-Cog13 and FAQ were used to approximate the iADRS instead of the ADAS-Cog14 and ADCS-iADL, based on the cognitive and functional scales collected in ADNI. In the solanezumab mild and moderate placebo data sets, the iADRS performed better than the other individual cognitive and functional scales collected in the EXPEDITION and EXPEDITION2 Studies. In all populations assessed, the addition of items or entire scales measuring function to items or entire scales measuring cognition improved the ability to detect disease progression. That is, composites combining cognition and function generally showed greater sensitivity in detecting decline than traditional cognitive-only or functional-only scales across MCI, mild AD, and moderate AD.
Therapeutic Sensitivity
Assessing iADRS as a Primary Efficacy Outcome in the Mild AD Populations of Solanezumab EXPEDITION, EXPEDITION2, and EXPEDITION-EXT Studies and Semagacestat IDENTITY Study
MMRM analysis of pooled mild AD population from EXPEDITION and EXPEDITION2 showed a statistically significant reduction in decline in the solanezumab-treated group relative to the placebo-treated group starting at Week 40 for the ADAS-Cog14 and from Week 64 for the ADCS-iADL. The iADRS showed similar results with a statistically significant reduction in decline between solanezumab- versus placebo-treated groups at Week 40 through Week 80 (Figure 2).
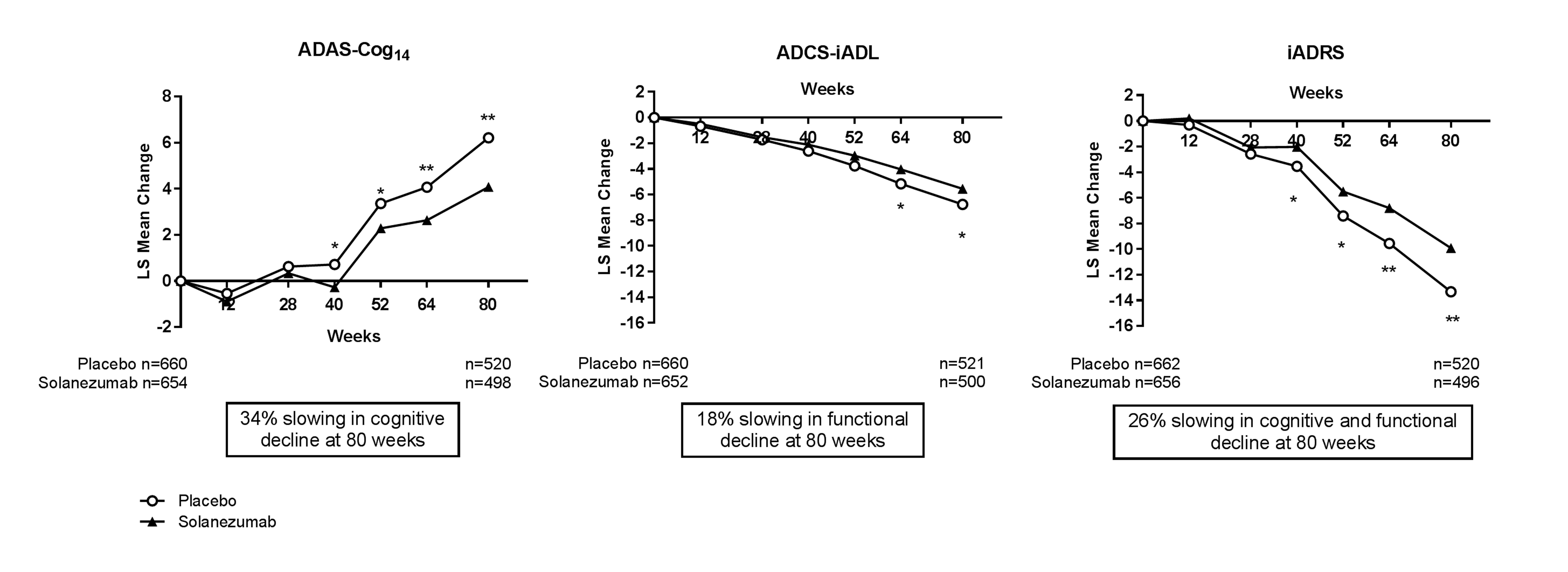
Figure 2. Repeated measures analysis of ADAS-Cog14, ADCS-iADL, and iADRS in the pooled mild AD population from solanezumab EXPEDITION and EXPEDITION2 Studies
Abbreviations: ADAS-Cog14 = 14-item Alzheimer’s Disease Assessment Scale-Cognitive subscale; ADCS-iADL = Alzheimer’s Disease Consortium Study-Activities of Daily Living, instrumental items; iADRS = integrated Alzheimer’s Disease Rating Scale; LS = least squares. *=p<.05; **=p<.01.
Significant or directionally consistent effects in cognition (ADAS-Cog14) and function (iADL) were observed in mild AD populations from the EXPEDITION and EXPEDITION2 Studies when each study was analyzed separately. However, treatment effects were significant for both studies when analyzed using the iADRS, and the overall results for these two identically designed studies appear more similar to each other when analyzed with the iADRS relative to each scale separately (Figure 3).
Figure 3. Repeated measures analysis of ADAS-Cog14, ADCS-iADL, and iADRS in the mild AD populations from solanezumab EXPEDITION and EXPEDITION2 Studies, by study
Abbreviations: ADAS-Cog14 = 14-item Alzheimer’s Disease Assessment Scale-Cognitive subscale; ADCS-iADL = Alzheimer’s Disease Consortium Study-Activities of Daily Living, instrumental items; iADRS = integrated Alzheimer’s Disease Rating Scale; LS = least squares. *=p<.05; **=p<.01.
The iADRS also improved the ability to detect treatment differences and noninferiority in the delayed-start analyses of the pooled mild AD data from the solanezumab EXPEDITION, EXPEDITION2, and EXPEDITION-EXT Studies compared with either cognition or function alone (Figure 4). The difference between early-start and delayed-start groups was statistically significant at the end of the placebo-controlled period (80 weeks). The difference between early-start and delayed-start groups at 108 weeks since randomization (Δ2, that is, 28 weeks in the delayed-start period) was also statistically significant. The noninferiority criterion was met at the primary analysis time point (108 weeks since randomization, Δ2, that is, 28 weeks in the delayed-start period), indicating the treatment difference in cognition and function at the end of the placebo-controlled period were preserved at 108 weeks within a pre-defined margin. Throughout the remainder of the delayed-start period, treatment differences for the iADRS were significant and noninferiority was met through 184 weeks (the entire period analyzed), whereas the ADAS-Cog14 and ADCS-iADL separately demonstrated statistical significance and noninferiority only through 132 weeks.
