F. Jessen1,2, J. Georges3, M. Wortmann4, S. Benham-Hermetz5
1. Medical Faculty, University Hospital Cologne, Cologne, Germany; 2. German Center for Neurodegenerative Diseases, Bonn|Cologne, Germany; 3. Executive Director of Alzheimer Europe, Luxembourg, Luxembourg; 4. CEO of Marc Wortmann Consultancy, Zeist, Netherlands; 5. Alzheimer’s Research UK, Cambridge, United Kingdom”
Corresponding Author: Samantha Benham-Hermetz, Alzheimer’s Research UK, 3 Riverside, Granta Park, Cambridge, United Kingdom CB21 6AD, Samantha.BenhamHermetz@alzheimersresearchuk.org, +44 (0)300 111 5666
J Prev Alz Dis 2022;3(9):550-555
Published online February 2, 2022, http://dx.doi.org/10.14283/jpad.2022.22
Abstract
Alzheimer’s Disease (AD) is the most common cause of dementia. Recent thinking portrays AD as a continuum consisting of three stages: an asymptomatic preclinical period, a mild cognitive impairment phase, and dementia, which can be further classified as mild, moderate or severe. While many studies explore the cognitive and functional aspects of AD, fully understanding AD pathophysiology, as well as the potential value of pharmacological and psycho-social interventions, requires a deeper understanding of patient and care partner priorities, particularly in the early stages where such interventions may have the greatest impact in slowing or delaying progression. Available studies highlight a diverse range of patient and care partner priorities, including impacts on their emotions, moods, and social lives. These priorities have not been systematically incorporated in the clinical and value assessments of potential interventions. We propose approaches to better understand the humanistic impact of AD including conducting additional research into the impacts of interventions from the point of view of patients and care partners, expanding notions of ‘value’ and improving health system capacity for diagnosis.
Key words: Alzheimer’s Disease, dementia, patient preferences, value frameworks.
Introduction
Recent estimates indicate that approximately 50 million people are living with dementia around the world, and this figure could surpass 150 million by 2050 (1). The global costs of dementia are staggering and similarly expected to continue to grow to increasingly unsustainable levels. For example, in 2015, dementia costs were estimated at US$818 billion—equivalent to 1.1% of global GDP (2). By 2030, it is estimated that the global cost of dementia could grow to US$2 trillion, which could impede socioeconomic development globally and overwhelm our health and social care systems (2, 3).
Alzheimer’s Disease (AD) is the most common cause of dementia, responsible for 60–70% of cases. Despite the lack of a “cure”, recent advances are collectively paving the way for effective interventions. For example, there are over 120 agents in clinical trials for the treatment of AD, of which almost 100 are disease-modifying therapies intended to slow or delay disease progression (4). Moreover, recent meta-analyses have found that 12 potentially modifiable risk factors account for approximately 40% of dementia cases worldwide (5).
These advances are raising the intriguing possibility of intervening early in AD during the period where patients have mild symptoms and before the more severe and devastating impacts of the disease begin. This could have tremendous benefits for patients, their families, and society.
However, a critical and often overlooked component of identifying and prioritizing effective interventions is the need to understand the outcomes that are regarded most important to the stakeholders most affected by the disease, including individuals with AD and the members of their families who currently or may eventually serve as care partners. Historically, there has been a wealth of understanding of the cognitive, and functional changes across the course of AD, which are meaningful and important endpoints that are extensively used in clinical research. However, a broader understanding of the difficulties that patients and their care partners face and what they value beyond these traditional measures could be of immense help in evaluating the full benefit of potential pharmacological and psycho-social interventions and enrich our understanding of AD pathophysiology. Such an understanding would be particularly helpful at the early disease stages where cognitive and functional impacts are relatively milder and where interventions aimed at slowing or delaying the progression of the disease would most likely need to be initiated.
In this paper, we provide a brief overview of the emerging body of literature on the AD continuum, outline what is understood about the impacts of the disease from the viewpoint of patients and their families (including current and future care partners) and outline the emerging implications for future research and policy.
