I. Yunusa1, N. Rashid2, V. Abler2, K. Rajagopalan3
1. Center for Outcomes Research & Evaluation, University of South Carolina College of Pharmacy, Columbia, South Carolina, USA; 2. Acadia Pharmaceuticals, Inc., San Diego, California, USA; 3. Anlitiks Inc., Dover, Massachusetts, USA
Corresponding Author: Krithika Rajagopalan, PhD, Anlitiks Inc., 18 Old Colony Dr, Dover, Massachusetts, 02030, USA, Phone: 508-314-8158, Email: kr.rajagopalan@anlitiks.com
J Prev Alz Dis 2021;4(8):520-533
Published online August 6, 2021, http://dx.doi.org/10.14283/jpad.2021.48
Abstract
Objectives: To evaluate the comparative efficacy, safety, tolerability, and effectiveness of atypical antipsychotics (AAPs) for the treatment of dementia related psychosis (DRP) in older adults.
Methods: In this systematic literature review (SLR), we qualitatively synthesized evidence on the comparative efficacy (based on neuropsychiatric inventory), tolerability (weight gain), and safety (cerebrovascular adverse events [CVAE], cardiovascular events, mortality, somnolence, extrapyramidal symptoms [EPS]) of AAPs used to treat DRP. We also assessed effectiveness based on all-cause discontinuations and discontinuations due to lack of efficacy or adverse events (AE). Published articles from through March 2021 from PubMed, EMBASE, PsycINFO, and Cochrane databases evaluated. We included double-blind, active-comparator/placebo-controlled randomized trials, open-label trials, and observational studies.
Results: This qualitative synthesis included 51 eligible studies with sample size of 13,334 and mean age of 79.36 years. Risperidone, olanzapine, quetiapine, and aripiprazole demonstrated numerically small improvement in psychotic symptoms among patients with DRP. Somnolence was the most reported AE for all the AAPs, with weight gain and tardive dyskinesia more common with olanzapine and risperidone, respectively. These AAPs are associated with falls, EPS, cognitive declines, CVAE, and mortality. Aripiprazole and olanzapine had lower odds of discontinuation due to lack of efficacy, with olanzapine having greater discontinuation odds due to AEs.
Conclusion: This SLR demonstrated that AAPs used off-label to treat DRP are associated with small numerical symptom improvement but with a high risk of AEs, including cognitive decline and potentially higher mortality. These results underscore the need for new treatments with a favorable benefit-risk profile for treating DRP.
Key words: Dementia-related psychosis, antipsychotics, atypical antipsychotics, hallucinations, delusions, safety, tolerability, efficacy, effectiveness.
Abbreviations: NPS:Neuropsychiatric symptoms; DRP: dementia-related psychosis; AP: antipsychotics; SLRs: systematic literature reviews; FDA: Food and Drug Administration; AAPs: atypical antipsychotics; EPS: extrapyramidal symptoms; APA: American Psychiatric Association; NPI: neuropsychiatric inventory; NPI-NH: neuropsychiatry index-nursing home; BPRS: Brief Psychiatric Rating Scale; CVAE: cerebrovascular events; AD: Alzheimer’s disease; VaD: vascular dementia; DLB: dementia with Lewy bodies; MD: mixed dementia; PD: Parkinson’s disease; NH: nursing home; LTC: long-term care; CGI: clinical global improvement; CATIE-AD: clinical antipsychotic trials of intervention effectiveness- Alzheimer’s disease; CGI-S: clinical global impression scale- severity; CGI-C: clinical global impression scale- change; PANSS: Positive and Negative Syndrome Scale; SAS: Simpson-Angus Scale; TEAEs: treatment-emergent adverse events; MMSE: Mini-Mental State Examination; BEHAVE-AD: Behavioral Symptoms in Alzheimer’s Disease; SUCRA: surface under cumulative ranking curve
Background
Worldwide, an estimated 50 million people currently live with dementia, which results in progressive loss of cognitive function severe enough to cause a decline in the patient’s ability to perform activities of daily living (1). It is estimated that 7.5M individuals have dementia of different types in the United States, and this number is expected to double by 2030, given the increase in the elderly population and rising life expectancy (2). While cognitive decline is the hallmark of dementia, neuropsychiatric symptoms (NPS) are common and can dominate its clinical presentation (3). NPS in patients with dementia includes dementia-related psychosis (DRP) that manifests as delusions (false fixed beliefs) or hallucinations (seeing or hearing things that others do not see or hear), and other behavioral symptoms such as agitation, aggression, depression, apathy, elation, anxiety, disinhibition, irritability, and aberrant motor behavior (4). Notably, about 2.4M people in the U.S. are estimated to have DRP (5). Data on patients with Alzheimer’s disease (AD) dementia suggest that 94% of patients experience delusions while 70% experience hallucinations (6, 7). DRP prevalence is also anticipated to grow significantly with the increasing rates of dementia, exerting significant distress to individuals and their families and potentially imposing an enormous economic burden to the society (8). Thus, interventions aimed at treating DRP could tremendously improve the health outcomes of patients, their families, and caregivers (3).
At the time of this analysis, there was no Food and Drug Administration (FDA) approved treatment for DRP. However, antipsychotics (APs), both typical antipsychotics (first-generation) and atypical antipsychotics [AAPs] (second-generation) are used off-label to treat hallucinations and delusions associated with DRP. Available evidence on the comparative efficacy of currently used AAPs versus placebo suggests that AAPs only offer a numerically small improvement in psychotic symptoms among patients with DRP (3). On the other hand, they are known to be associated with significant safety risks related to treatment-emergent cerebrovascular adverse events (CVAE) such as stroke, and a higher risk of mortality (9, 10). In recognition of the unfavorable benefit-risk profile of current off-label AAPs, they all carry an FDA boxed warning on the increased risk of mortality among the elderly with dementia (9). Furthermore, years of research on these medications suggest that each of them report a unique side effect profile that ranges from extrapyramidal symptoms (EPS) (more associated with typical APs) to weight gain, hyperlipidemia, impaired glucose metabolism including potential insulin resistance, diabetes, tardive dyskinesia, sedation, hyperprolactinemia, orthostatic hypotension, sexual dysfunction, increased rate of fractures and cognitive deterioration, among others (11-13). Considering this, the American Psychiatric Association (APA) guidelines recommend the short-term use of pharmacological interventions only after nonpharmacological interventions such as cognitive, behavioral, and environmental therapies have been attempted first to treat NPS such as DRP (14). Additionally, they also provide instructions for gradual dose reduction or taper.
This study focused on assessing the outcomes of the off-label use of AAPs in treating DRP. Previously published SLRs and network meta-analyses (NMA) assessed AAPs’ relative benefits and safety on a broader NPS population from randomized controlled trials (RCTs) which assessed fewer outcomes (15, 16). As they evaluated the overall NPS of dementia, previous studies assessed total neuropsychiatric inventory (NPI) or neuropsychiatry inventory-nursing home (NPI-NH) score as opposed to their psychosis subscale which would be more informative in assessing DRP. The SLRs demonstrated that the trade-offs between the benefits of treating NPS did not adequately offset the risks associated with AAPs. While Yunusa et al. 2019 (15) found significant benefits for total NPI score, and Brief Psychiatric Rating Scale (BPRS) with some AAPs compared to placebo; there were a greater risk of adverse outcomes of CVAE. A recently published SLR and NMA by Watt et al. (16) suggest that, in subgroups of persons with dementia, AAPs are associated with greater harm (i.e., falls, fractures, and CVAE) than antidepressants and anticonvulsants (medications used in place of AAPs for treating NPS of dementia). Other agent (anticonvulsants, dextromethorphan-quinidine) combinations were also associated with an adverse safety profile compared to placebo. However, no single comprehensive SLR has been conducted to comparatively assess efficacy, tolerability, safety, and, most of all, AAPs’ effectiveness in treating symptoms of DRP. Furthermore, it is crucial to consider that additional clinical trials have been published since the most recent SLR (17). This SLR aimed to comprehensively compare the efficacy, effectiveness, safety, and tolerability of different AAPs used to treat DRP by including open label and non-double blind studies along with RCTs to assess a broader gamut of outcomes to reflect real-world data and address critical knowledge gaps and provide the most recent review to date.
