S. Tomaszewski1, S. Gauthier2, A. Wimo3, P. Rosa-Neto2
1. Faculty of Medicine, McGill University, Montreal, QC, Canada; 2. Alzheimer Disease Research Unit, McGill Centre for Studies in Aging, Douglas Mental Health Research Institute, Montreal, QC, Canada; 3. Karolinska Institute, Stockholm, Sweden
Corresponding Author: Serge Gauthier, McGill Center for Studies in Aging, Douglas Mental Health University Institute, Douglas Hospital, Verdun, QC, Canada. Phone: +1 514-766-2010; Fax: +1 514-888-4050. Email: serge.gauthier@mcgill.ca
J Prev Alz Dis 2016;3(3):164-172
Published online November 27, 2015, http://dx.doi.org/10.14283/jpad.2015.85
Abstract
Current drugs for treatment of mild to severe dementia of the Alzheimer’s type include cholinesterase inhibitors and the NMDA non-competitive receptor antagonist memantine. There is controversy as to the additive benefit of these symptomatic drugs, and their effects are clinically modest. Patients with Alzheimer’s disease (AD) are known to have characteristic pathology, including senile plaques with amyloid beta-protein aggregates and neurofibrillary tangles with assembled tau proteins, which start in the hippocampus and spread to neighboring areas. Amyloid and tau modifying drugs are under clinical testing. Based on this pathophysiology, it is crucial to investigate whether anti-amyloid and anti-tau combined therapy would show efficacy in early stage of AD, beyond what could be achieved with anti-amyloid or anti-tau monotherapy. It is equally important to consider the socio-economic implications of such a combination therapy, if effective. We hypothesize that the high costs of combination therapy for early-stage AD patients will require societal and public health initiatives to ensure universal access to AD treatment. In order to better predict these socio-economic implications, we summarize the management of other combination therapies used for tuberculosis, HIV/AIDS, and breast cancer, based on a database search of PubMed and other relevant sources. We put forward a framework for testing a potential anti-amyloid and anti-tau disease modifying combination therapy for early-stage AD patients and present an analysis of the socio-economic implications of such a combination therapy.
Key words: Alzheimer’s disease, dementia, combination therapy, anti-amyloid, anti-tau, socio-economic considerations, tuberculosis, HIV/AIDS, breast cancer.
Introduction
Alzheimer’s disease (AD) comes third on the scale of most expensive disorders in the United States, outranked only by cancer and coronary heart disease. The care given to AD patients can use up to 75% of household income (1). In the United Kingdom, dementia is found to be the number one brain-related disorder in terms of costliness, followed by mood and psychotic disorders (2). Worldwide, dementia affects as many as 46.8 million patients in 2015 and remains on the rise; the prevalence is expected to double every two decades, in parallel with population aging – the strongest risk factor contributing to the dementia epidemic (3). This prediction underpins the growing socio-economic impact of AD on patients, society, and healthcare systems in both the developed and developing world.
To address the Alzheimer’s epidemic with its growing human burden and associated economic tribulations, health ministers from eight top industrialized nations, have committed at the G8 Dementia Summit in UK (2013) to increasing research efforts with the goal to develop a cure or a disease modifying treatment for dementia within the next decade, by 2025 (4). In parallel, the socio-economic challenges with current symptomatic AD therapy and potential future AD drug combinations should guide the elaboration of public health policies, advocating for the quality of life of AD patients and working towards lowering the heavy burden of AD on patients, families, and society as a whole. Similar challenges have been encountered when dealing with tuberculosis (TB), HIV/AIDS, and breast cancer, due to the high cost of combination therapies implicated in the management of those diseases.
This position paper outlines potential solutions to a hypothetical AD combination treatment inspired by the management of other diseases that rely on multiple-drug therapies.
Rationale for combination therapy in AD
Currently, the recommended drug therapy for AD patients includes a cholinesterase inhibitor, such as donepezil, rivastigmine, or galantamine. There is ongoing controversy about the additive benefit of NMDA receptor antagonist memantine in the moderate to severe stages of AD. To date, research in drug development has been centered on monotherapies but there is emerging interest in testing drug combinations in AD (4) and this has been encouraged by the regulators (5).
In a multifactorial disease like Alzheimer’s involving various genetic and environmental risk factors, with resulting amyloid and tau abnormalities, it seems valid to target more than one element in the disease pathology to potentially better the therapeutic outcome. An analogous logic is applied in cancer care, where combination therapies act on multiple pathways to target cancer cells for apoptosis as efficiently as possible, while decreasing, as a side-benefit, the likelihood of drug resistance (6). The potential disease modifying combination therapy for AD, similarly to cancer combination regimens (6), will have to be closely tested for efficacy and safety, considering the potential risks of overlapping toxicity between agents and/or antagonistic neutralizing effects. Recent epidemiological results support the multi-factorial shape of AD and indicate that cardiovascular risk factors also may be involved in the pathogenesis of AD (and other dementias) (7); this may be one explanation to the possible, but not yet surely confirmed decline in prevalence of dementia (8, 9).
Anti-tau and anti-amyloid combination
Alzheimer’s patients present with two hallmark neuropathological findings: senile plaques and neurofibrillary tangles (NFTs). Senile plaques are aggregates of toxic fragments of the amyloid precursor protein, which collect progressively in the brain, often years before the symptomatic phase of AD. On the other hand, NFTs with intracellular hyperphosphorylated tau aggregates are found throughout the brain of AD patients and account for the disruption of neuronal function (10). Based on this pathological process (also shown in figure 1), it becomes intuitive to predict that disease modifying effects might be obtained by halting the progression of brain amyloid and tau pathology. Thus, the following protocol will outline the step-by-step approach to a future trial on the therapeutic effectiveness of anti-amyloid and anti-tau combination therapy, as compared to an anti-amyloid and anti-tau monotherapy.