Understanding AD as a Continuum
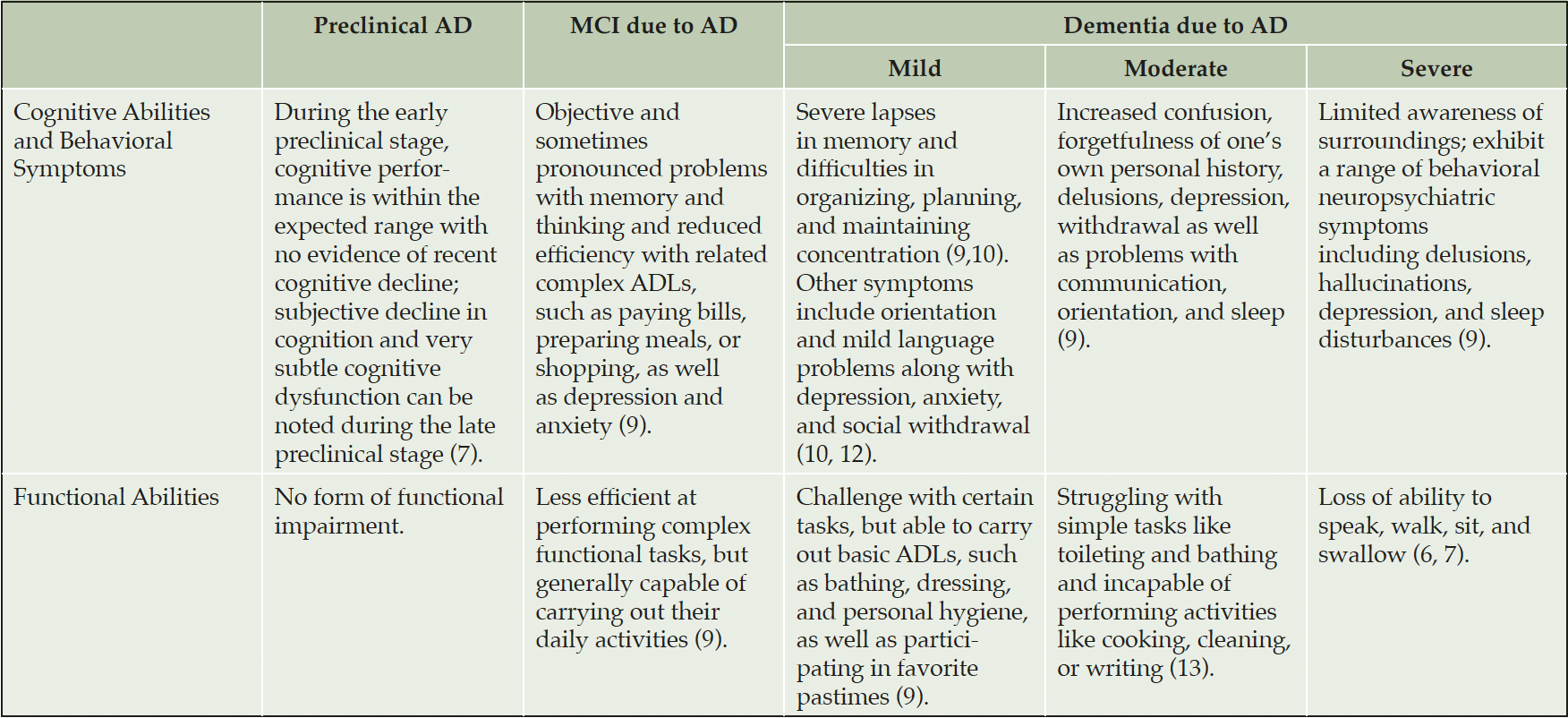
Recent thinking on AD reframes the disease as a pathologic continuum that can be divided into three stages: a long asymptomatic preclinical period, with subtle symptom manifestation in the late phase, followed by a prodromal or mild cognitive impairment (MCI) phase, and then dementia (6–8). The latter can then be divided into mild, moderate, and severe stages.
As the disease progresses across this continuum, individuals with AD experience changes in behavior, cognition, and functional ability (Table 1), intensifying the need for care (6, 7, 9). Individuals in the preclinical and MCI stages of the disease can live independently (9). Patients with mild dementia may require supportive care, but are still capable of doing things for themselves that maintain their sense of independence and self-worth (10). As AD progresses to later stages of dementia, the need for support is greatly amplified, with patients in the severe stages often requiring around-the-clock care from care partners who may, in turn, suffer from physical and mental health issues and often have a reduced quality of life (QoL) (11).
The severe cognitive and functional decline described above results in a progressive loss of autonomy and the ability to engage in social activities. Care partners themselves frequently need to contribute extensive (unpaid) time to care for patients with AD, and this can result in an immense emotional, social, and financial burden (3). The impact of disease progression on QoL for both patients and their care partners has been studied using standardized QoL scales and questionnaires (3,14). Mirroring the clinical and functional impacts described above, the QoL of patients and care partners generally begins to decline as patients progress from MCI and mild AD to more severe disease stages (15–17). However, modest decreases in QoL have been noted in the early stages of AD, and appear to be driven by memory or mood impairments as opposed to social or health issues (15–17). There are several practical challenges associated with using standardized QoL scales in AD, and there is no consensus on the most appropriate methodology to use (3,14), which may confound a full understanding of how the disease affects individuals. Therefore, there is a need to better understand the disease impact from the perspectives of patients and their care partners to develop a full understanding of AD and inform the development of interventions that meet the needs of those impacted by AD. Several studies have begun to explore this issue and are discussed below.