Methods
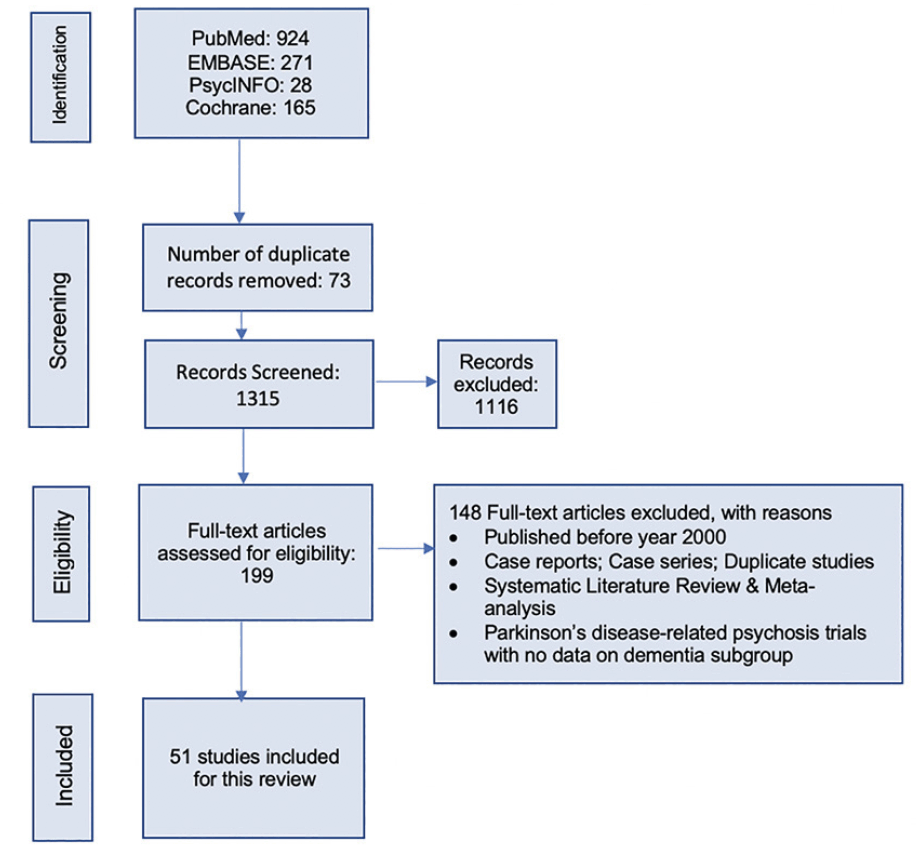
This systematic literature review followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines (18). The study selection process is illustrated in Figure 1. A protocol for this SLR was developed internally by the study team before starting the review, and it guided the conduct of the study.
Eligibility Criteria
The Patients/Population, Intervention, Comparator, Outcome, and Study design (PICOS) framework, was used as an eligibility criterion to search, select, and review relevant studies. Included participants from the studies (age ≥ 40, those living in the community or nursing home [NH]) had to have dementia of the following type: AD dementia, frontotemporal dementia, vascular dementia (VaD), dementia with Lewy bodies (DLB) and Parkinson’s disease (PD) dementia. The interventions included were both typical and atypical antipsychotics with or without multiple comparator groups and outcomes related to efficacy, tolerability, safety, and effectiveness. Finally, we excluded studies other than double-blind, active-comparator or placebo-controlled RCTs, open-label trials, or observational studies. Efficacy was assessed as improvement in DRP symptoms related to psychosis (i.e., hallucinations and delusions) and the NPI-psychosis subscale, NPI-NH psychosis subscale, BPRS psychosis factor subscale, and BEHAVE-AD (Behavioral Symptoms in Alzheimer’s Disease) psychosis subscale were used to measure improvement in psychotic symptoms in patients. The psychosis subscale is a combination of the delusion and hallucination subscales. Additionally, measures of adverse effects were assessed as tolerability (i.e., weight gain due to AAPs) and safety outcomes (i.e., somnolence, EPS including tardive dyskinesia, cognition, CVAE, falls, and mortality, among others). Effectiveness was summarized from all-cause discontinuations and discontinuations due to lack of efficacy or safety.
Data Sources and Literature Search Strategy
The literature search was conducted in MEDLINE/PubMed (Appendix. 1), PsycINFO, EMBASE, and Cochrane Central Register of Controlled Trials from January 2000 to March 2021. The search was limited to articles published in English. Databases were searched using predefined search terms to identify published studies evaluating the safety and effectiveness of antipsychotics used to treat DRP of any type (PD dementia, VaD, DLB, AD dementia, and frontotemporal dementia). Search strategies were developed using medical subject headings (MeSH) terms (PubMed and Cochrane library), Emtree terms (EMBASE and PsycINFO), and text words related to antipsychotic treatment in DRP.
Key search terms to define the patient population included dementia, NPS, DRP of any type including psychosis related to PD dementia, VaD, DLB, AD dementia and frontotemporal dementia, hallucinations, delusions, agitation, and aggression. Search terms for interventions (medications) included typical antipsychotics, haloperidol, acetophenazine, carbamazepine, chlorpromazine, chlorprothixene, fluphenazine, loxapine, mesoridazine, molindone, perphenazine, prochlorperazine, promazine, thioridazine, thiothixene, trifluoperazine, atypical antipsychotics, aripiprazole, clozapine, ziprasidone, risperidone, quetiapine, olanzapine, pimavanserin, asenapine, brexpiprazole, cariprazine, paliperidone, and lurasidone.
Search, Study selection, Data Extraction
Two researchers independently searched the indexed electronic databases through the Anlitiks SLR platform, a proprietary internal search engine within Anlitiks that allows integrated searches of selected index databases such as Medline/PubMed and allows the searches for the index databases such as Cochrane review separately, as applicable. Articles were also independently screened against predefined eligibility criteria in two phases, title/abstract screening (Phase 1) and full-text screening (Phase 2). References of all eligible articles were searched to identify the possibility of any missing articles. Subsequently, we extracted data from eligible articles that passed Phase 2 screening using an apriori standardized data extraction form in Microsoft Excel. A third reviewer resolved any disagreement in the information extracted from the articles by both researchers. Data were extracted from both the secondary analysis and original trials. In case of missing information, authors were contacted where necessary. Outcomes related to efficacy, safety, tolerability, and effectiveness were reviewed. Effectiveness outcome was assessed as AP withdrawal or discontinuations, time to AP discontinuations, AP switches or augmentation, as well as relapses of NPS. Also, the availability of data on other effectiveness measures such as hospitalizations, ER visits, and other health resource use was evaluated. Lastly, outcomes like measures of cardiometabolic disturbances (e.g., hyperglycemia, dyslipidemia), sedation, somnolence, and cardiovascular events (e.g., heart attacks), CVAE (e.g., stroke), EPS including tardive dyskinesia, falls and fractures, urinary incontinence, urinary tract infection, as well as measures of cognitive decline, were assessed. In cases where outcomes were reported as a composite measure (e.g., CVAE), we also tried to extract data for the individual outcomes (i.e., stroke, transient ischemic attack, etc.) constituting the composite measure, if available in the published articles. Outcome data were extracted regardless of the reporting format, i.e., either as a dichotomous, categorical, or continuous variable.
Risk of bias and Study Quality Assessment
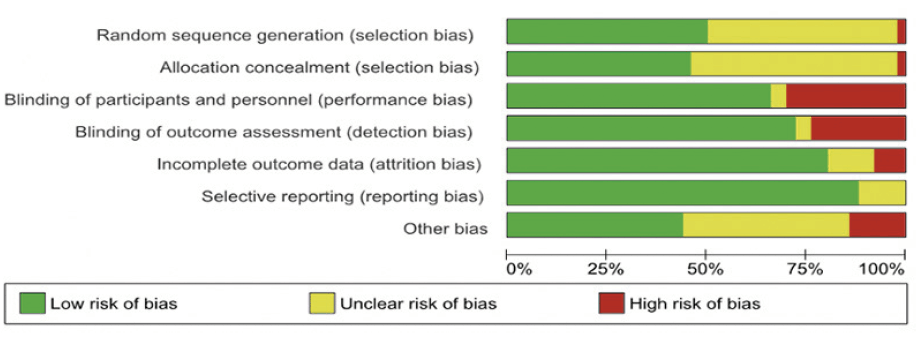
The Cochrane Risk of Bias tool (19) was used to assess the risk of bias in 49 original trials using seven domains (namely sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and ‘other sources of bias’s). Accordingly, the risk of bias was categorized on a three-level categorical scale viz. – ‘Low risk’ of bias; ‘High risk’ of bias, or ‘Unclear risk’ of bias. The summary of the risk of bias assessment is shown in Figure 2, and the study-level risk of bias is shown in a tabular form in Appendix 2. The observational studies were assessed using the Newcastle-Ottawa scale for the assessment of the quality of observational studies (20) as shown in Appendix 3.