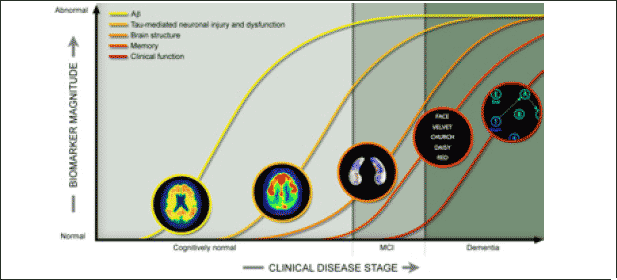
Figure 1. Different biomarker levels throughout the phases of AD, following normal clinical progression (Source: http://adni.loni.usc.edu/study-design/background-rationale/)
Protocol to demonstrate benefit of combination therapy in AD
Anti-amyloid and anti-tau combination therapy could be tested clinically following a specific framework summarized in table 1.
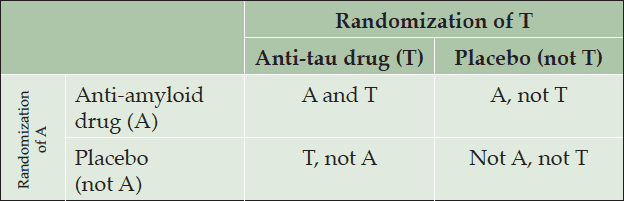
Table 1. 2 x 2 factorial design testing the effectiveness of anti-amyloid (A) and anti-tau (T) combination therapy versus A or T monotherapy
(Adapted from http://handbook.cochrane.org/chapter_16/16_5_6_factorial_trials.htm)
The target population could be selected from older patients with amnestic mild cognitive impairment (MCI) with positive biomarker suggestive of AD pathology, such as low CSF Aβ42 and high phospho-tau levels (11).
Potential alternative trial populations could be the subjects living in Antioquia, Colombia, who carry the presenilin-1 E280A mutation (PS1)—a genetic change that translates into early-onset familial AD (EOFAD) with severe cognitive decline early in life (often starting in the third decade) and severe amyloid-β and tau pathology (12)—or the more heterogeneous population of mutation carriers in the Dominantly Inherited Alzheimer Network (DIAN; (13)).
This potential drug combination could be tested according to a 2 x 2 factorial design (shown in table 2), the ideal framework for comparing the effectiveness of a drug combination versus monotherapy. The idea would be to separate the sample population into four subgroups receiving the following regimens: (1) anti-amyloid (A) and anti-tau (T) drug combination therapy, (2) anti-amyloid (A) drug combined with placebo, (3) anti-tau (T) drug combined with placebo, and (4) two placebos.
Clinical outcomes could be assessed using three distinct tools comparing differences between baseline and 12 months of treatment. The Clinical Dementia Rating – Sum of Boxes (CDR-SB) score, as a composite scale for the cognitive and functional state of AD patients, could serve as a primary outcome measure. Supportive secondary outcome measures could include biomarkers in the CSF, namely Aβ1-42 and phospho-tau-181, as well as the score on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog), a tool for evaluating cognitive impairment.
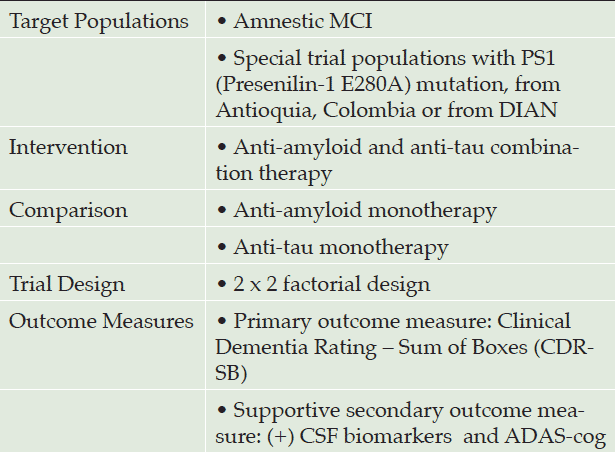
Table 2. Summary of Drug Protocol for AD disease modifying anti-amyloid and anti-tau combination therapy
Lessons from combination therapy in tuberculosis, HIV/AIDS and breast cancer
To maximize the quality of life of AD patients, it is crucial to anticipate the potential financial burden of an anti-amyloid and anti-tau combination drug therapy as well as be ready to propose effective solutions to mitigate costs. Thus, we will look at socio-economic success stories in TB, HIV/AIDS and breast cancer care in order to guide the elaboration of effective cost-lowering strategies for AD patients.
Tuberculosis
Tuberculosis (TB) is a valid public health concern considering its infectious nature and its large patient population. In 2004, 8.9 million new cases were estimated worldwide. Despite the existence of standard TB regimens, tuberculosis remains the treatable infectious disease with the highest mortality (14). To fight this epidemic, WHO set up the Stop TB partnership (2001) which proposed a Global Plan to Stop TB, 2001-2005 (with further developments for 2006-2015), advocating six key WHO-recommended elements, for instance improving disease detection and cure, involving all care providers, and investing in research (15). These global disease-specific targets, guiding public health initiatives, complement the United Nations’ Millennium Development Goal (MDG) 6, whose objective is to stop and progressively reverse the incidence of major infectious diseases, such as malaria, HIV/AIDS, and TB, by 2015 (16).