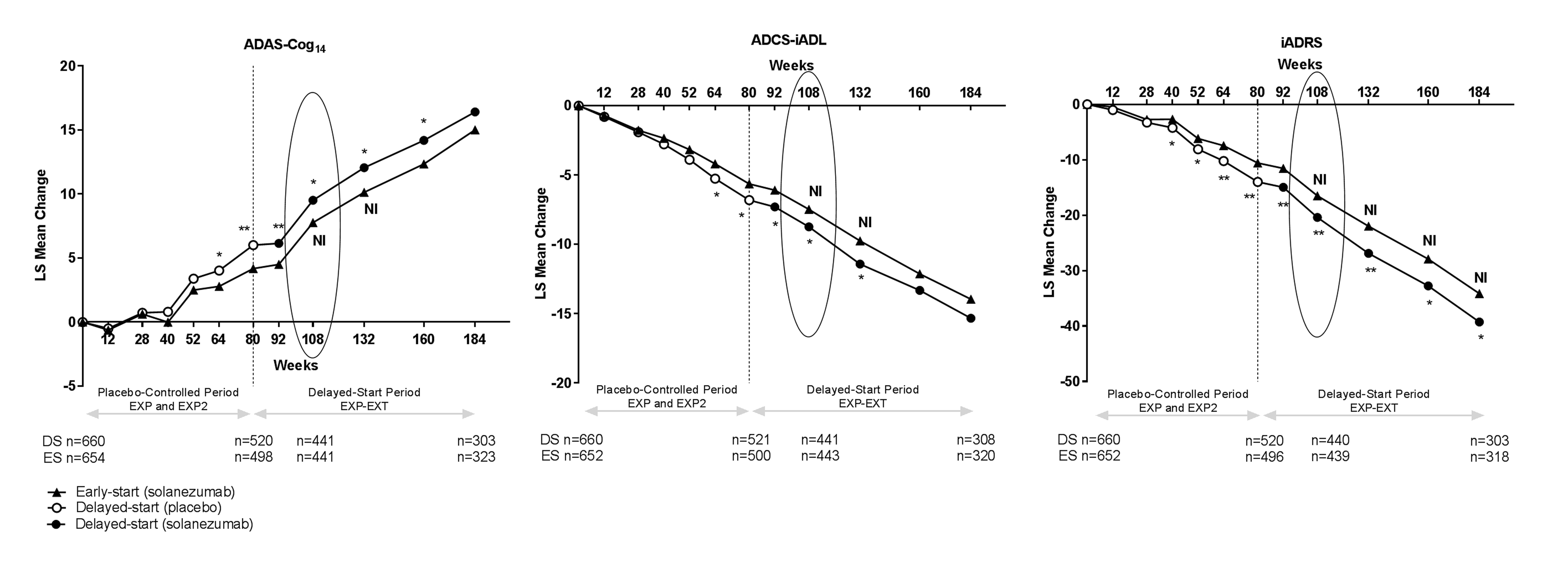
Figure 4. Delayed-start analysis of iADRS in the pooled mild AD population from solanezumab EXPEDITION, EXPEDITION2, and EXPEDITION-EXT Studies
Abbreviations: ADAS-Cog14 = 14-item Alzheimer’s Disease Assessment Scale – Cognitive subscale; ADCS-iADL = Alzheimer’s Disease Cooperative Study – instrumental items of the Activities of Daily Living Inventory; DS = delayed start; ES = early start; EXP = EXPEDITION; EXP2 = EXPEDITION2; EXP-EXT = EXPEDITION-EXT; iADRS = integrated Alzheimer’s Disease Rating Scale; LS = least squares; NI = noninferiority criterion met. *=p<.05; **=p<.01; Primary delayed-start analysis time point circled.
The iADRS was also able to detect treatment-related worsening in the semagacestat IDENTITY Study at all time points from 28 weeks through 76 weeks. When cognition and function were analyzed separately using the ADAS-Cog14 and the ADCS-iADL, significant treatment-related worsening was observed less consistently (Figure 5).
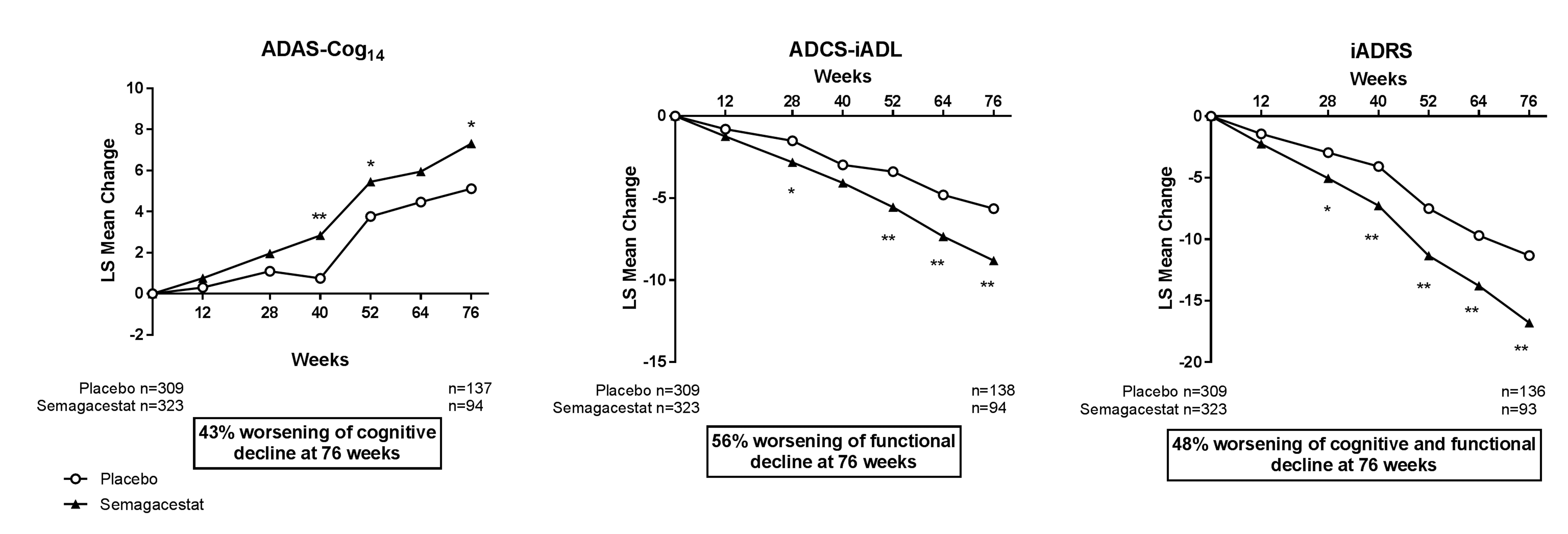
Figure 5. Repeated measures analysis of ADAS-Cog14, ADCS- iADL, and iADRS in the mild AD population from semagacestat IDENTITY Study
Abbreviations: ADAS-Cog14 = 14-item Alzheimer’s Disease Assessment Scale – Cognitive subscale; ADCS-iADL = Alzheimer’s Disease Cooperative Study – instrumental items of the Activities of Daily Living Inventory; iADRS = integrated Alzheimer’s Disease Rating Scale; LS = least squares. *=p<.05; **=p<.01.