The figure depicts pooled study quality assessment of all the RCT included in the study.
Results
Study Selection
The initial search yielded a total of 1,388 citations, and after removing the duplicates, 1,315 titles and abstracts were screened for eligibility against pre-defined inclusion/exclusion criteria. A total of 199 articles were eligible for full-text review. Overall, 51 studies 17, 21-77) published between January 2000-March 2021 were included in the qualitative synthesis that met the inclusion/exclusion criteria.
Study Characteristics
Figure 1 illustrates the search yield and study attrition for the selection of eligible studies. Both checklist (Appendix 4,5) (18) and flow diagram from the PRISMA ensured transparency in selecting articles, and the quality standards were followed. Of the total included 51 original studies, 39 were randomized trials, 10 were open-label trials, and 2 were observational studies. Information from 8 post-hoc analyses of the randomized trials were also extracted. Among the identified studies, 21 were conducted in institutionalized settings such as the NH or long-term care (LTC) facilities, while the rest were conducted among community-dwelling, or outpatient settings. Based on assessment of study quality, it was found that 10% of the studies included in the SLR were of low quality.
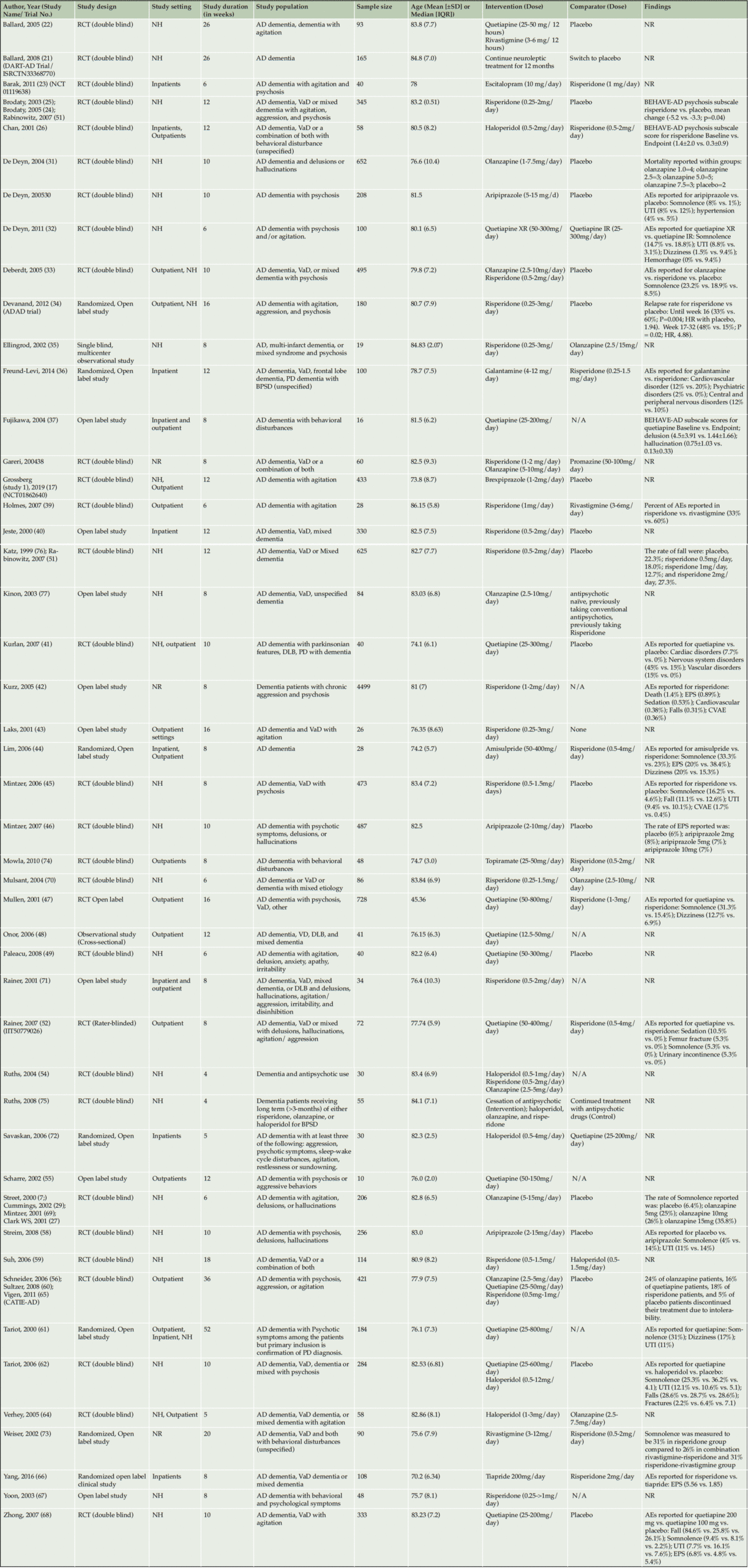
From the included studies ranging from 4 to 52 weeks, there were a total of 13,334 patients (sample size ranged from 10 to 4499) with mean age of 79.36 years. Most patients in the included studies were diagnosed with AD dementia, with some studies on VaD, DLB, PD-related dementia, or mixed dementia (MD). There were two studies that had PD dementia-related psychosis as their primary inclusion criteria. Of the 42 parallel-group studies, 21 were placebo-controlled studies, and 21 had active-controls. Overall, there were 30 trials of risperidone, 14 of quetiapine, 10 of olanzapine, 3 of aripiprazole. There was one trial each for ziprasidone, tiapride, and brexpiprazole. The summary of all included study characteristics is described in Table 1, and the Table 2 depicts the outcome measures assessed for individual AAPs.
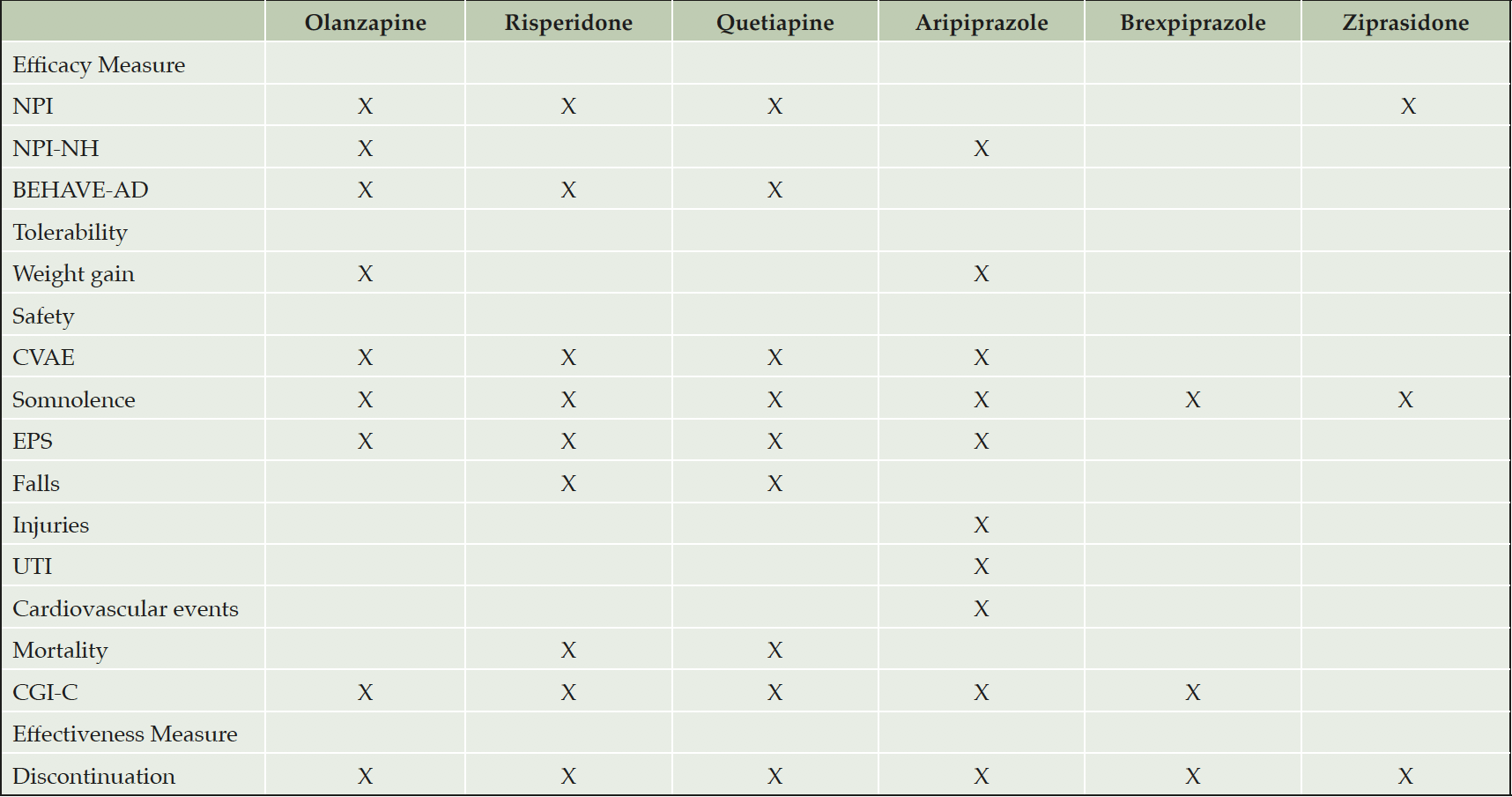
While the SLR was intended to include outcome measures such as quality of life (QoL), functioning and caregiver burden outcomes, and hospital admissions and emergency department visits, interestingly, little has been reported in the literature about the role of APs and their impact on these outcomes. Of note, the review resulted in a reasonable number of available publications for the six commonly used AAPs (i.e., olanzapine, risperidone, quetiapine, aripiprazole, ziprasidone, and brexpiprazole) and only one AP (i.e., haloperidol). Therefore, this SLR primarily focused on the qualitative assessment of comparative efficacy, tolerability, safety, and effectiveness of AAPs in the treatment of DRP.