Out of the 10 anti-TB drugs approved by the U.S. Food and Drug Administration (FDA), the current first-line treatment for tuberculosis consists of a two-month combination therapy of isoniazid (INH), rifampin (RIF), ethambutol (EMB), and pyrazinamide (PZA), followed by a four-month INH and RIF regimen (17). In the 1950s, a variety of novel TB drugs with distinct mechanisms of action were developed, paving the path for a new combination therapy (18). Already in 1966, a pivotal paper was published regarding the combination of isoniazid with para-amino salicylic acid (PAS) for patients with pulmonary TB. This combination turned out to be equally safe for administration at home as in a sanatorium, under supervision of the health care team, without real risk of onward transmission to close family contacts. Thus, this pivotal study served as the ground base for the introduction of ambulatory care in TB (19). One year later, rifampicin, a new bactericidal antituberculous drug, was synthesized, marking a major breakthrough in TB drug development. When RIF was included in the TB cocktail-drug treatment, along with another potent agent, PZA, the new resulting regimen was a revelation: it increased the long-term cure rates to 95% or more, while shortening the duration of treatment by over a half (20).
Initially, those advances, despite their therapeutic benefits, imposed a large financial burden on TB patients, namely due to the high cost of the first-line combination therapy. Other direct expenditures involve the costs for: diagnostics and follow-up tests, inpatient care, diet supplements, transportation to doctor visits, to name a few. Indirect expenditures, such as lost productivity, are also a huge concern in TB given that the patient population is quite young and a large portion is on sickness leave from work until recovery (21).
To alleviate part of the financial struggle experienced by many TB victims, partnerships on a local, national and international level have mobilized to ensure free or at least more affordable antituberculous drug combinations, especially in high TB-burden countries. The Global Drug Facility (GDF), established by the Stop TB partnership in 2001, is one such initiative that offers antituberculous drugs for free or at little cost to countries in need (22, 23). GDF also strengthens national TB programmes by helping them with proper drug distribution and administration (22). GDF, in line with the mission of the Stop TB partnership, managed to grant drugs at lower prices thanks to the expansion of the drug supplier base, bulk purchasing, and competitive bidding at an international level (23).
In the early 1990s, China implemented the WHO-recommended TB control strategy, DOTS (Directly Observed Treatment – Shortcourse), thus becoming a pioneer country in offering free TB services. The DOTS approach is an example of public health intervention aimed at better global disease control. It comprises five main elements centered on sustained political mobilization with strategic partnerships and proper funding, improved diagnostics, standard supervised therapy with adequate patient support, continued drug supply, and monitoring systems with data reporting for outcome measurement. An expansion to this strategy, DOTS-plus (1999), addresses the high cost of second-line TB drugs for multidrug-resistant TB (MDR-TB) patients. Many countries have also appealed to external bodies for financial support to allow proper TB management. In Swaziland, a South-African sovereign state, the government has entered into partnership with international nongovernmental organizations in order to maximize access to medicines and TB care services throughout the country (24).
Another cost-lowering strategy involves non-profit generic companies and philanthropic donors. For instance, the Global Drug Facility (GDF) provides drugs at low prices thanks to the generosity of donors who help fund medicine stockpiles for the treatment of many illnesses, TB included (23). The Global Fund to fight AIDS, Tuberculosis and Malaria (2002) as well as other international sources of financial aid have a variety of grants available to support TB care in candidate countries (24).
In the United States, two fixed-dose combinations have been approved for use in TB management by the Food and Drug Administration (FDA): Rifamate© (isoniazid and rifampin) and Rifater© (isoniazid, rifampin, and pyrazinamide). According to expert opinion, while no evidence would suggest a superior pharmacologic activity and therapeutic effect of fixed-dose combinations compared to individual medicines, such drug formulations are recommended in cases where DOT is administered daily or, on the contrary, when DOT is not given at all. Using fixed-dose combinations reduces the amount of capsules or tablets that a patient has to take, thus offering an advantage in terms of easier drug administration and improved adherence, as well as potentially decreasing the likelihood of drug resistance since patients cannot mistakenly take one type of pill selectively out of the other drugs prescribed in their combination therapy (17, 25).
It can be argued that TB drug combinations should be provided for free to all affected patients, since many of them would not be able to afford the treatment otherwise. Another argument is of a social construct; the logic is that “treatment has benefits that extend to society as a whole (cure prevents transmission to others)” given the infectious nature of TB (26). Strategies to mitigate the costs of drug combinations also indirectly promote an increased access to medicines by the very principle that less expensive drugs can be more easily afforded by a larger patient population. Another method used in TB care to optimize treatment accessibility is the inclusion of anti-TB drugs into the WHO Model List of Essential Medicines (27). WHO formulates an “Access Framework” (2004) around that model list to promote universal access of these essential medicines by advocating strategic selection and use of these drugs, along with reasonable prices, continued funding, and efficient management and delivery of the drug supply (28). A wide variety of organizations (international, nongovernmental, non-profit, etc.), such as UNICEF, UNHCR and UNFPA, use the Model List as a basis for their drug supply system (29).
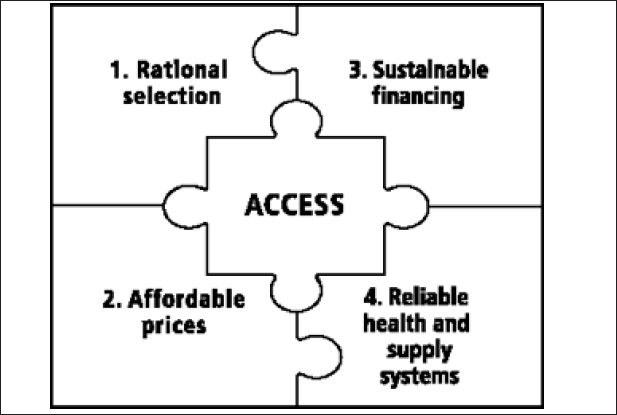
Figure 2. Four key elements of WHO’s “Access Framework” meant to maximize access to essential medicines – thus reflecting Millennium Development Goals, Target 17 (Source: http://apps.who.int/medicinedocs/en/d/Js4962e/#Js4962e)
HIV/AIDS
The worldwide prevalence of HIV was estimated to be 35.3 million in 2012, with 70.8% living in Sub-Saharan Africa, compared to 31.0 million in 2002. In parallel, new cases of infection decreased from 3.3 million to 2.3 million within the same time period. Clearly, progress has been made thanks to the introduction of combination antiretroviral (ARV) therapies in the late 1990s, which transformed HIV into a chronic yet controllable condition. The increased longevity thus achieved explains the growing prevalence of HIV/AIDS (30).