Assessing iADRS as a Primary Efficacy Outcome in the Donepezil ADCS-MCI Study
Results from analyses of MCI APOE ε4 carriers in the donepezil ADCS-MCI Study show that the modified iADRS (calculated using ADAS-Cog14 and ADCS-iADL-MCI) was effective in tracking disease progression and in detecting an early symptomatic treatment benefit in this MCI population. This benefit was not maintained over time in either the cognitive or functional domains or the modified iADRS (Figure 6).
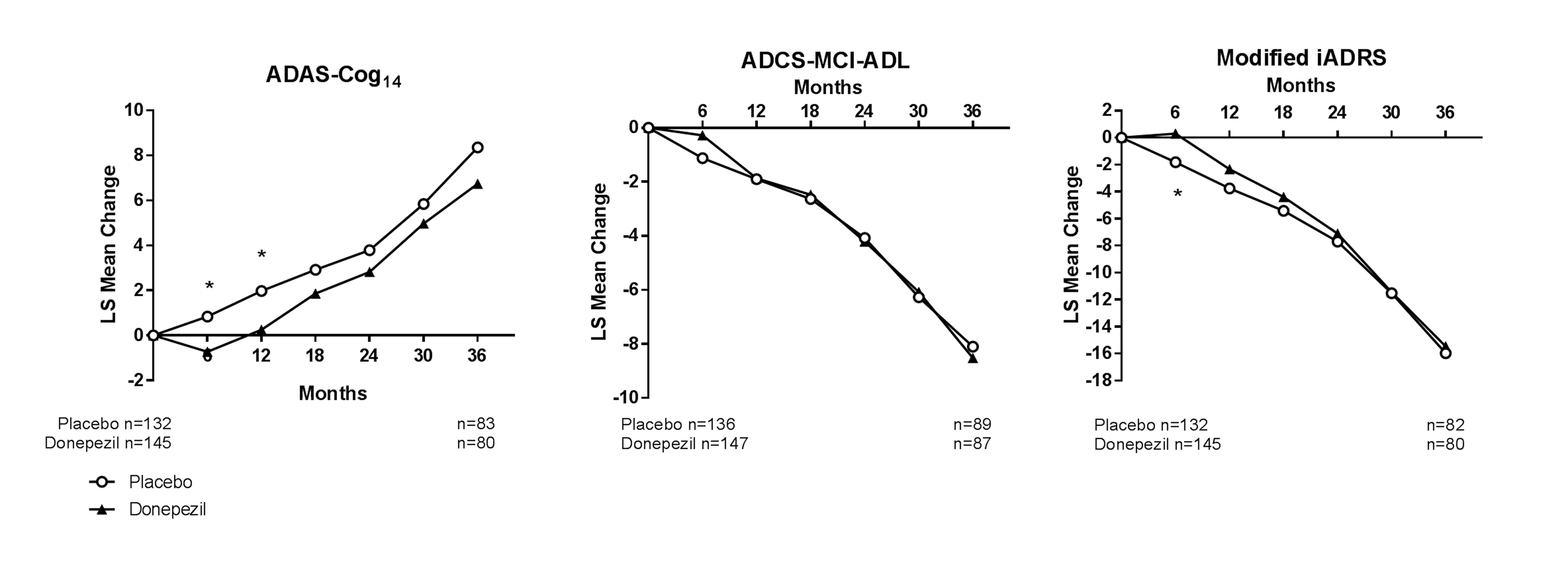
Figure 6. Repeated measures analysis of ADAS-Cog14, ADCS-MCI-ADL, and modified iADRS in the MCI+APOE ε4 carrier population from the donepezil ADCS-MCI Study
Abbreviations: ADAS-Cog14 = 14-item Alzheimer’s Disease Assessment Scale – Cognitive subscale; ADCS-ADL-MCI = modified version of the Alzheimer’s Disease Consortium Study Activities of Daily Living inventory for a mild cognitive impairment population; LS = least squares; Modified iADRS = integrated Alzheimer’s Disease Rating Scale calculated using the ADAS-Cog14 and the ADCS-ADL-MCI. *=p<.05.
Psychometric Properties of the iADRS
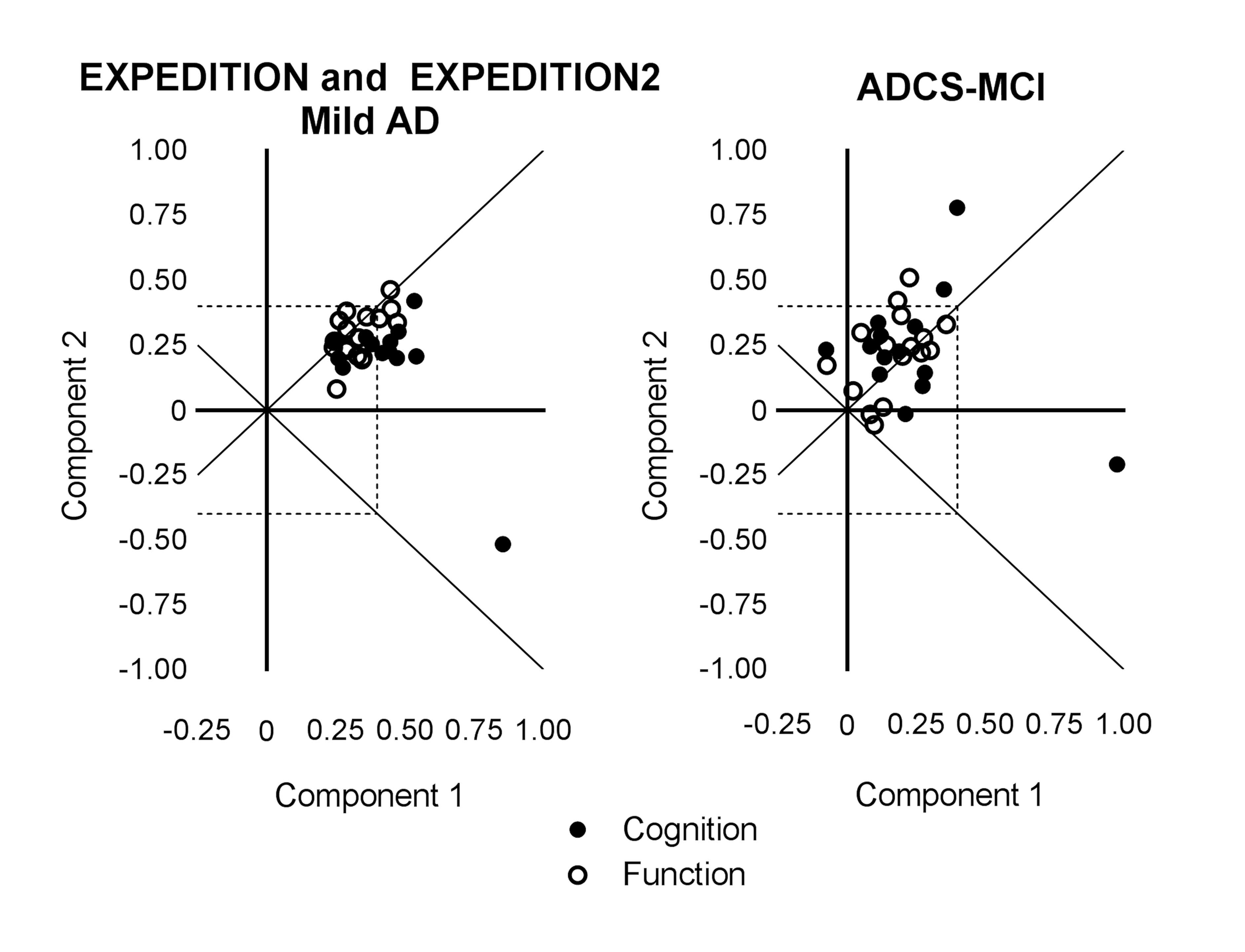
Principal component analyses of baseline solanezumab EXPEDITION and EXPEDITION2 data demonstrated the iADRS can be separated into two principal components with Component 1 representing primarily cognitive items (explaining 30% of the total variance) and Component 2 representing instrumental function (explaining 13% of the total variance) (Table 1, Figure S1). The items most highly loaded on Component 1 were from the ADAS-Cog, and the items most highly loaded on Component 2 were from the ADCS-iADL.