Abbreviations and full names; RCT- Randomized control trials; NR- No reported findings relevant to the study; NH- Nursing home; DLB – dementia with Lewy bodies; BPSD – behavioral and psychological symptoms of dementia; VaD- Vascular dementia; AD- Alzheimer’s disease; PD- Parkinson’s Disease; BEHAVE-AD- Behavioral Symptoms in Alzheimer’s Disease; AE- Adverse Events; EPS- Extrapyramidal symptoms; UTI- Urinary tract infection; CVAE- Cerebrovascular adverse events; Olz- Olanzapine; N/A- Not applicable
The table depicts the individual outcomes assessed for every atypical antipsychotic (AAP); Abbreviations: NPI (Neuropsychiatric inventory); NPI-NH (Neuropsychiatric inventory- nursing home subscale); BEHAVE-AD (Behavioral Symptoms in Alzheimer’s Disease); CVAE (Cerebrovascular adverse events); EPS (Extrapyramidal symptoms); UTI (Urinary tract infection); CGI-C (Clinical Global Impression – Corrections). The ‘X’ represent outcome measures that were studied for corresponding AAPs. The blank cells represent the outcome measures that were not studied for corresponding AAPs.
Olanzapine
A total of 10 studies (4 placebo-controlled and 6 active comparator [e.g., risperidone, haloperidol, and promazine] studies) were included in the review (27, 29, 31, 33, 38, 54, 56, 57, 60, 64, 65, 69, 70, 75, 77). Placebo comparison studies suggest that lower doses of olanzapine have a greater effect on improving psychotic symptoms than higher doses (27, 29, 31, 57, 60). RCT of individuals with AD dementia who were retrospectively identified as meeting the DLB criteria (N =29) found that individuals treated with 5 mg olanzapine/day (N =10) showed greater reductions in NPI delusion subscale (−3.8 points) and hallucination (−5.9 points) subscale scores when compared with placebo (N =10) (29). As per De Deyn et al. 2004, in a double-blind study of 652 patients with delusions or hallucinations associated with AD dementia, olanzapine 7.5 mg/day significantly decreased psychotic symptoms (31). While according to Street et al., another randomized placebo comparison study (N =206) of olanzapine (5,10, or 15mg/day) among AD patients with psychosis and/or agitation/ aggression, it was reported that low-dose olanzapine (5 and 10 mg/d) showed significant improvement on the NPI-NH psychosis total , subscale compared to placebo; on the other hand, improvement with olanzapine (15 mg/day) was not significantly greater than placebo for this subscale (57). As per the study published by Verhey et al. 2006 (64), olanzapine was found to improve the NPI psychosis subscale score compared to placebo.
Reported AEs associated with olanzapine were high risk of CVAE (33, 60), somnolence (27, 57, 69), weight gain (31, 38), and EPS (56, 57). For example, Street et al. 200057 found that somnolence was significantly more common among all patients receiving olanzapine, but gait disturbance occurred in those receiving 5 or 15 mg/d (57) compared to placebo and 10mg/d of olanzapine. Additionally, the effects of olanzapine on cognitive symptoms were found to be inconsistent. Although Vigen et al. 2011 (65) found that olanzapine and other AAPs (risperidone) were associated with the worsening of cognitive symptoms, Clark et al. 2001 (27) reported that olanzapine was not associated with a decline in cognition compared to placebo. According to Deberdt et al. (33), olanzapine was associated with a significant increase in CVAE and mortality in comparison with placebo.
For effectiveness outcomes, De Deyn et al. 2004 (31) reported that olanzapine had significantly lower rate of discontinuation due to lack of efficacy as compared to placebo. According to the CATIE-AD trial (56, 60, 65), which compared olanzapine, quetiapine, and risperidone to placebo, olanzapine had the highest rate of discontinuation due to AEs.
Risperidone
A total of 30 studies (8 placebo-controlled, 18 active-comparator [i.e., quetiapine, haloperidol, olanzapine, and rivastigmine, escitalopram, galantamine, promazine, topiramate, amisulpride, citalopram, tiapride], and 4 single-arm) were included in the review (21, 23-26, 28, 33-36, 38-40, 42-45, 47, 50-52, 54, 56, 59, 60, 63, 65-67, 70, 71, 73-76). Placebo-comparison efficacy studies of risperidone found that in most cases, risperidone was associated with improved psychosis symptoms, compared to placebo (25, 51, 60). In the clinical antipsychotic trials of intervention effectiveness- Alzheimer’s disease (CATIE-AD) study, 421 outpatients, were included with AD and psychosis or agitated/aggressive behavior. Compared to placebo, greater improvements were seen with risperidone in the BPRS psychosis factor subscale (60). In a randomized placebo comparison study of risperidone (n=345), a significant reduction in BEHAVE-AD psychotic symptoms subscale (p=0.004) was seen with risperidone (25). In a secondary analysis of a 12-week, randomized controlled trial of individuals with AD, mean change at endpoint in BEHAVE-AD psychosis subscale was higher in risperidone group compared to placebo (-5.2 vs. -3.3; p=0.039) (24). Another secondary exploratory analysis of data on 479 nursing-home patients with psychosis of AD from three 12-week, double-blind, placebo-controlled clinical trials reported risperidone to be effective on the BEHAVE-AD delusion and hallucination subscales (51). In a multicenter, randomized, double-blind placebo-controlled trial of nursing home residents diagnosed with AD and psychosis (45), both risperidone group and placebo groups showed significant improvements on the BEHAVE-AD psychosis subscale (45). Overall, these studies reported that risperidone is moderately effective in treating various symptoms associated with psychosis. Interestingly, in a study by Deberdt et al. 2005 (33), olanzapine, risperidone, and placebo treatment reported improved NPI-NH psychosis subscale scores, though no significant changes emerged across treatments, including placebo-comparisons (33). Furthermore, significant reductions were found in the NPI hallucination and delusion subscales scores for amisulpride and risperidone according to Lim et al. 2006 (44).
The AE profile of risperidone demonstrated inconsistent results on the various safety outcomes depending on the number and type of measures. Jeste et al. (40) observed that the incidence of persistent tardive dyskinesia with risperidone appeared to be much lower than that seen in elderly patients treated with conventional neuroleptics (40). In a comparative study of risperidone vs. haloperidol, Suh et al. 2006 (59) reported that the risk of antipsychotic-induced Parkinsonism was significantly lower with risperidone (59). Yoon et al. (67) found that risperidone treatment was generally well tolerated, although EPS were noted in a dose-dependent manner (67). On the other hand, Teranishi et al. 2013 (63) found that drug-induced EPS increased significantly in the risperidone group (63) As far as cognitive decline, there were no differences between placebo and the APs, i.e., risperidone, thioridazine, haloperidol, chlorpromazine, and trifluoperazine in the DART-AD trial (21).