The standard ARV regimen prescribed nowadays is a triple therapy; it involves one non-nucleoside reverse transcriptase inhibitor (NNRTI), integrase inhibitor or protease inhibitor (PI), combined with two nucleoside reverse transcriptase inhibitors (NRTIs) (30). As shown in the Merck protocol 035, a pivotal clinical trial, despite the pill burden and the associated treatment cost, the benefit of delayed disease progression with a triple therapy of two NRTIs with a PI (versus two NRTIs or a PI alone) can partially dwarf the costs of treatment. Indeed, according to an economic model developed by Cook et al (31) which was applied to patients from the Merck Protocol 035, the costs of triple therapy, given that suppression lasts up to five years, would exceed the costs of double therapy, yet 81% could be discounted based on the fact that fewer cases progressed to AIDS.
To further fight the spread of HIV/AIDS and promote universal access to treatment, similarly to the AIDS targets of the Millennium Development Goal 6 (16), the Joint United Nations Programme on HIV/AIDS (UNAIDS) launched in 2012 a global action plan called Treatment 2015. This initiative included a specific target known as “15 by 15” meaning that 15 million patients will have access to HIV antiretroviral drug regimens by 2015. Despite many challenges, this “15 by 15” goal turned into a success story, and even better, it made history, because it was the first time that a quantifiable treatment objective in global health had been met before its deadline (32).
To make ARV drugs more affordable, especially for low-income nations, in 2001, former United Nations Secretary-General Kofi Annan brought forward the idea of a global fund for HIV treatment that would offer free drugs after having bought them at little cost. He argued that ARV drugs remained expensive for disadvantaged patient populations, even after price reductions by pharmaceutical companies. This valid argument underpinned the need for a drastic change in the health economic management of ARV drug therapies; one year later, in 2002, the Global Fund to Fight AIDS, Tuberculosis and Malaria was established. In the next decade, US$ 20 billion have been pledged, the majority of funds going into HIV/AIDs treatment and care services. This amount is the largest pledge that has ever been made for a single health condition in such a short time period (24).
Additional funding for ARV drugs, which are the basis of HIV/AIDS treatment, has been made possible by the United States President’s Emergency Plan for AIDS Relief (PEPFAR), which works in close collaboration with UNAIDS, UNICEF, and more broadly with WHO. From 2003 to 2011, PEPFAR dedicated sustained efforts into procuring the funds necessary for the purchase of ARV drugs to prevent mother-child transmission, thus successfully protecting 340,000 babies from HIV infection. Thanks to PEPFAR, during 2004-2011, the US government has collected and disbursed the unforeseen value of US$ 32 billion for HIV (24). As part of PEPFAR, the Supply Chain Management System (SCMS) was launched in 2005. This initiative is focused on strengthening and creating new drug supply chains for HIV to ensure accessibility and best-value of ARV drugs, HIV test kits and other products (33).
Analyzing the situation of AIDS, Berwick (58) postulates that price reductions should go beyond the public sector; they should be applied across the board. In a more radical tone, the author claims that AIDS—–the most deadly epidemic in all history—–could be quickly resolved if pharmaceutical companies decided to improve the fate of the world and provide HIV drugs for free to disadvantaged populations in lower-income countries (58). Actually, five world-leading pharmaceutical companies agreed to decrease prices of HIV drugs in sub-Saharan Africa, some even offering 90% price reductions (34).
There seems to be a need for putting more pressure on pharmaceutical companies to sell quality drugs for less. In 1986, Burroughs Wellcome & Co. synthesized a new very expensive drug, Zidovudine, initially intended for cancer care, and accidentally observed to delay progression to AIDS via its effect on maintaining a low viral load. The high annual cost of US$10,000 per patient linked with a Zidovudine regimen would have prevented many HIV victims from using such drugs, had it not been for social pressure from activists that forced the pharmaceutical company to lower the price by 20%. Despite alleviating part of the financial burden, this drug remains highly unaffordable in resource-poor countries (24).
To alleviate the financial burden of HIV combination therapy and other associated medical or nonmedical costs, the HIV/AIDS Policy, Coordination and Programs Division, under the Public Health Agency of Canada, offers five funds to help fight this chronic illness, as part of a federal mission plan, the “Federal Initiative to Address HIV/AIDS in Canada” (35). Nonprofit generic companies, subsidized by philanthropic foundations, could also help ensure lower drug prices and greater accessibility to treatment. This has been already achieved with considerable success in Africa, when dealing with the AIDS epidemic (36).
To attain the most affordable prices for essential medicines such as the ARV drugs, it is crucial to have generic competition for bio-equivalent drugs (58). Equally, comparative drug price information needs to be made publicly available to inform the patient population on their options, thus helping them make cost-effective decisions. The International Drug Price Indicator Guide (updated annually after its first edition in 1986) is one such tool developed by the Management Sciences for Health (MSH), a non-profit international health organization, working with WHO since 2000. This guide comprises a price comparison of different pharmaceutical products, diagnostic tests, and other medical tools, from many suppliers (both commercial and non-profit), for the prevention and treatment of various prevalent illnesses like HIV/AIDS. This price list is used as a basis for the publication on “Sources and Prices of Selected Drugs and Diagnostics for People Living with HIV/AIDS”. Both these documents help patients select the least expensive option for their prescribed regimen, while also improving the drug procurement system on a societal dimension, by encouraging competition and negotiations for the best price (28, 33).