Principal component analysis performed using baseline data from the donepezil ADCS-MCI Study data set (MCI APOE ε4 carriers) showed that three items had significant loading values (≥0.4) at baseline, all from the ADAS-Cog (Table 1, Figure S1). Thus, all significant items represented cognitive items and no items measuring function had a significant loading in this data set, suggesting that in this MCI data set, the observed variability at baseline was primarily driven by cognition and not by function.
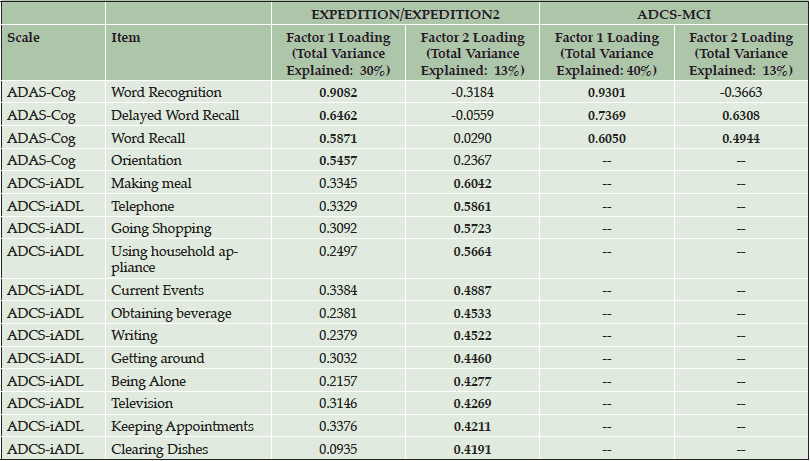
Table 1. Baseline Principal Components and Significant Loadings (≥0.4 or ≤-0.4) for Items in the iADRS from Mild AD Patients in the Solanezumab EXPEDITION and EXPEDITION2 Studies and MCI APOE ε4 Carrier Patients in the Donepezil ADCS-MCI Study
Abbreviations: ADAS-Cog = Alzheimer’s Disease Assessment Scale, Cognitive Subscale; ADCS-iADL = Alzheimer’s Disease Cooperative Study-Activities of Daily Living inventory, instrumental items; MCI = mild cognitive impairment. Significant factor loadings (≥0.4 or ≤ -0.4) in bold. Values for items not significant for either factor in a data set are not reported.
Principal component analysis using change from baseline data from the pooled mild AD placebo-treated subjects from solanezumab EXPEDITION and EXPEDITION2 Studies and donepezil ADCS-MCI Study showed the ADAS-Cog and ADCS-iADL items load on a single component, indicating a correlation between change in cognition and change in function. Therefore, the observed variance of the change score was driven by both cognition and function. (Table S1 and Figure S2).
It is possible that an imbalance of the relative contributions of cognition and function could exist within the iADRS because the overall score ranges for the ADAS-Cog and ADCS-iADL differ (0 to 90 points for the ADAS-Cog14 and 0 to 56 points for the ADCS-iADL). However, in the solanezumab EXPEDITION and EXPEDITION2 and semagacestat IDENTITY mild and moderate AD data sets, the observed baseline and change from baseline domain scores were within the same scale width or dynamic range, demonstrating the iADRS total score reflects approximately equal contributions from both subscales (Table 2). Similarly, analysis of the donepezil ADCS-MCI Study demonstrated approximately equal contributions from the ADAS-Cog and a version of the ADCS-ADL modified for an MCI population (ADCS-ADL-MCI) (Table S2).
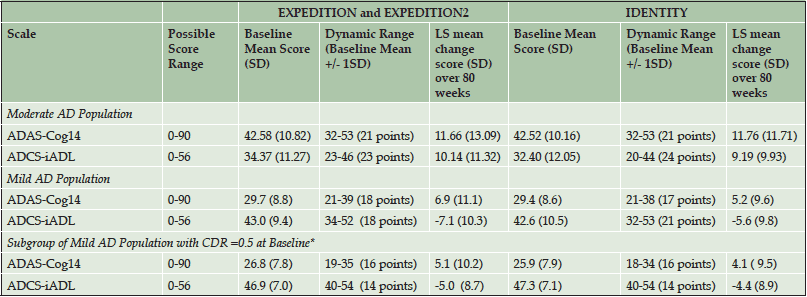
Table 2. Dynamic Ranges of iADRS Component Subscores (ADAS-Cog14 and ADCS-iADL) in Bootstrapped Data Sets from Solanezumab EXPEDITION and EXPEDITION2 Studies and Semagacestat IDENTITY Study
Abbreviations: ADAS-Cog14 = 14-item Alzheimer’s Disease Assessment Scale – Cognitive Subscale; ADCS-iADL = Alzheimer’s Disease Cooperative Study- Activities of Daily Living inventory, instrumental items; CDR = Clinical Dementia Rating scale; SD = standard deviation; *CDR score =0.5 is often used to denote MCI. All patients in the solanezumab EXPEDITION and EXPEDITION2 Studies and semagacestat IDENTITY Study mild AD data sets were diagnosed with mild AD (MMSE score 20-26), but the patients in the subgroup also had a CDR score =0.5, indicating they were likely the mildest mild AD patients and may have similar characteristics to MCI patients.
In item analyses, as a percentage of the maximum point value at baseline, mild patients in the EXPEDITION and EXPEDITION2 and IDENTITY Studies were most cognitively impaired on the ADAS-Cog14 delayed recall, word recall, and word recognition items (Table 3); on the ADCS-iADL, they were most functionally impaired on the reading item (Table 4). Decline in cognition over 80 and 76 weeks (in the solanezumab and semagacestat studies, respectively) was relatively evenly distributed across all individual items of the ADAS-Cog14. However, the relative contributions of specific item change scores to the total score differed slightly between the datasets (note that in semagacestat, almost no change was observed for the maze and delayed recall item). Similarly, decline in function was distributed across all individual items of this functional scale. Thus, although every item made a contribution to the total scale in assessment of disease progression, the strength of those contributions varied by item and across data sets. Treatment effect was observed across all individual cognitive items in the mild AD population of the solanezumab EXPEDITION and EXPEDITION2 Studies as indicated by negative or less change, whereas treatment effect in the opposite direction (worsening) was observed for 11 of the 14 items in the IDENTITY trial (Table 5). With the exception of three iADL items in the EXPEDITION and EXPEDITION2 Studies and one item in the IDENTITY Study, treatment effect was observed on all iADL items in both data sets (Table 6). The magnitude of the treatment differences varied by item and dataset.