In a study of participants with DLB, risperidone experienced higher overall neurologic effects and worsening of neuropsychiatric symptoms (28). Other reported AEs for risperidone included falls,59,63 EPS (26, 38, 44, 56, 59) somnolence (25, 38, 44, 45, 59) and CVAE (42). In the long-term follow-up of the DART-AD trial, Kaplan-Meier estimates of mortality showed a significantly increased risk of mortality for patients among patients randomized to continue antipsychotic treatment on risperidone compared with those randomized to placebo.
In terms of effectiveness measures, results with risperidone were mixed for outcomes such as discontinuations, time to discontinuations, and or treatment augmentation and switches. In a study by Culo et al. 2010 (28), a significantly higher proportion of participants with DLB (68%) discontinued risperidone prematurely than AD (50%) patients on risperidone, and discontinuation rates were comparable in DLB participants with psychosis that were treated with citalopram (71%) or risperidone (65%). According to the CATIE-AD trials (56, 60, 65), it was reported that risperidone had higher rate of all cause discontinuation and discontinuation due to lack of efficacy compared to placebo. In terms of relapse prevention, Devanand et al. 2012 (34) found that risperidone was associated with a lower rate of psychotic relapse than placebo (60% vs. 33%). To our knowledge, apart from the Devanand study, there have been no other studies of psychotic relapse prevention in patients with DRP.
Quetiapine
A total of 14 studies (6 placebo-controlled, 4 active-comparator [e.g., risperidone, rivastigmine, quetiapine, haloperidol], and 4 single-arm) were reviewed for quetiapine (22, 32, 37, 41, 47-49, 52, 55, 56, 60-62, 65, 68, 72). Efficacy studies for quetiapine in improving psychosis, have had mixed reports; while some studies reported favorable effects on symptom improvements, others showed quetiapine to be ineffective in improving psychotic symptoms. For example, Scharre et al. 2002 (55) found that patients on quetiapine showed a significant reduction in NPI-NH delusion subscale scores, after receiving doses of 50 to 150 mg (55). In another 10-week, double-blind, fixed-dose study, elderly institutionalized patients with dementia and agitation randomized to quetiapine 200mg/day, 100mg/day, or placebo, quetiapine 200mg was associated with clinically greater improvements in the NPI-NH psychosis subscale scores.68 Fujikawa et al. 2005 (37) found significant improvements with quetiapine in the BEHAVE-AD subscales of delusions.
However, other studies found that quetiapine did not improve psychosis compared with placebo (41, 62). For example, Tariot et al. 2006 showed that quetiapine, haloperidol, and placebo demonstrated similar levels of improvement in psychotic symptoms (i.e., no difference between placebo), as reported by mean NPI-NH2 (i.e. hallucination and delusion) subscale, in patients with possible AD from baseline to week 10 (62).
The most commonly reported adverse effects for quetiapine were somnolence (61, 62) death (61, 62) CVAE (52), and EPS (37, 49, 55, 56). While Zhong et al. 2007 (68) found that incidence of CVAE, postural hypotension, and falls were similar among quetiapine and placebo groups while mortality was numerically higher in the quetiapine group; however, these rates were not statistically significant (68).
In terms of effectiveness, Tariot et al. 2000 (61) reported that only 89 (48%) patients (n=184) on quetiapine completed treatment through 52 weeks. The main reasons for antipsychotic withdrawal or discontinuations included lack of efficacy (19%), AEs (15%), failure to return for follow-up (13%). Somnolence (31%), dizziness (17%), postural hypotension (15%) and EPS (13%).61 Onor et al. 2007 (48) found that clinically significant orthostatic hypotension (for patients on quetiapine) led to the discontinuation of 5 patients from their observational study (n =41) (48).
Aripiprazole
There were 3 studies of aripiprazole compared with placebo. Efficacy results for aripiprazole appear inconsistent across studies (Streim et al. 2008; De Deyn et al. 2005; Mintzer et al. 2007) (30, 46, 58). In a randomized, double-blind, placebo-controlled multicenter trial of 487 institutionalized AD patients with psychosis, Mintzer et al. 2007 (46) found that Aripiprazole 10 mg/day showed significantly greater improvements than placebo on the NPI-NH Psychosis Subscale for baseline scores compared to Week 10 scores (-6.87 versus -5.13; p =0.013) and NPI-NH Psychosis response rate (65 versus 50; p =0.019). However, in the study reported by De Deyn et al., (30) compared to placebo aripiprazole showed similar improvements in psychotic symptoms as assessed by NPI psychosis subscale scores but significantly greater improvements from baseline in BPRS psychosis subscale scores at the study endpoint (30). Additionally, Streim et al. 2008 (58) also found conflicting results to that reported by Mintzer (46), with no significant differences in mean change from baseline score on the NPI-NH Psychosis Subscale between aripiprazole and placebo.
As it relates to the safety and tolerability of aripiprazole, Streim et al, reported comparable rates for treatment-emergent adverse events (TEAEs) between aripiprazole and placebo, except for somnolence (aripiprazole, 14%; placebo, 4%) (58). While in the study reported by De Deyn et al. 2005 (30), only mild somnolence was observed. Moreover, the AEs were generally mild to moderate in severity and included (aripiprazole vs. placebo): urinary tract infection (8% vs. 12%), accidental injury (8% vs. 5%), somnolence (8% vs. 1%), and bronchitis (6% vs. 3%) (30). There were no significant differences from placebo in EPS, or clinically significant ECG abnormalities, vital signs, or weight (30). In a study by Mintzer et al. 2007 (46), CVAE was a reported outcome for the aripiprazole-treated population while no patients from the placebo group suffered from the same. Other AEs seen in the aripiprazole group were asthenia, agitation, and EPS (46).
Effectiveness outcomes reported in these studies did not show any clear patterns. In the De Deyn et al. study (30), the number of patients discontinuing due to AEs, lack of efficacy, or withdrawal of consent was similar in the aripiprazole and placebo groups (30). AEs leading to greater than 2% discontinuation in the aripiprazole or placebo group were asthenia (4%, aripiprazole 10mg/dl) and agitation (4%, placebo), respectively (46).
Brexpiprazole
There was one reported publication of brexpiprazole that reported the results of two separate studies Grossberg et al. 2020 (17) assessed the efficacy, safety, and tolerability of brexpiprazole in patients with agitation in Alzheimer’s dementia (AAD) in two 12-week, randomized, double-blind, placebo-controlled, parallel-arm studies. While one study was a fixed-dose study (Study 1: 433 randomized), the second one was a flexible-dose study (Study 2: 270 randomized) of patients with AAD; in a care facility or community-based setting. Since the main focus of our study was psychosis and Grossberg et a., 2020 focused on patients with agitation, only safety outcomes reported in the study were considered for review.
TEAEs among patients receiving brexpiprazole were headache, insomnia, and somnolence. In general, most TEAEs were mild or moderate in severity. The studies found that brexpiprazole 2 mg/day has the potential to be efficacious, safe, and well-tolerated in the treatment of agitation in AD dementia (17). All cause discontinuations reported across both studies did not show numerical differences between brexpiprazole and placebo groups for both studies. However, discontinuation due to adverse events was reported to be higher for brexpiprazole as compared to placebo.
Ziprasidone
Rocha et al. 2006 (53) evaluated the efficacy and tolerability of ziprasidone in a 7-week open-label trial. For the patients included in the study, the mean NPI delusion subscale score fell significantly from 4.88 to 2.28 i.e., -53% from baseline to day 49 (p < 0.01). However, of the 25 patients who participated, 10 discontinued the study. The main reason for discontinuation was AEs. The most frequent AEs were somnolence, gastrointestinal symptoms, and parkinsonism (39, 53).
Discussion
Published SLRs of AAP use among dementia patients largely focused on the gamut of NPS (i.e., psychosis – delusions and hallucinations, agitation/aggression, depression, anxiety, and irritability, among others) and selected safety parameters (such as CVAE, stroke, and mortality) from RCTs or observational studies. This qualitative synthesis adds to previously published SLRs in many ways. First, it is the most comprehensive, recent review of APs as a treatment of dementia related psychosis. Second, this SLR is intended to review real-world effects by including publications of RCTs, open-label trials, and observational studies, including single-arm or comparator-arm trials. Third, this is a comparative review of placebo-AP or AP-AP differences in efficacy, safety, tolerability, and effectiveness in treating DRP. The final and most important difference is that this SLR examined effectiveness outcomes such as differences in all-cause discontinuations and discontinuations due to lack of efficacy or discontinuation due to AEs, and time to relapses between the different AAPs.