Turning to patent rules, Brazil can serve as a case study for the use of compulsory licensing as a way of exerting positive pressure on pharmaceutical companies; the Brazilian government managed on many occasions to lower prices of ARV drugs under the threat of breaking the companies’ drug patents and allowing domestic production of their products. Thanks to this negotiation tactic, Brazil was able to force lower drug prices for nelfinavir and efavirenz on Roche and Merck, the respective drug manufacturers. Compulsory licensing, although acceptable under Brazil’s patent law, became a reason of concern for pharmaceutical companies and other stakeholders. This led the World Trade Organization (WTO) to open a discussion panel on compulsory licencing, following a request from the United States on behalf of its pharmaceutical companies. This panel was dedicated to assessing the validity of Brazil’s appeal to compulsory licensing in light of the Trade-Related Aspects of Intellectual Property Rights (TRIPS) agreement—–a binding law ensuring that copyright, patent and other property rights are protected. Despite the clear political and financial dilemma, soon after, WTO dropped its dispute panel regarding Brazil’s clashing interpretation of the TRIPS agreement. In November 2001, the Doha Declaration was released to clarify when a state is justified in resorting to compulsory licencing: the conclusion is that this “negotiating tool” can be used to address public health emergencies. More commonly, Brazil brings drug prices down by encouraging domestic production of medicines at a lower cost, thus largely reducing the need for international import. In 1999 for instance, nearly half of national ARV drugs were produced in state or privately-run firms (37).
There are currently over 20 distinct ARV drugs, out of which a few have been joined together into fixed-dose combination (FDC) products. FDCs have the advantage of facilitating distribution and administration due to a lower pill burden, and potentially decreasing treatment costs. However, only selected ARV drugs can be combined given the possibility of antagonistic reactions and overlapping toxicity. Despite the potential risks of FDCs, if not properly tested for efficacy and safety, some newer FDCs (still waiting for FDA approval) have already been marketed in resource-poor countries where HIV reached crisis levels in order to benefit from the indispensable advantages of FDCs (38).
Breast Cancer
Cancer care is a very costly endeavor, especially now that patients tend to survive longer, thus prolonging the treatment period (39). Breast cancer, specifically, is the main cause of cancer-related mortality among women throughout the world. Incidence rates are highest in high-income countries and although low at baseline in more resource-limited settings they are on the rise (40). This is why a new approach in health-care economics needs to be considered. Many governments have already taken some steps in this direction in their respective National Cancer Plans. Also known as “national cancer control programmes” by the World Health Organisation, they lay out strategies on how to best deal with new cancer cases. Although they differ slightly across the different jurisdictions, they typically emphasize prevention as well as early detection to increase likelihood of a successful treatment and thus lower costs (41).
Pharmacologic treatment of breast cancer includes a large number of anticancer agents with distinct mechanisms of action and varying effects on tumor cells. Common examples are: “methotrexate, 5-fluorouracil (5-FU), cyclophosphamide, anthracyclines, taxanes, trastuzumab, tamoxifen, and aromatase inhibitors” (42). These and other medications are often combined to maximize treatment outcomes by targeting different cell pathways and receptors. The majority of drug combinations are not approved by the FDA, unlike the individual drugs that they consist of, yet they are still commonly used. Standard chemotherapy regimens can include any of the following combinations: AC (Adriamycin (A) + Cyclophosphamide (C)), AC-T (A + C + Paclitaxel (Taxol)), CAF (C + A + Fluorouracil (F)), CMF (C + Methotrexate + F), FEC (F + Epirubicin Hydrochloride + C), and TAC (Docetaxel (Taxotere) + A + C) (43).
Addressing cancer therapies, an American study mentions the need for better evidence-based national guidelines; one suggestion is to replace the current available list of treatment options for each type of cancer with a comparative cost-effective analysis of different generic drugs, including risks and benefits for patients, which would facilitate treatment choices (36). The Breast Health Global Initiative (BHGI) works on a specific set of guidelines, culturally adapted to poorer nations that lack sustainable healthcare systems, to improve health outcomes for patients with breast cancer (44).
An interesting field in development is the study of pharmacogenomics that looks at predictive factors to maximize treatment responsiveness, thus potentially increasing the cost-effectiveness of otherwise costly combination therapies. Before opting for genetic screening, an economic analysis is needed to evaluate the quantity of savings gained from a more targeted treatment, i.e. administered to patients on the basis of whether they express or do not express a specific protein predictive of therapeutic success, with respect to the additional costs of testing and decreased revenue for drug producers linked with a more restricted patient selection. The resulting disadvantage of genetic testing for pharmaceutical companies may force them to raise the prices of the drug combinations in question. This likely outcome needs to be weighed against the societal and health benefits. The concept of pharmacogenetics is growing in importance in oncological conditions (and HIV/AIDS, to a slightly lesser extent) (45).
When conducting a socio-economic analysis of combination therapies, it is crucial to adopt a far-reaching view on their financial impact. For example, although offering an adjuvant therapy implies additional immediate costs, on the long term such a regimen (if optimal) can be cost-saving, because it has the potential to inhibit metastases and limit recurrences of tumor (39).