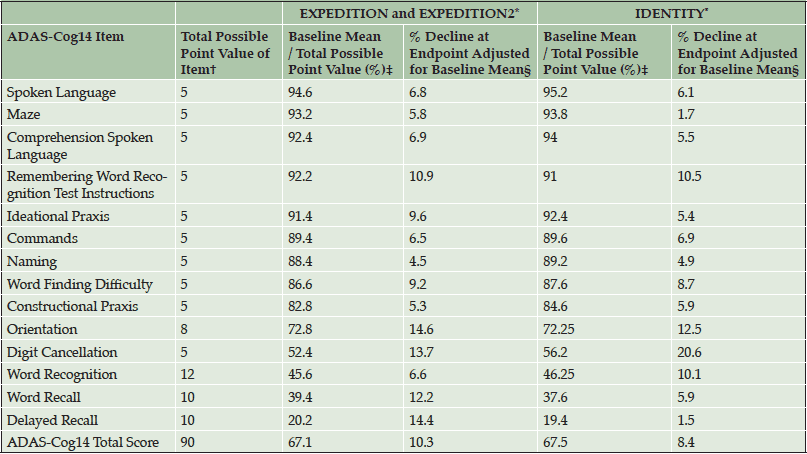
Table 3. Performance of Individual Items of ADAS-Cog14 in Mild AD Placebo-Treated Patients from Solanezumab EXPEDITION and EXPEDITION2 Studies and Semagacestat IDENTITY Study in Disease Progression Analyses
Abbreviations: ADAS-Cog14 = 14-item Alzheimer’s Disease Assessment Scale – Cognitive Subscale. *Endpoint in EXPEDITION and EXPEDITION2 was at 80 weeks. Endpoint in IDENTITY was at 76 weeks. †Higher point values indicate greater impairment. ‡Because a higher score on the ADAS-Cog14 indicates greater cognitive impairment, this value was transformed such that lower scores indicate greater cognitive impairment by subtracting from 100. Thus, 0% indicates the maximum degree of cognitive impairment on that item and 100% indicates no cognitive impairment on that item (to align with the ADCS-iADL). §The percentage decline at endpoint adjusted for baseline mean was calculated as LS mean change / (total possible point value – baseline mean) * 100.
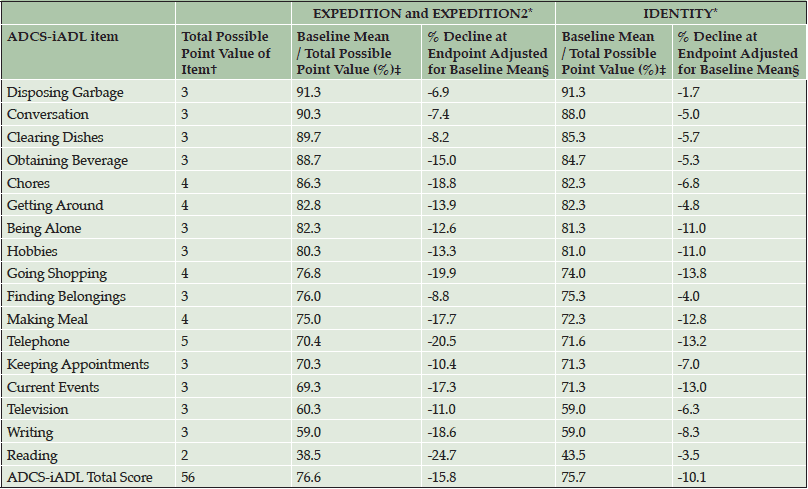
Table 4. Performance of Individual Items of ADCS-iADL in Mild AD Placebo-Treated Patients from Solanezumab EXPEDITION and EXPEDITION2 Studies and Semagacestat IDENTITY Study in Disease Progression Analyses
Abbreviations: ADCS-iADL = Alzheimer’s Disease Cooperative Study- Activities of Daily Living inventory, instrumental items. * Endpoint in EXPEDITION and EXPEDITION2 was at 80 weeks. Endpoint in IDENTITY was at 76 weeks. † Lower point values indicate greater impairment. ‡ 0% indicates the maximum degree of cognitive impairment on that item and 100% indicates no cognitive impairment on that item. § The percentage decline at endpoint adjusted for baseline mean was calculated as LS mean change / (total possible point value – baseline mean) * 100.
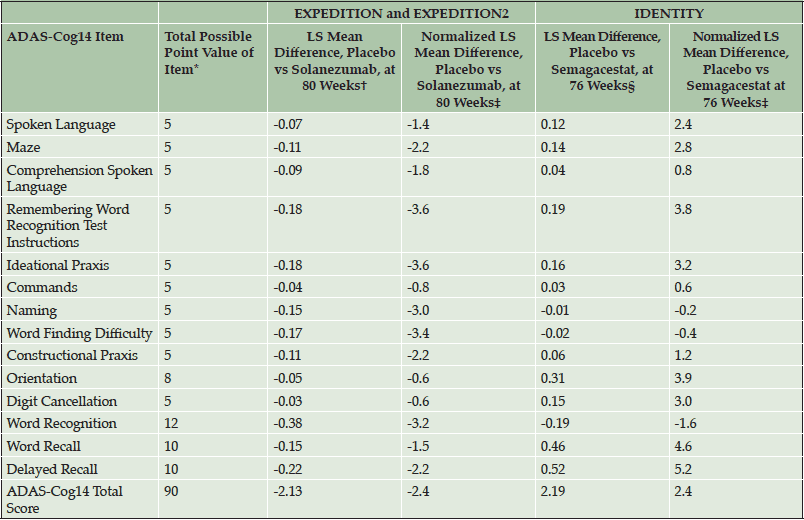
Table 5. Performance of Individual Items of ADAS-Cog14 in Mild AD Patients from Solanezumab EXPEDITION and EXPEDITION2 Studies and Semagacestat IDENTITY Study in Treatment Effect Analyses
Abbreviations: ADAS-Cog14 = 14-item Alzheimer’s Disease Assessment Scale, Cognitive Subscale; LS = least square. *Higher point values indicate greater impairment. †Negative value indicates positive treatment effect in favor of solanezumab. ‡Normalized LS mean difference was calculated as LS mean difference / total possible point value of item * 100. §Positive value indicates positive treatment effect in favor of placebo.