This SLR showed that off-label therapies such as risperidone, olanzapine, quetiapine, and aripiprazole demonstrated numerically small psychotic symptom improvements among DRP patients; however, only risperidone was reported to have symptom improvements consistently while also showing a significant increase in EPS. This is supported by the fact that although it is not approved by the FDA, for short-term treatment of aggression in AD, risperidone is the only licensed drug in countries like UK, Canada, and Australia if aggression poses a risk or the person has not responded to non-drug approaches (78, 79). Both quetiapine and aripiprazole reported mixed results, and lower dose olanzapine showed greater symptom improvements than higher doses. These results are consistent with the previously published NMA of 17 studies (5373 patients) conducted by Yunusa et al. 2019 (15), comparing different AAPs (risperidone, olanzapine, aripiprazole, and quetiapine) that reported no statistically significant differences between the AAPs in terms of NPI symptoms scores. While no drug-drug differences were found across measures of efficacy and safety among aripiprazole, olanzapine, quetiapine, and risperidone, placebo-drug differences were found for some drugs for specific outcomes. The surface under the cumulative ranking curve estimated relative ranking of treatments from Yunusa’s study suggested that aripiprazole might be the most effective and safe AAP and that olanzapine provides the least effect overall; however, these results should be interpreted with caution where point estimates (OR and SMD) show that there is no statistically significant difference between placebo-drug and drug-drug comparisons from the synthesis of the 17 trials.
Interestingly, the current review, consistent with other studies, suggested that olanzapine may potentially exhibit a dose-response relationship in symptom improvements despite demonstrating only slight symptom improvements with pooled doses. Specifically, doses <10mg of olanzapine were significantly better than placebo in terms of neuropsychiatric symptom improvements (NPI), and doses >10mg were not different from placebo according to two studies (31, 57). While a dose range was found to be effective, no single effective dose was reported in these primary studies. It is not clear why higher doses of olanzapine had similar effects to placebo; it is plausible that the higher rates of AEs reported for olanzapine may have tempered with the effectiveness of olanzapine in psychotic symptom improvements. An NMA along with a meta-regression of these outcomes by dose level differences may be contemplated in the future to test the hypothesis.
While published SLRs and NMAs by Yunusa (15) and Watt (16) suggest that APs may be associated with a significant risk of strokes, falls, fractures, CVAEs, or death, and the umbrella review by Papola et al. (80) suggest an association between the use of APs and fractures, stroke, and cardiac death, the current review of AAP tolerability and safety outcomes included more outcomes such as weight gain, metabolic disturbances (e.g., hyperglycemia, dyslipidemia), sedation, somnolence, and cardiovascular events, CVAE, EPS including tardive dyskinesia, falls and fractures, urinary incontinence, urinary tract infection and cognitive decline. Not surprisingly, our review showed that somnolence was the most reported AE for all the major APs, with weight gain and tardive dyskinesia being more commonly reported for olanzapine and risperidone, respectively. Other AEs reported for all AAPs were EPS, falls, and CVAEs, except for brexpiprazole. Furthermore, studies also show that these APs may be associated with greater cognitive declines (38, 41, 60, 81) and potentially increased mortality (21, 33, 68) in patients with DRP. Although our study was qualitative in nature, Maust et al., reported that, about 27-50 patients need to be treated with AAPs in order for one person to die (9). In alignment with a previous study, this review also suggests that other AEs among patients receiving olanzapine include CVAE and EPS (81). While falls, EPS, and CVAE were also reported for risperidone. In addition to somnolence, commonly reported adverse effects of quetiapine were EPS, dizziness, postural hypotension, and death. AEs associated with aripiprazole are somnolence, urinary tract infection, accidental injury, somnolence, bronchitis, CVAE, akathisia, asthenia, agitation, and EPS. All these qualitative findings suggest the major AAPs that are currently used off-label for treating DRP may have an unfavorable benefit-risk based on multiple outcome measures, including CVAE, and mortality. These findings suggest that a quantitative review through an NMA may be needed.
The current study is an extension of previous reviews by examining additional measures of effectiveness defined as AP withdrawal or discontinuations, time to discontinuations, AP switches or augmentation, as well as psychotic relapses. While our review attempted to review data on other effectiveness measures such as hospitalizations, ER visits, and other health resource use, it was limited by the paucity of data on these outcomes. Available data suggest that compared to placebo, odds of all-cause discontinuations were lower with aripiprazole while olanzapine, quetiapine, risperidone, and brexpiprazole reported no differences. While aripiprazole and olanzapine had lower discontinuation odds due to lack of efficacy, olanzapine had higher discontinuation odds due to lack of safety. Compared to placebo, odds of discontinuation were found to be higher for aripiprazole, olanzapine, risperidone, quetiapine and brexpiprazole. Based on the reported information, it appears that risperidone had a lower likelihood of relapse of NPS than placebo. Studies of olanzapine, quetiapine, brexpiprazole did not report treatment effects based on relapse of psychosis.
For multiple drug comparator studies from the CATIE-AD trial, effectiveness for olanzapine, quetiapine and risperidone was measured through discontinuation or effects of AAP withdrawal. Ruths et al. 2004 (54) reported that for olanzapine and risperidone most patients’ behavioral scores remained stable after the withdrawal of APs from nursing home (NH) patients with dementia. However, impairment in the patient’s nighttime and daytime activity was reported as sleep problems and restlessness (54). In another double-blind, placebo-controlled trial of 421 outpatients with AD and psychosis or aggression/agitation, Schneider et al. 2006 (56) found no significant differences in time to all-cause discontinuation (i.e., discontinuation for any reason) among olanzapine, quetiapine and risperidone.
As with any research, this SLR has a few limitations. Specifically, the SLR included trials beyond the gold standard for double-blind, randomized trials and thus resulted in 10% of studies of low-quality being included with high risk of bias for blinding of participants and personnel. This could be attributed to the inclusion of non-blinded and open label studies in the SLR. It is anticipated that the small proportion of low-quality studies included in this qualitative review of the SLR are not likely to change the overall conclusions. However, a sensitivity analysis may be considered during quantitative NMA, if any, based on this SLR’s findings to evaluate the impact of low-quality studies included in the SLR. It is recommended that the NMA ought to consider issues of heterogeneity, transitivity, and other inherent problems to overcome any intrinsic study-related biases. Notwithstanding these few limitations, this review represents the most comprehensive analysis to-date.
Conclusions
Consistent with previous findings, the overall evidence from this SLR suggest that currently used AAPs described in this study confer non-significant benefits in treating dementia related hallucinations and delusions. Additionally, they are associated with a high risk of significant AEs, accelerated cognitive decline, and potentially higher mortality among patients with DRP. Furthermore, antipsychotic effectiveness may be poor, given the potentially high rates of all-cause discontinuations and discontinuations due to AEs reported in the studies. Overall, these qualitative findings suggest that these AAPs used as off-label treatments for the vulnerable DRP population may have an unfavorable benefit-risk profile and require quantitative confirmation through an NMA of data derived from this SLR. These results also underscore the potential unmet need for new treatment options with an improved benefit-risk profile for the treatment of DRP.
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Competing interests: NR and VA are employees of Acadia Pharmaceuticals, Inc. which has funded the research. The authors confirm that funding has in no way influenced the outcome. There are no conflicts of interest associated with this publication.
Funding: The research was funded by Acadia Pharmaceuticals Inc. Employees of Acadia Pharmaceuticals Inc. were a part of the research team for development of concept, study design, study selection and interpretation of the results.
Authors’ contributions: IY, NR, VA, KR development of concept and study design; IY, and KR literature search; IY, NR, VA, KR study selection, and interpretation of data; IY, NR, VD, KR preparation of manuscript; All authors critically reviewed and approved the final version of the paper.
Acknowledgements: Author KR is an employee, while IY is a former employee of Anlitiks Inc which is a part of the funded research group.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Association Asd. Alzheimer’s disease facts and figures report. https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf. Accessed accessed June 26 2020.
2. Prince M, Ali GC, Guerchet M, Prina AM, Albanese E, Wu YT. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res Ther. 2016;8(1):23.
3. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. Jama. 2005;293(5):596-608.
4. Kales HC, Gitlin LN, Lyketsos CG, Detroit Expert Panel on A, Management of Neuropsychiatric Symptoms of D. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
5. Small GW. Managing the Burden of Dementia-Related Delusions and Hallucinations. Supplement to The Journal of Family Practice.69(7).
6. Paulsen JS, Salmon DP, Thal LJ, et al. Incidence of and risk factors for hallucinations and delusions in patients with probable AD. Neurology. 2000;54(10):1965-1971.