Combination therapy for AD
A large number of patients, reaching epidemic proportions, are suffering from dementia; as of 2015, there were 46.8 million cases of dementia worldwide (3), exceeding highly prevalent diseases like tuberculosis and HIV/AIDS.». This number is expected to reach 74.7 million by 2030, almost doubling in the space of 20 years (3). To counter the high associated worldwide cost of dementia, of the amount of US$ 818 billion in 2015 (3), different cost-lowering strategies must be applied, based on success stories from other prevalent conditions, such as TB, HIV/AIDS, and breast cancer. There is also no state of opposition with the potentially positive effects of prevention activities in AD and other dementias as indicated by the FINGER study (46) and a pharmaceutical combined disease modifying treatment.
As in other illnesses, effective management of AD costs requires putting pressure on pharmaceutical companies by encouraging competition for bio-equivalent drugs. Also, another measure is to stimulate competition at the level of drug regulators, which can be achieved by privatizing existing certification boards. Privatised regulators would come up with specific standards of regulation based on the choice requirements of those who select drug regimens, thus making the approval process both smoother and faster. In the same vein, development costs will be potentially reduced, thus allowing more drugs to be produced for a given monetary value – this means the drug supply will become less expensive and come closer to matching the demand, thus increasing access to medicines (47).
While a potential anti-amyloid and anti-tau combination therapy for early-stage AD patients would imply a higher cost of prescription medications, if indeed found to have a disease modifying effect, we would expect the resulting caregiver time needed to be reduced, thus balancing out this increase in drug cost, or at least relieving some of the financial burden on society.
Delaying the onset and evolution of AD by 1 year via preventive interventions and early therapeutics can save around 9 million people worldwide from having an Alzheimer’s diagnosis in 2050. This may serve as a cost-benefit argument for encouraging preventive measures in dementia care (48). Similarly, delaying institutionalization at the moderate to severe AD phases, even only by one month, was shown to generate cost savings of US$1863 monthly. From a socio-economic perspective, it appears advantageous to prolong home care as much as possible (given that the condition of the patient permits it); it can help reduce direct healthcare costs as well as decrease the global burden of disease by enhancing the quality of life of AD patients, for whom home care is often the number one preference (49). However, cost-effectiveness per se does not necessarily imply that cost savings must be achieved. There is a societal willingness to pay (WTP) for improvement in care. An effective disease modifying treatment will probably prolong survival and since treatment will start in predementia states (where costs of care also without treatment are rather low), the aggregated treatment cost will be significant (50). Even if the total aggregated costs during the whole period, from early symptoms to death, may be higher with than without disease modifying treatment, it may be regarded as cost-effective due to significant effects in the outcomes, supporting good value for money.
In the event that the anti-amyloid and anti-tau drug combination, proposed in this paper, is found be an effective disease modifying therapy, the use of pharmacogenomics would become more ethically acceptable, since a positive genetic test would no longer “[condemn] an innocent person to death without his being able to escape his fate” as would be the case in the absence of preventive or curative treatment (10).
However, to study the long-term cost-effectiveness of a combination therapy is not easy. Several designs need to be used (51). While efficacy is analysed in phase 3 trials, cost-effectiveness analysis based on empirical within trial data is often taking place in phase 4 trials. Since resource use and cost data are frequently skewed, the power analysis will show that the needed sample sizes are much larger than for clinical efficacy measures (52). Furthermore, to cover the whole survival in controlled trials is not possible and thus health economic modelling is needed (53).
New genetic tests exist to check the status of the apolipoprotein E type 4 gene, the main genetic indicator of risk for AD (10). The ApoE gene can also serve as a predictive factor to select patients who are more likely to respond to a symptomatic and/or disease modifying drug therapy (54). Furthermore, developing biomarkers for diagnostic and prognostic purposes could help improve detection of disease (55) while decreasing costs, by being used as a replacement for the more expensive PET scans. The use of effective biomarkers is also essential to avoid cases meeting clinical criteria for dementia without Alzheimer’s disease pathophysiology in the early diagnostics of AD (56). However, it remains elusive which biomarkers would be sufficient to identify carriers of AD pathophysiology.
There is also a clear need for improving the drug development process to maximize efficiency and decrease costs. A potential solution to this challenge is brought forward by Accelerating Medicines Partnership (AMP), which involves the collaboration between four types of actors: US National Institutes of Health (NIH), US Food and Drug Administration (FDA), 10 pharmaceutical industries, and a group of non-profit organizations, working in three disease areas: AD, type 2 diabetes, and autoimmune disorders of rheumatoid arthritis and systemic lupus erythematosus. All partners accept to pool their data on biomarkers and potential drug targets thus increasing the likelihood of developing targeted therapies without the typical failure rate. Clearly, it is important to ensure an optimal choice of drug target and design before conducting the actual trial, and AMP facilitates this task. Normally, over 95% of candidate drugs do not pass the extensive testing which spans over many years; those that fail in the late phase clinical trials are responsible for the greatest waste of money and time. Thus, improving the drug development process is an essential cost-lowering strategy needed to revolutionize the health economics of AD (57). This is of great importance since 58% of people with AD and other dementias worldwide presently live in low and middle income countries and this proportion is estimated to increase to 63% in 2030 and 68% in 2050 (3).
Access to treatment with the current drugs is today limited in many countries. If a combination disease modifying treatment will enter the market, its price will be significant for most people with dementia worldwide and the whole issue of pricing and reimbursement will be crucial. Cost-lowering strategies used or predicted to be of use in TB, HIV/AIDS and breast cancer are equally applicable to Alzheimer’s disease. These include: local, national or international partnerships aiming to provide more affordable drugs, drug price indicators with cost-effective data, inclusion of anti-tau and anti-amyloid drug combination in WHO’s list of essential medicines, appeal to philanthropic donors, public health policies, compulsory licensing, fixed-dose combinations, maximization of domestic production, improved drug distribution to remote areas or underprivileged populations, and raised awareness of the disease and its management among patients and caregivers.