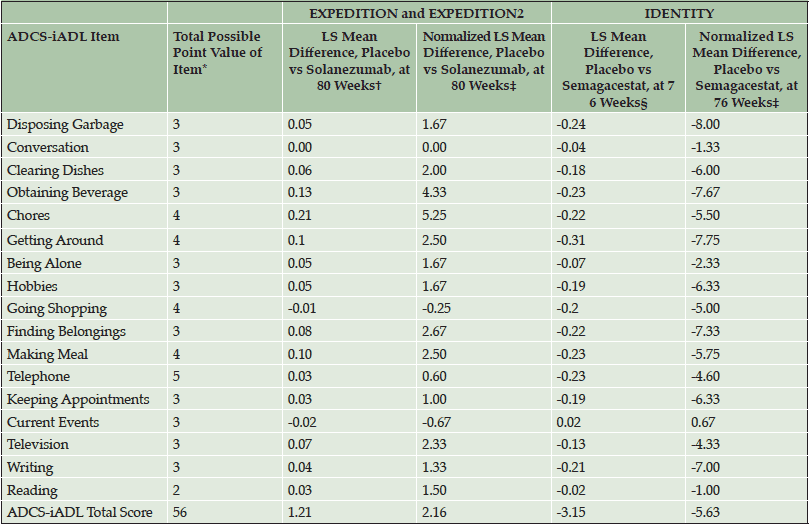
Table 6. Individual Item Scores of ADCS-iADL in Mild AD Patients from Solanezumab EXPEDITION and EXPEDITION2 Studies and Semagacestat IDENTITY Study in Treatment Effect Analyses
Abbreviations: ADCS-iADL = Alzheimer’s Disease Cooperative Study- Activities of Daily Living inventory, instrumental items; LS = least square. *Lower point values indicate greater impairment. †Positive value indicates positive treatment effect in favor of solanezumab. ‡Normalized LS mean difference was calculated as LS mean difference / total possible point value of item * 100. § Negative value indicates positive treatment effect in favor of placebo.
Discussion
The iADRS was developed using a theory-driven approach to create a single composite including two core domains of AD: cognition and function. The inclusion of the full ADAS-Cog14 and ADCS-iADL as components of iADRS was supported by analyses comparing it to the performance of other various cognition plus function composites in ADNI and EXPEDITION/EXPEDITION2 data sets. Because the iADRS is defined as a simple linear combination of the widely accepted ADAS-Cog and ADCS-iADL, it provides an overall measure of AD impairment (total score) as well as individual subscores for cognition and function. Analyses using EXPEDITION, EXPEDITION2, EXPEDITION-EXT, IDENTITY, and ADCS-MCI data sets demonstrated that the iADRS is sensitive to disease progression and to beneficial as well as detrimental treatment effects. Additionally, it has acceptable psychometric properties.
In development and validation of the iADRS, the 13-item and 14-item versions of the ADAS-Cog were used in various analyses, based on which version was collected in the respective data set. To maintain comparability across studies, both the ADAS-Cog13 and the ADAS-Cog14 were used in the calculation of iADRS where possible (solanezumab and semagacestat studies). No significant differences in results were found between the ADAS-Cog13 version and the ADAS-Cog14 version of iADRS (data not shown). Thus, iADRS can be applied in studies using either the ADAS-Cog13 or the ADAS-Cog14. If the ADAS-Cog13 is used, the total score for that version (85 points) should be used as the constant in the calculation of the iADRS. Similarly, both the FAQ and ADCS-iADL were used to test the concept of function plus cognition in a single composite based on availability in different data sets. Because no data set included both the FAQ and the ADCS-iADL, we were not able to directly compare performance. The ADCS-iADL may be more useful for the iADRS because it has shown its ability to show treatment differences, both positive and negative. Furthermore, it is more granular than the FAQ; thus, the scale range is better matched to the ADAS-Cog. This finding increases the likelihood that cognition and function will be measured equally in the composite.
Several basic test construction strategies were applied in the development and validation of the iADRS, including the evaluation of the contributions of individual items, the contributions of the cognitive and functional subscales, and principal component analysis. Furthermore, six separate datasets comprising AD subjects at different stages of the disease spectrum (MCI, mild AD, and moderate AD) as well as clinical trial subjects and subjects in observational studies were used in the creation and validation of the iADRS, resulting in heterogeneity of subjects and robust sample sizes.
All items of the ADAS-Cog14 and ADCS-iADL are included in the iADRS without weighting of individual items. Because the components are standard, accepted, and therapeutically responsive instruments, this construct yields face validity and ease of interpretation of the composite and also of its individual components. Analyses were performed to understand the contribution of every item in the iADRS to determine whether the ability of iADRS to detect disease progression and treatment effects could be improved by removing the least responsive items. Within and across datasets we found inconsistencies between items identified as most sensitive in the tracking of disease progression and treatment effect, suggesting that it would not be advantageous to select a subset of individual items to include in a composite. For example, an item-based composite with good sensitivity to detect disease progression might not be very sensitive for detecting a treatment difference. Likewise, an item-based composite with good sensitivity to detect disease progression and/or treatment effect in one data set might not be sensitive in another, even within the same stage of disease. Thus, selection of specific individual items of cognitive and functional scales to track disease progression and treatment effect was not supported by these analyses, and the iADRS includes all items from the subscales it comprises.
Despite differences in the possible total scores for the two subscales (ADAS-Cog14 and ADCS-iADL) and different baseline means (indicating that subjects were at different points within the scales at baseline), the observed baseline scores were within the same dynamic range across data sets, demonstrating the iADRS total score reflects approximately equal contributions from both cognitive and functional subscales. An advantage of equal contributions from both domains is that normalization of individual scales (by dividing the raw score at each time point by the total possible score for that scale to enforce equal contribution) is not necessary, and direct comparison across studies using the iADRS is possible.
The iADRS has demonstrated utility in the detection of disease progression across a broad range of the symptomatic disease spectrum, demonstrating suitability of its use in studies of mixed-spectrum populations. The sensitivity of the iADRS to treatment effects has been demonstrated in MCI and mild AD populations; however, we were not able to test its sensitivity to treatment effects in a moderate AD population because our data sets did not include a treatment trial with an observed treatment effect in moderate AD. Because most studies of potentially disease-modifying therapies span at least 18 months, substantial proportions of patients are likely to progress along the AD continuum. Thus, a tool sensitive across multiple stages of the continuum has obvious benefits for these long-term studies. Currently, a trial data set with robust treatment effects in MCI as well as in the later moderate stage to adequately test the performance of treatment detection is lacking.
The application of widely used scales to form the iADRS allowed validation across a large number of observational and treatment studies. However, the main limitation of these analyses is that the iADRS has not yet been used prospectively as an outcome measure. Prospective use of the iADRS as an outcome measure will provide further opportunity to characterize its performance in the detection of disease progression and treatment differences.
The use of a measure that integrates assessments of various core disease processes is common in clinical practice and the study of potential treatments for other chronic neurological disorders. For example, the Unified Parkinson Disease Rating Scale (UPDRS) (20), which measures various aspects of Parkinson’s disease including motor function, activities of daily living, and mentation, has been used as a primary outcome measure in clinical trials that assess symptomatic benefits of a new intervention, or to slow or delay disease progression (21). In other therapeutic areas, such as rheumatoid arthritis (22) and cardiology (23, 24), the approach of combining several endpoints into a single integrated primary outcome is accepted by regulators and within the fields of practice.