7. Wilson RS, Gilley DW, Bennett DA, Beckett LA, Evans DA. Hallucinations, delusions, and cognitive decline in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2000;69(2):172-177.
8. Scarmeas N, Brandt J, Albert M, et al. Delusions and Hallucinations Are Associated With Worse Outcome in Alzheimer Disease. Archives of Neurology. 2005;62(10):1601-1608.
9. Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438-445.
10. Hsu WT, Esmaily-Fard A, Lai CC, et al. Antipsychotics and the Risk of Cerebrovascular Accident: A Systematic Review and Meta-Analysis of Observational Studies. J Am Med Dir Assoc. 2017;18(8):692-699.
11. Mittal V, Kurup L, Williamson D, Muralee S, Tampi RR. Risk of cerebrovascular adverse events and death in elderly patients with dementia when treated with antipsychotic medications: a literature review of evidence. Am J Alzheimers Dis Other Demen. 2011;26(1):10-28.
12. Citrome L, Eramo A, Francois C, et al. Lack of tolerable treatment options for patients with schizophrenia. Neuropsychiatric disease and treatment. 2015;11:3095.
13. Papola D, Ostuzzi G, Thabane L, Guyatt G, Barbui C. Antipsychotic drug exposure and risk of fracture: a systematic review and meta-analysis of observational studies. Int Clin Psychopharmacol. 2018;33(4):181-196.
14. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the Use of Antipsychotics to Treat Agitation or Psychosis in Patients With Dementia. Focus (Am Psychiatr Publ). 2017;15(1):81-84.
15. Yunusa I, Alsumali A, Garba AE, Regestein QR, Eguale T. Assessment of Reported Comparative Effectiveness and Safety of Atypical Antipsychotics in the Treatment of Behavioral and Psychological Symptoms of Dementia: A Network Meta-analysis. JAMA Netw Open. 2019;2(3):e190828.
16. Watt JA, Goodarzi Z, Veroniki AA, et al. Safety of pharmacologic interventions for neuropsychiatric symptoms in dementia: a systematic review and network meta-analysis. BMC Geriatr. 2020;20(1):212.
17. Grossberg GT, Kohegyi E, Mergel V, et al. Efficacy and safety of brexpiprazole for the treatment of agitation in Alzheimer’s dementia: two 12-week, randomized, double-blind, placebo-controlled trials. The American Journal of Geriatric Psychiatry. 2020;28(4):383-400.
18. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021:105906.
19. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
20. Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. ᅟ. 2000;ᅟ.
21. Ballard C, Lana MM, Theodoulou M, et al. A randomised, blinded, placebo-controlled trial in dementia patients continuing or stopping neuroleptics (the DART-AD trial). PLoS Med. 2008;5(4):e76.
22. Ballard C, Margallo-Lana M, Juszczak E, et al. Quetiapine and rivastigmine and cognitive decline in Alzheimer’s disease: randomised double blind placebo controlled trial. Bmj. 2005;330(7496):874.
23. Barak Y, Plopski I, Tadger S, Paleacu D. Escitalopram versus risperidone for the treatment of behavioral and psychotic symptoms associated with Alzheimer’s disease: a randomized double-blind pilot study. International psychogeriatrics. 2011;23(9):1515-1519.
24. Brodaty H, Ames D, Snowdon J, et al. Risperidone for psychosis of Alzheimer’s disease and mixed dementia: results of a double-blind, placebo-controlled trial. International Journal of Geriatric Psychiatry: A journal of the psychiatry of late life and allied sciences. 2005;20(12):1153-1157.
25. Brodaty H, Ames D, Snowdon J, et al. A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. The Journal of clinical psychiatry. 2003.
26. Chan Wc, Lam LCw, Choy CNp, Leung VPy, Li Sw, Chiu HFk. A double-blind randomised comparison of risperidone and haloperidol in the treatment of behavioural and psychological symptoms in Chinese dementia patients. International journal of geriatric psychiatry. 2001;16(12):1156-1162.
27. Clark WS, Street JS, Feldman PD, Breier A. The effects of olanzapine in reducing the emergence of psychosis among nursing home patients with Alzheimer’s disease. The Journal of clinical psychiatry. 2001.
28. Culo S, Mulsant BH, Rosen J, et al. Treating neuropsychiatric symptoms in dementia with Lewy bodies: a randomized controlled-trial. Alzheimer Disease & Associated Disorders. 2010;24(4):360-364.
29. Cummings JL, Street J, Masterman D, Clark WS. Efficacy of olanzapine in the treatment of psychosis in dementia with Lewy bodies. Dementia and geriatric cognitive disorders. 2002;13(2):67-73.
30. De Deyn P, Jeste DV, Swanink R, et al. Aripiprazole for the treatment of psychosis in patients with Alzheimer’s disease: a randomized, placebo-controlled study. Journal of clinical psychopharmacology. 2005;25(5):463-467.
31. De Deyn PP, Carrasco MM, Deberdt W, et al. Olanzapine versus placebo in the treatment of psychosis with or without associated behavioral disturbances in patients with Alzheimer’s disease. International journal of geriatric psychiatry. 2004;19(2):115-126.
32. De Deyn PP, Eriksson H, Svensson H. Tolerability of extended-release quetiapine fumarate compared with immediate-release quetiapine fumarate in older patients with Alzheimer’s disease with symptoms of psychosis and/or agitation: a randomised, double-blind, parallel-group study. Int J Geriatr Psychiatry. 2012;27(3):296-304.
33. Deberdt WG, Dysken MW, Rappaport SA, et al. Comparison of olanzapine and risperidone in the treatment of psychosis and associated behavioral disturbances in patients with dementia. The American journal of geriatric psychiatry. 2005;13(8):722-730.
34. Devanand D, Mintzer J, Schultz SK, et al. Relapse risk after discontinuation of risperidone in Alzheimer’s disease. New England Journal of Medicine. 2012;367(16):1497-1507.
35. Ellingrod VL, Schultz SK, Ekstam-Smith K, Kutscher E, Turvey C, Arndt S. Comparison of risperidone with olanzapine in elderly patients with dementia and psychosis. Pharmacotherapy. 2002;22(1):1-5.
36. Freund-Levi Y, Jedenius E, Tysen-Bäckström AC, Lärksäter M, Wahlund L-O, Eriksdotter M. Galantamine versus risperidone treatment of neuropsychiatric symptoms in patients with probable dementia: an open randomized trial. The American journal of geriatric psychiatry. 2014;22(4):341-348.
37. Fujikawa T, Takahashi T, Kinoshita A, et al. Quetiapine treatment for behavioral and psychological symptoms in patients with senile dementia of Alzheimer type. Neuropsychobiology. 2004;49(4):201-204.
38. Gareri P, Cotroneo A, Lacava R, et al. Comparison of the efficacy of new and conventional antipsychotic drugs in the treatment of behavioral and psychological symptoms of dementia (BPSD). Archives of gerontology and geriatrics Supplement. 2004(9):207.
39. Holmes C, Wilkinson D, Dean C, et al. Risperidone and rivastigmine and agitated behaviour in severe Alzheimer’s disease: a randomised double blind placebo controlled study. Int J Geriatr Psychiatry. 2007;22(4):380-381.
40. Jeste DV, Okamoto A, Napolitano J, Kane JM, Martinez RA. Low incidence of persistent tardive dyskinesia in elderly patients with dementia treated with risperidone. American Journal of Psychiatry. 2000;157(7):1150-1155.
41. Kurlan R, Cummings J, Raman R, Thal L. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology. 2007;68(17):1356-1363.
42. Kurz A, Schwalen S, Schmitt A. Effects of risperidone on behavioral and psychological symptoms associated with dementia in clinical practice. International psychogeriatrics. 2005;17(4):605.
43. Laks J, Engelhardt E, Marinho V, et al. Efficacy and safety of risperidone oral solution in agitation associated with dementia in the elderly. Arquivos de neuro-psiquiatria. 2001;59(4):859-864.
44. Lim H-K, Pae C-U, Lee C, Lee C-U. Amisulpride versus risperidone treatment for behavioral and psychological symptoms in patients with dementia of the Alzheimer type: a randomized, open, prospective study. Neuropsychobiology. 2006;54(4):247-251.
45. Mintzer J, Greenspan A, Caers I, et al. Risperidone in the treatment of psychosis of Alzheimer disease: results from a prospective clinical trial. The American journal of geriatric psychiatry. 2006;14(3):280-291.