AD, Alzheimer’s Disease; MCI, Mild Cognitive Impairment; ADLs, Activities of Daily Living
What Actually Matters to Patients and Their Care Partners?
While there are several relevant reports in the literature, the Real-World Outcomes Across the AD Spectrum for Better Care (ROADMAP) initiative recently conducted a meta-analysis of the literature to better understand what outcomes are prioritized by patients across the AD spectrum (18). The authors of that report uncovered 34 published studies that had elicited information either directly from AD patients or other stakeholders, such as care partners and health professionals, using surveys, focus groups, or interviews. In addition to this comprehensive work, several novel studies have been published and have further shed light on this critical topic (19–22). Below, we provide a brief synthesis of the key insights emerging from this research before addressing implications.
The ROADMAP meta-analysis uncovered 32 different outcomes considered important to stakeholders organized across seven overarching domains: Cognition; Functioning and dependency, Behavioral and neuropsychiatric; Patient length of and quality of life; Care partner-oriented outcomes; Health, social care, and treatment-related outcomes; and Social issues (18). Outcomes included those typically assessed in clinical trials such as those related to cognition (e.g. memory decline, communication) as well as functional aspects and ADLs (e.g. cooking, finances, and self-hygiene) (18). However, other priorities were uncovered in the analysis, including the importance of maintaining patient QoL, the impact of mental health issues, maintenance of patient identity and personality, and maintaining a quality patient-care partner relationship (18). One limitation of the ROADMAP meta-analysis is that it only focused on studies published between 2008 and 2017, and may have therefore excluded older studies (18).
The recent What Matters Most (WMM) study from the Alzheimer’s Disease Patient and Caregiver Engagement (AD PACE) initiative provides additional evidence to support the above findings (19). The WMM study conducted qualitative interviews with patients and care partners across five groups, ranging from individuals with underlying pathology but no symptoms to those with severe dementia (19). Only care partners participated in interviews focused on understanding the impact of the later stages of the disease (19). This study first identified and then assessed 42 concepts that ranged from domains of memory, function, emotional well-being, and behavioral manifestations (19). Of note, several concepts reveal different activities that contribute to levels of patient independence (19). In participants with MCI or mild AD, more than half of the 42-items were rated as very important or extremely important, and even the least-important items were more than moderately important on average (19). Items considered important included those related to cognition and ADLs/function but also those related to emotions, mood, and social lives. These findings illustrate the range of experiences and abilities that are important to patients (19).
Very few studies have examined what could constitute a meaningful delay in disease progression from the perspective of patients. This issue was explored in the WMM study in which all participants were asked what an ideal “treatment for AD” would do for them (19). Across the AD continuum, improved memory, disease modification, and remaining independent (including the ability to perform daily activities) were considered the most important AD treatment outcomes to individuals (19).
AD is a complex neurodegenerative disorder with a diverse range of issues and priorities, suggesting that there are nuanced impacts that extend to families, future informal care partners, as well as informal care partners’ children. For example, the WMM study found that all care partners reported being impacted in some way (e.g., the need to supervise or drive the patient, changing roles, impacts on daily chores) (19). These findings add to the growing body of literature on the intense burden the disease can place on care partners (3).
Along with the established deterioration in clinical measures of cognition and function described in the previous section, the frequency of cognitive issues reported by patients increased as the severity of the disease grew (19). As noted above, the impacts on patients extend to emotional and social dimensions, which were also reported more often in later disease stages than in earlier stages. Despite the limited dataset, the above findings suggest that the impact of AD on patients begins early in the disease course and intensifies over time.
Recent work through the ROADMAP initiative has built upon the research discussed above through the development of a Data Cube (22) that may provide a more comprehensive picture of the outcomes important to patients and their care partners and how these evolve across the AD continuum. This framework provides a three-dimensional overview of prioritized outcomes, the relevance of these outcomes to different disease stages, and heat maps for the availability of real world-data for outcomes of interest (22). The Data Cube illustrates that the outcome domains most captured with existing data sets include those related to cognition, function, and independence, behavioral and neuropsychiatric symptoms, treatment, and comorbidities, and mortality. However, less data is available for several outcomes, including significant disease-related life events, medical investigations, use of health and social care, and patient QoL. Most notably, the domain with the least amount of data was care partner-oriented outcomes such as QoL. Additional research and real-world data platforms like the Data Cube will be critical to refining our understanding of what outcomes are most relevant to patients and their care partners across the entire AD continuum.
What are the Implications for Future Research and Policy?