Taken together, these analyses demonstrate that the combination of cognitive and functional measures in the iADRS accurately and sensitively captures AD progression and treatment effects; thus the iADRS provides a useful integrated measurement tool for the AD research community.
Acknowledgements: The authors thank Kristin Wrobleski (Eli Lilly and Company) for scientific review, Michael Case (Eli Lilly and Company) for statistical review, and Laura Ramsey (Eli Lilly and Company) for assistance with article preparation.
Funding: Eli Lilly and Company (Indianapolis, Indiana, USA) provided funding for this project and was involved in study conduct and analysis and preparation and approval of the final manuscript.
Conflict of interests: A. M. Wessels, E. R. Siemers, P. Yu, S. W. Andersen, K. C. Holdridge, J. R. Sims, and K. Sundell are employees and minor shareholders of Eli Lilly and Company. Y. Stern reports personal fees from Eli Lilly and Company. D. M. Rentz reports consulting for Eli Lilly and Company. B. Dubois reports grants from Eli Lilly and Company and Pfizer. R. W. Jones reports grants from the UK Alzheimer’s Society, Economic and Social Research Council, and National Institute for Health Research; grants, personal fees, and non-financial support from Eli Lilly; grants, personal fees, and non-financial support from Servier; grants, personal fees, and non-financial support from Pfizer; personal fees and non-financial support from Lundbeck; personal fees and non-financial support from Novartis; grants from Genentech; grants from Boehringer Ingelheim; grants from Tau Rx; grants, personal fees, and non-financial support from AC Immune; and personal fees and non-financial support from Roche Pharmaceuticals. J. Cummings reports grants from from Avid Radiopharmaceuticals and Teva Pharmaceuticals; consultation to Abbvie, Acadia, ADAMAS, Alzheon, Anavex, Avanir, Biogen-Idec, Biotie, Boehinger-Ingelheim, Chase, Eisai, Forum, Genentech, Grifols, Intracellular Therapies, Lilly, Lundbeck, Merck, Neurotrope, Novartis, Nutricia, Otsuka, QR Pharma, Resverlogix, Roche, Suven, Takeda, Toyoma, GE Healthcare, and MedAvante; stock ownership in ADAMAS, Prana, Sonexa, MedAvante, Neurotrax, and Neurokos; copyright ownership of the Neuropsychiatric Inventory; and expert witness consultation regarding olanzapine and ropinerol. P.S. Aisen reports grants from Eli Lilly and Company.
Ethical standards: Ethical review board approval and informed consent of subjects were reported in the primary publications of the studies from which data were used in the current analyses.
References
1. Donohue MC, Sperling RA, Salmon DP, et al.; Australian Imaging, Biomarkers, and Lifestyle Flagship Study of Ageing; Alzheimer’s Disease Neuroimaging Initiative; Alzheimer’s Disease Cooperative Study. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol 2014;71(8):961-970.
2. Sperling RA, Rentz DM, Johnson KA, et al. The A4 study: Stopping AD before symptoms begin? Sci Transl Med 2014;6(228):28fs13.
3. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry 1984;141(11):1356-1364.
4. Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord 1997;11(Suppl 2):S13-21.
5. Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Jones RN, Mukherjee S, Curtis SM, Harvey D, Weiner M, Mungas D; Alzheimer’s Disease Neuroimaging Initiative. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav. 2012;6(4):502-516.
6. Gibbons LE, Carle AC, Mackin RS, et al.; Alzheimer’s Disease Neuroimaging Initiative. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav 2012;6(4):517-527.
7. Skinner J, Carvalho JO, Potter GG, et al.; Alzheimer’s Disease Neuroimaging Initiative. The Alzheimer’s Disease Assessment Scale-Cognitive-Plus (ADAS-Cog-Plus): an expansion of the ADAS-Cog to improve responsiveness in MCI. Brain Imaging Behav 2012;6(4):489-501.
8. Logovinsky V, Hendrix S, Perdomo C, Wang J, Satlin A. New composite score demonstrates sensitivity to disease progression and treatment effects. Presented at the AD/PD Conference on March 7, 2013.
9. Raghavan N, Samtani MN, Farnum M, et al. The ADAS-Cog revisited: Novel Composite scales based on ADAS-Cog to improve efficiency in MCI and early AD trials. Alzheimer Dement 2013;9(1 Suppl):S21-31.
10. Hendrix S, Ellison N, Stanworth S, et al. Methodological Aspects of the Phase II Study AFF006 Evaluating Amyloid-beta -Targeting Vaccine AFFITOPE® AD02 in Early Alzheimer’s Disease – Prospective Use of Novel Composite Scales. J Prev Alz Dis 2015;2(2):91-102.
11. Pfeffer RI, Kurosaki TT, Harrach CH Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol 1982;37(3):323-329.
12. Weiner M, Veitch DP, Aisen PS, et al.; Alzheimer’s Disease Neuroimaging Initiative. The Alzheimer’s Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimers Dement 2013;9(5):e111-e194.
13. Doody RS, Thomas RG, Farlow M et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med 2014;370(4):311-321.
14. Siemers ER, Sundell KL, Carlson C, Case M, Sethuraman G, Liu-Seifert H, Dowsett SA, Pontecorvo MJ, Dean RA, Demattos R. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer’s disease patients. Alzheimer’s Dement 2015 (in press).
15. Liu-Seifert H, Siemers E, Holdridge KC, et al. Delayed-start analysis: Mild Alzheimer’s disease patients in solanezumab trials, 3.5 years. Alzheimers Dement (NY) 2015;1(2)-111-121.
16. Doody RS, Raman R, Farlow M, et al.; Alzheimer’s Disease Cooperative Study Steering Committee, Siemers E, Sethuraman G, Mohs R; Semagacestat Study Group. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med 2013;369(4):341-350.
17. Petersen RC, Thomas RG, Grundman M, et al.; Alzheimer’s Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005;352(23):2379-2388.
18. Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997; 53:983-997.
19. Stevens JP. Applied multivariate statistics for the social sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1992.
20. Fahn S, Elton RL, Members of the UPDRS Development Committee. The Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, eds. Recent developments in Parkinson’s disease. Vol. 2. Florham Park, NJ: Macmillan Healthcare Information, 1987: 153-63.
21. Olanow CW, Rascol O, Hauser R, Feigin PD, Jankovic J, Lang A, et al. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med 2009;361:1268–78.
22. Felson DT, Anderson JJ, Boers M, Bet al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995, 38:727–735.
23. DeMets DL. Califf RM. Lessons learned from recent cardiovascular clinical trials: part 1. Circulation. 2002;106:746-751.