46. Mintzer JE, Tune LE, Breder CD, et al. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: a multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. The American Journal of Geriatric Psychiatry. 2007;15(11):918-931.
47. Mullen J, Jibson MD, Sweitzer D. A comparison of the relative safety, efficacy, and tolerability of quetiapine and risperidone in outpatients with schizophrenia and other psychotic disorders: the quetiapine experience with safety and tolerability (QUEST) study. Clin Ther. 2001;23(11):1839-1854.
48. Onor ML, Saina M, Aguglia E. Efficacy and tolerability of quetiapine in the treatment of behavioral and psychological symptoms of dementia. American Journal of Alzheimer’s Disease & Other Dementias®. 2007;21(6):448-453.
49. Paleacu D, Barak Y, Mirecky I, Mazeh D. Quetiapine treatment for behavioural and psychological symptoms of dementia in Alzheimer’s disease patients: a 6-week, double-blind, placebo-controlled study. International Journal of Geriatric Psychiatry: A journal of the psychiatry of late life and allied sciences. 2008;23(4):393-400.
50. Pollock BG, Mulsant BH, Rosen J, et al. A double-blind comparison of citalopram and risperidone for the treatment of behavioral and psychotic symptoms associated with dementia. The American Journal of Geriatric Psychiatry. 2007;15(11):942-952.
51. Rabinowitz J, Katz I, De Deyn PP, Greenspan A, Brodaty H. Treating behavioral and psychological symptoms in patients with psychosis of Alzheimer’s disease using risperidone. International psychogeriatrics. 2007;19(2):227-240.
52. Rainer M, Haushofer M, Pfolz H, Struhal C, Wick W. Quetiapine versus risperidone in elderly patients with behavioural and psychological symptoms of dementia: efficacy, safety and cognitive function. European psychiatry. 2007;22(6):395-403.
53. Rocha FL, Hara C, Ramos MG, et al. An exploratory open-label trial of ziprasidone for the treatment of behavioral and psychological symptoms of dementia. Dementia and geriatric cognitive disorders. 2006;22(5-6):445-448.
54. Ruths S, Straand J, Nygaard HA, Bjorvatn B, Pallesen S. Effect of antipsychotic withdrawal on behavior and sleep/wake activity in nursing home residents with dementia: a randomized, placebo-controlled, double-blinded study The Bergen District Nursing Home Study. J Am Geriatr Soc. 2004;52(10):1737-1743.
55. Scharre DW, Chang S-I. Cognitive and behavioral effects of quetiapine in Alzheimer disease patients. Alzheimer Disease & Associated Disorders. 2002;16(2):128-130.
56. Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. New England Journal of Medicine. 2006;355(15):1525-1538.
57. Street JS, Clark WS, Gannon KS, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. Archives of General Psychiatry. 2000;57(10):968-976.
58. Streim JE, Porsteinsson AP, Breder CD, et al. A randomized, double-blind, placebo-controlled study of aripiprazole for the treatment of psychosis in nursing home patients with Alzheimer disease. The American Journal of Geriatric Psychiatry. 2008;16(7):537-550.
59. Suh GH, Greenspan AJ, Choi SK. Comparative efficacy of risperidone versus haloperidol on behavioural and psychological symptoms of dementia. International Journal of Geriatric Psychiatry: A journal of the psychiatry of late life and allied sciences. 2006;21(7):654-660.
60. Sultzer DL, Davis SM, Tariot PN, et al. Clinical symptom responses to atypical antipsychotic medications in Alzheimer’s disease: phase 1 outcomes from the CATIE-AD effectiveness trial. American Journal of Psychiatry. 2008;165(7):844-854.
61. Tariot PN, Salzman C, Yeung PP, Pute J, Rak IW. Long-term use of quetiapine in elderly patients with psychotic disorders. Clinical Therapeutics. 2000;22(9):1068-1084.
62. Tariot PN, Schneider L, Katz IR, et al. Quetiapine treatment of psychosis associated with dementia: a double-blind, randomized, placebo-controlled clinical trial. The American journal of geriatric psychiatry. 2006;14(9):767-776.
63. Teranishi M, Kurita M, Nishino S, et al. Efficacy and tolerability of risperidone, yokukansan, and fluvoxamine for the treatment of behavioral and psychological symptoms of dementia: a blinded, randomized trial. Journal of clinical psychopharmacology. 2013;33(5):600-607.
64. Verhey FR, Verkaaik M, Lousberg R. Olanzapine versus haloperidol in the treatment of agitation in elderly patients with dementia: results of a randomized controlled double-blind trial. Dementia and geriatric cognitive disorders. 2006;21(1):1-8.
65. Vigen CL, Mack WJ, Keefe RS, et al. Cognitive effects of atypical antipsychotic medications in patients with Alzheimer’s disease: outcomes from CATIE-AD. American Journal of Psychiatry. 2011;168(8):831-839.
66. Yang X-P, Wang Y-T, Liu D-C, et al. Comparison of the effects of tiapride and risperidone on psycho-behavioral symptoms of senile dementia. Int J Clin Exp Med. 2016;9(6):9593-9597.
67. Yoon JS, Kim JM, Lee H, Shin IS, Choi SK. Risperidone use in Korean patients with Alzheimer’s disease: optimal dosage and effect on behavioural and psychological symptoms, cognitive function and activities of daily living. Human Psychopharmacology: Clinical and Experimental. 2003;18(8):627-633.
68. Zhong KX, Tariot P, Mintzer J, Minkwitz M, Devine N. Quetiapine to treat agitation in dementia: a randomized, double-blind, placebo-controlled study. Current Alzheimer Research. 2007;4(1):81-93.
69. Mintzer J, Faison W, Street JS, Sutton VK, Breier A. Olanzapine in the treatment of anxiety symptoms due to Alzheimer’s disease: a post hoc analysis. International journal of geriatric psychiatry. 2001;16(S1):S71-S77.
70. Mulsant BH, Gharabawi GM, Bossie CA, et al. Correlates of anticholinergic activity in patients with dementia and psychosis treated with risperidone or olanzapine. J Clin Psychiatry. 2004;65(12):1708-1714.
71. Rainer MK, Masching AJ, Ertl MG, Kraxberger E, Haushofer M. Effect of risperidone on behavioral and psychological symptoms and cognitive function in dementia. J Clin Psychiatry. 2001;62(11):894-900.
72. Savaskan E, Schnitzler C, Schröder C, Cajochen C, Müller-Spahn F, Wirz-Justice A. Treatment of behavioural, cognitive and circadian rest-activity cycle disturbances in Alzheimer’s disease: haloperidol vs. quetiapine. Int J Neuropsychopharmacol. 2006;9(5):507-516.
73. Weiser M, Rotmensch HH, Korczyn AD, Hartman R, Cicin-Sain A, Anand R. A pilot, randomized, open-label trial assessing safety and pharmakokinetic parameters of co-administration of rivastigmine with risperidone in dementia patients with behavioral disturbances. Int J Geriatr Psychiatry. 2002;17(4):343-346.
74. Mowla A, Pani A. Comparison of topiramate and risperidone for the treatment of behavioral disturbances of patients with Alzheimer disease: a double-blind, randomized clinical trial. J Clin Psychopharmacol. 2010;30(1):40-43.
75. Ruths S, Straand J, Nygaard HA, Aarsland D. Stopping antipsychotic drug therapy in demented nursing home patients: a randomized, placebo-controlled study–the Bergen District Nursing Home Study (BEDNURS). Int J Geriatr Psychiatry. 2008;23(9):889-895.
76. Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. Risperidone Study Group. J Clin Psychiatry. 1999;60(2):107-115.
77. Kinon BJ, Stauffer VL, McGuire HC, Kaiser CJ, Dickson RA, Kennedy JS. The effects of antipsychotic drug treatment on prolactin concentrations in elderly patients. J Am Med Dir Assoc. 2003;4(4):189-194.
78. Antipsychotic drugs for behavioural and psychological symptoms. https://www.alzheimers.org.uk/about-dementia/treatments/drugs/antipsychotic-drugs. Accessed March 27, 2021.
79. Corbett A, Burns A, Ballard C. Don’t use antipsychotics routinely to treat agitation and aggression in people with dementia. BMJ. 2014;349:g6420.
80. Papola D, Ostuzzi G, Gastaldon C, et al. Antipsychotic use and risk of life-threatening medical events: umbrella review of observational studies. Acta Psychiatr Scand. 2019;140(3):227-243.
81. Ballard C, Howard R. Neuroleptic drugs in dementia: benefits and harm. Nat Rev Neurosci. 2006;7(6):492-500.