Conclusion
When designing a protocol for a potential disease modifying therapy, the socio-economic impact of such a pharmacological construct also has to be speculated and evaluated prior to its chemical development and introduction into the market, in order to reduce its future monetary burden and increase access to such therapy. This research achieves exactly that. It goes beyond the current symptomatic treatment alternatives for AD; it introduces a framework for testing a potential anti-tau and anti-amyloid disease modifying combination therapy for early-stage AD patients and includes a socio-economic analysis of such a combination therapy, based on the cost-lowering strategies used in other prevalent diseases, such as TB, HIV/AIDS, and breast cancer.
While a cost-effectiveness analysis of the potential anti-amyloid and anti-tau combination therapy is important for treatment decisions, it cannot be the only determining factor that will guide physicians to prescribe the given regimen or not. Optimizing the quality of AD care does not mean blindly applying cost-effectiveness study results to clinical practice. Patient and family goals and expectations need to be considered case by case in order to provide the most human and compassionate care in Alzheimer’s disease.
Disclosures: The authors declare no conflict of interests.
Acknowledgements: SG and PRN receive peer-reviewed funding from the CIHR, and the ELSI program of the Canadian Consortium for Neurodegeneration in Aging (CCNA) funded the summer studentship of ST.
References
1. Castro DM, Dillon C, Machnicki G, Allegri RF. The economic cost of Alzheimer’s disease: family or public health burden?. Dement. Neuropsychol. 2010;4(4):262-267.
2. Fineberg NA, Haddad PM, Carpenter L, Gannon B, Sharpe R, Young AH, et al. The size, burden and cost of disorders of the brain in the UK. J Psychopharmacology. 2013;27(9):761-70.
3. Alzheimer’s Disease International. World Alzheimer report 2015 the global impact of dementia: an analysis of prevalence, incidence, cost and trends. 2015;1-88.
4. Long R. Finding a path for the cure for dementia: an independent report into an integrated approach to dementia research [Internet]. 2015;1-28.
5. U.S. Food and Drug Administration. Guidance for industry Alzheimer’s disease: developing drugs for the treatment of early stage disease (Draft Guidance). New Hampshire: U.S. Department of Health and Human Services FDA/Center for Drug Evaluation and Research. 2013.
6. LoRusso PM, Canetta R, Wagner JA, Balogh EP, Nass SJ, Boerner SA, et al. Accelerating cancer therapy development: the importance of combination strategies and collaboration. Summary of an Institute of Medicine workshop. Clin Cancer Res. 2012;18(22):6101-9.
7. Kivipelto M, Mangialasche F, Solomon A, Johansson G, Lannfelt L, Fratiglioni L, et al. 9th Key symposium introduction: updating Alzheimer’s disease diagnosis implications for prevention and treatment. J Intern Med. 2014;275(3):202-3.
8. Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382(9902):1405-12.
9. Rocca WA, Petersen RC, Knopman DS, Hebert LE, Evans DA, Hall KS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7(1):80-93.
10. Poirier J, Gauthier S. Alzheimer’s disease : the complete introduction. Toronto: Dundurn; 2014.
11. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):270-9.
12. Sepulveda-Falla D, Glatzel M, Lopera F. Phenotypic profile of early-onset familial Alzheimer’s disease caused by presenilin-1 E280A mutation. J Alzheimers Dis. 2012;32(1):1-12.
13. Bateman RJ, Xiong C, Benzinger TLS, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Eng J Med. 2012;367(9):795-804.
14. Dye C. Global epidemiology of tuberculosis. The Lancet.367(9514):938-40.
15. Stop TB partnership. The global plan to stop TB, 2006-2015 [Internet]. World Health Organization; 2015:1-172. Available from: http://www.stoptb.org/assets/documents/global/plan/globalplanfinal.pdf
16. World Health Organization. MDG 6: combat HIV/AIDS, malaria and other diseases [Internet]. World Health Organization; 2014. Available from: http://www.who.int/topics/millennium_development_goals/diseases/en/.
17. Centers for Disease Control and Prevention. Core curriculum on Tuberculosis: what the clinician should know [Internet]. 2013;139-187. Available from: http://www.cdc.gov/tb/education/corecurr/pdf/corecurr_all.pdf.
18. Zumla A, Nahid P, Cole ST. Advances in the development of new tuberculosis drugs and treatment regimens. Nat Rev Drug Discov. 2013;12(5):388-404.
19. Kamat SR, Dawson JJ, Devadatta S, Fox W, Janardhanam B, Radhakrishna S, et al. A controlled study of the influence of segregation of tuberculous patients for one year on the attack rate of tuberculosis in a 5-year period in close family contacts in South India. Bull World Health Organ. 1966;34(4):517-32.
20. Geraint D. Chemotherapy including drug-resistant therapy. Clinical Tuberculosis, 5th ed: CRC Press; 2014. p. 229-40.
21. Tiemersma EW, Collins D, van den Hof S. Costs faced by (multidrug resistant) tuberculosis patients during diagnosis and treatment: report from a pilot study in Ethiopia, Indonesia and Kazakhstan. [place unknown]:KNCV Tuberculosis Foundation/Management Sciences for Health; 2014.
22. Matiru R, Ryan T. The Global Drug Facility: a unique, holistic and pioneering approach to drug procurement and management. Bull World Health Org. 2007;85(5):348-53.
23. Lunte K, Cordier-Lassalle T, Keravec J. Reducing the price of treatment for multidrug-resistant tuberculosis through the Global Drug Facility. Bull World Health Org. 2015;93(4):279-82.
24. World Health Organization. Bugs, drugs, & smoke: stories from public health [Internet]. World Health Organization; 2011. Available from: http://whqlibdoc.who.int/publications/2012/9789241564366_eng.pdf.