24. Dahlof B, Devereux RB, Kjeldsen SE. et al. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:995-1003.
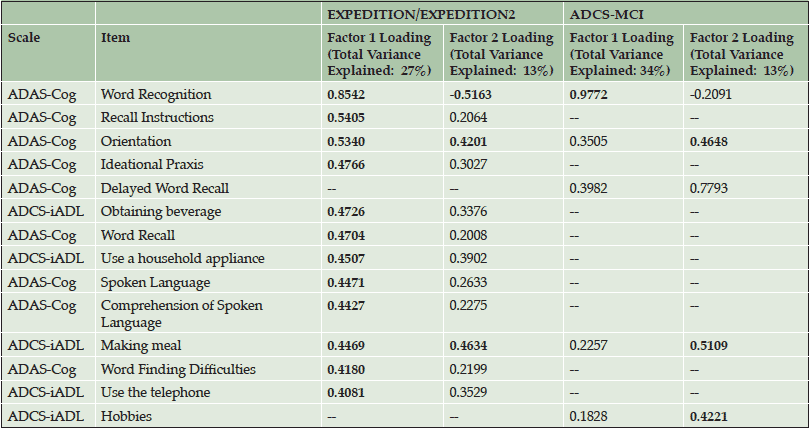
Table S1. Change from Baseline Principal Components and Significant Loadings (≥0.4 or ≤-0.4) for Items in the iADRS from Mild AD Patients in the Solanezumab EXPEDITION and EXPEDITION2 Studies and MCI APOE ε4 Carrier Patients in the Donepezil ADCS-MCI Study
Abbreviations: ADAS-Cog = Alzheimer’s Disease Assessment Scale, Cognitive Subscale; ADCS-iADL = Alzheimer’s Disease Cooperative Study-Activities of Daily Living inventory, instrumental items; MCI = mild cognitive impairment. NOTE: Significant factor loadings (≥0.4 or ≤0.4) in bold. Values for items not significant for either factor in a data set are not reported.
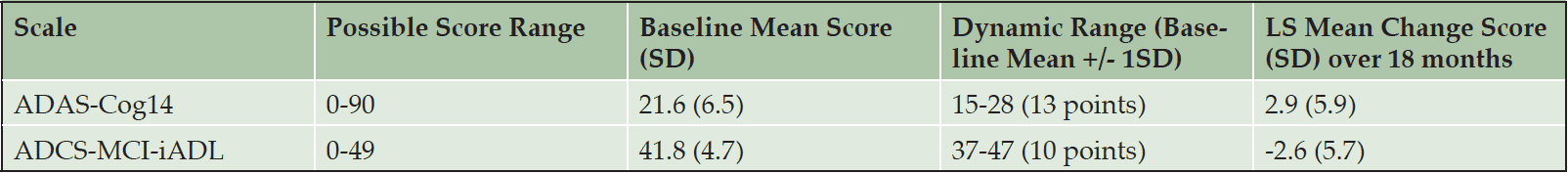
Table S2. Dynamic Ranges of iADRS Component Subscores (ADAS-Cog14 and ADCS-iADL) in MCI APOE ε4 Carrier Patients in the Donepezil ADCS-MCI Study
Abbreviations: ADAS-Cog14 = 14-item Alzheimer’s Disease Assessment Scale – Cognitive Subscale; ADCS-MCI-iADL = Alzheimer’s Disease Cooperative Study – mild cognitive impairment – Activities of Daily Living; LS = least squares; SD = standard deviation.
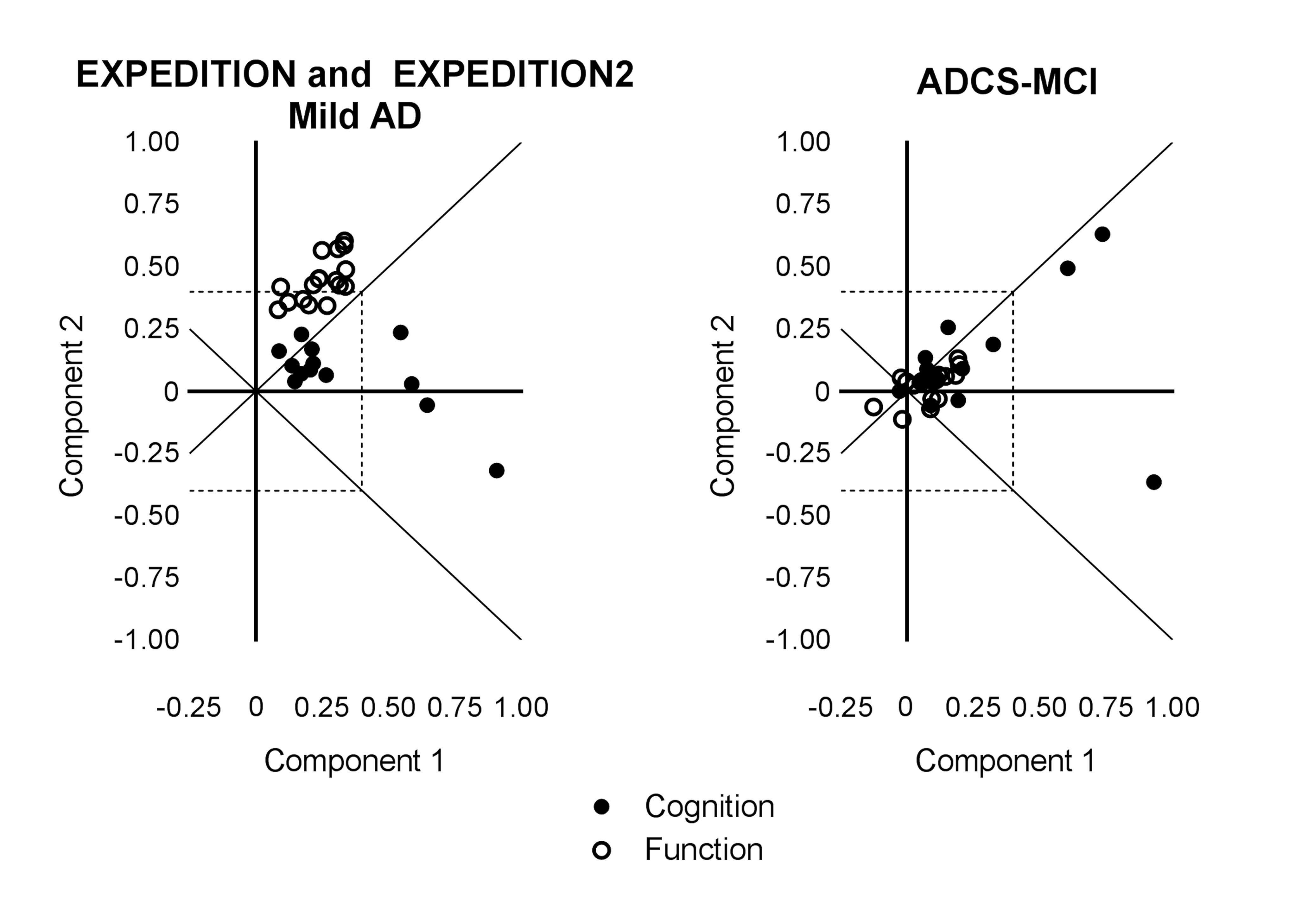
Figure S1. Baseline Principal Components and Loadings for Items in the iADRS from Mild AD Patients in the Solanezumab EXPEDITION and EXPEDITION2 Studies (left) and MCI APOE ε4 Carrier Patients in the Donepezil ADCS-MCI Study (right)