25. Mukund U, Diana W. WHO’s Stop TB Strategy. Tuberculosis. Lung Biology in Health and Disease: CRC Press; 2009. p. 300-24.
26. Singh JA, Bhan A, Upshur R. Diagnosis of drug-resistant TB and provision of second-line TB treatment in India: some ethical considerations. Indian J Med Ethics. 2013;10(2):110-4.
27. World Health Organization, Stop TB Initiative. Treatment of tuberculosis: guidelines. 4th ed. Geneva: World Health Organization; 2010.
28. van Boxtel CJ, Santoso B, Edwards IR. Drug benefits and risks: international textbook of clinical pharmacology. 2nd ed. Amsterdam; Uppsala, Sweden: IOS Press; Uppsala Monitoring Centre; 2008.
29. World Health Organization. Essential medicines [Internet]. World Health Organization; 2015. Available from: http://www.who.int/medicines/services/essmedicines_def/en/.
30. Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384(9939):258-71.
31. Cook J, Dasbach E, Coplan P, Markson L, Yin D, Meibohm A, et al. Modeling the long-term outcomes and costs of HIV antiretroviral therapy using HIV RNA levels: application to a clinical trial. AIDS Res Hum Retroviruses. 1999;15(6):499-508.
32. UNAIDS. “15 by 15”: a global target achieved [Internet]. Geneva: Joint United Nations Programme on HIV/AIDS; 2015. Available from: http://www.unaids.org/sites/default/files/media_asset/UNAIDS_15by15_en.pdf.
33. Frye JE, editor. International drug price indicator guide [Internet]. Cambridge, MA: Management for Services for Health/WHO; 2010. Available from: http://apps.who.int/medicinedocs/documents/s18714en/s18714en.pdf.
34. Gottlieb S. Companies reduce prices for HIV drugs in developing countries. Bull World Health Org. 2000;78(6):862.
35. Public Health Agency of Canada. National HIV/AIDS grants and contributions funds [Internet]. Public Health Agency of Canada; 2006. Available from: http://www.phac-aspc.gc.ca/aids-sida/funding/national_fund-eng.php.
36. Siddiqui M, Rajkumar SV. The high cost of cancer drugs and what we can do about it. Mayo Clin Proc. 2012;87(10):935-43.
37. Perlman D, Roy A. The practice of international health : a case-based orientation. Oxford; New York: Oxford University Press; 2009.
38. U.S Food and Drug Administration. Guidance for industry – fixed dose combination and co-packaged drug products for treatment of HIV [Internet]. Silver Spring: U.S. Food and Drug Administration: 2004.
39. Beckmann MW, Lux MP. Health economics in breast cancer. Breast Care (Basel). 2013;8(1):5-6.
40. Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2(3):133-40.
41. World Health Organization. National cancer control programmes [Internet]. Geneva: World Health Organization; 2015. Available from: http://www.who.int/cancer/nccp/en/.
42. Navolanic PM, McCubrey JA. Pharmacological breast cancer therapy (review). Int J Oncol. 2005;27(5):1341-4.
43. National Cancer Institute. Drugs approved for breast cancer [Internet]. National Cancer Institute. 2014. Available from: http://www.cancer.gov/about-cancer/treatment/drugs/breast#3.
44. Galukande M, Kiguli-Malwadde E. Rethinking breast cancer screening strategies in resource-limited settings. Afr Health Sci. 2010;10(1):89-92.
45. Danzon P, Towse A. The economics of gene therapy and of pharmacogenetics. Value Health. 2002;5(1):5-13.
46. Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-63.
47. Sauer C, Sauer RM. Reducing barriers to the development of high quality, low cost medicines: a proposal for reforming the drug approval process [Internet]. London: Hanway Print Center; 2005.
48. Andrieu S, Coley N, Lovestone S, Aisen PS, Vellas B. Prevention of sporadic Alzheimer’s disease: lessons learned from clinical trials and future directions. Lancet Neurol. 2015;14(9):926-44.
49. Zhu CW, Sano M. Economic considerations in the management of Alzheimer’s disease. Clin Interv Aging. 2006;1(2):143-54.
50. Skoldunger A, Johnell K, Winblad B, Wimo A. Mortality and treatment costs have a great impact on the cost-effectiveness of disease modifying treatment in Alzheimer’s disease – a simulation study. Curr Alzheimer Res. 2013;10(2):207-16.
51. Wimo A. Long-term effects of Alzheimer’s disease treatment. Lancet Neurol. 2015:1-2.
52. Briggs A. Economic evaluation and clinical trials: size matters (editorial). BMJ. 2000;321(7273):1362-3.
53. Wimo A, Ballard C, Brayne C, Gauthier S, Handels R, Jones RW, et al. Health economic evaluation of treatments for Alzheimer’s disease: impact of new diagnostic criteria. J Intern Med. 2014;275(3):304-16.
54. Liu C-C, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms, and therapy. Nat Rev Neurol. 2013;9(2):106-18.
55. Fougère B VJ, Delrieu AJ, Sinclair A, Wimo CJ, Herman H, et al. The road ahead to cure and prevent Alzheimer’s disease: implementing prevention into primary care. Prev Alz Dis. 2015; 2(3):199-211.
56. Salloway S, Sperling R, Brashear HR. Phase 3 trials of solanezumab and bapineuzumab for Alzheimer’s disease. N Engl J Med. 2014;370(15):1460.
57. National Institutes of Health. Accelerating medicines partnership [Internet]. Bethesda: U.S. Department of Health and Human Services; 2015. Available from: http://nih.gov/science/amp/index.htm.
58. Berwick D. «We all have AIDS»: case for reducing the cost of HIV drugs to zero. BMJ. 2002;324(7331):214-6.