As outlined above, there is a growing interest in better understanding the humanistic impact of AD, particularly in the early stages of the disease. Such an understanding is in line with the WHO’s ‘Global action plan on the public health response to dementia’ that underscores the importance of a person-centered approach to meet the needs and preferences of people with dementia and their families and care partners (4). Moreover, given the potential arrival of high-cost DMTs and biomarkers for AD, an understanding of the humanistic aspects of the disease could support health systems around the world in evaluating the value of these innovations. Below we outline proposals for future research and policy.
Complementing or standardizing outcome measures to better incorporate the patient voice in clinical research
A recent review found that in clinical trials for dementia and MCI, cognitive outcomes were reported in 70% of studies, yet only 29% and 13% reported measures of ADL or functional performance and QoL measures, respectively (23). Furthermore, AD clinical trials are designed per the FDA and EMA guidelines which heavily focus on cognitive elements. Cognitive performance, particularly improved memory, is clearly meaningful to patients and their care partners (19); however, as summarized in this article, a broader understanding what patients value beyond cognitive outcomes is of great interest to AD research and to determine the full benefit of interventions in AD (e.g. impact on social life, moods, emotions), particularly at the early stages where cognitive functions are relatively preserved.
However, despite growth in the number and types of outcome measurements used in dementia research, including the use of over 40 instruments related to QoL, several studies have criticized existing instruments for not fully reflecting what is most important for patients and their care partners (18, 20, 21, 23–25). For example, Harding et al. (2020) examined almost 350 outcome measurement instruments used in dementia research and found that none were deemed to have sufficient face validity when compared to the priorities of patients and other stakeholders (20). Similarly, Reilly et al. (2020) adopted an innovative Delphi-style process to build consensus on a ‘core outcome set’ for use when evaluating non-pharmacological health and social care interventions for people with dementia living at home. The research process involved not only patients but also other stakeholder groups (e.g. health professionals, policymakers, researchers). Ultimately, 13 outcomes were identified and organized into four conceptual categories: friendly neighbourhood and home, independence, self-managing dementia symptoms, and QoL. Echoing the results of other studies, many of these outcomes related to social health; indeed, only two cognitive outcomes remained in the final set of 13 alertness, knowing where you are).
Indeed, there have been calls for the creation of new patient-reported outcome measures for use in clinical trials, particularly for patients in early disease stages (21). Other researchers have argued for the employment of instruments rooted in the concept of ‘well-being’ and positive psychology, which may offer a broader scope and include insights based on psychological outcomes in AD compared to ‘quality of life’ measures (26). Given the lack of consensus in the literature, there is an urgent need for stakeholders, including patients, researchers, and policymakers to come to a consensus on what patient-centred outcome measurements should be assessed and what instruments should be used when evaluating interventions in clinical studies. One promising example of a patient-centred outcome that has been explored in clinical trials for dementia is the Goal Attainment Scale (GAS) (27). In this approach, personal goals are defined together with patients and their care partners and then used to measure the effect of interventions. In line with the findings presented in this paper, studies using GAS have found that the goals prioritized by patients and their care partners can be non-medical in nature and focus on, for example, aspects of quality of life or care partner burden (28). The strength of GAS is that person-specific goals, which can be very individualized and can differ from person to person, can be identified.
Expanding notions of ‘value’ when evaluating interventions for AD
Concerns have recently been raised that researchers and policymakers are not comprehensively capturing and describing the full scope and magnitude of the burden of AD (3). As we argue in this paper, better incorporating the well-being of AD on patients and care partners would further improve our ability to assess value in AD. Unfortunately, the patient perspective is not systematically included in cost-effectiveness analyses (CEA), which often rely on quality-adjusted life-years (QALYs) as a key measure of benefits that may not adequately capture the impact of AD on both patients and their care partners (3, 29).
Encouragingly, there are positive signs that decision-makers are increasingly open to expanded notions of value, including incorporating patient and care partner perspectives. For example, in a recent study, focus groups with Health Technology Assessment (HTA) representatives from Germany, Belgium, and Canada revealed an interest in the use of patient preferences to support decision-making (29); moreover, a recent review found that the inclusion of care partner health outcomes in NICE technology appraisals and highly specialized technologies does indeed occur, albeit relatively infrequently (30). A recent report by the ISPOR Special Task Force aimed to broaden the view of what constitutes value in health care and to spur new research on incorporating additional elements of value into CEA (31). We propose that HTA bodies build on this momentum and investigate novel value frameworks for AD that incorporate the perspectives of patients and care partners impacted by the disease.
Enhancing health system capacity for timely and accurate diagnosis of AD.
This article has thus far focused on outlining what matters to AD patients and their care partners and how this could potentially improve the way we do research, collect data and assess the value of interventions; however, a key limiting factor for implementing such aspects is that patients need to be diagnosed in the first place. It is estimated that as much as 50% of all people living with dementia never receive a formal diagnosis (32). Such statistics are disconcerting given the growing evidence that the timely recognition of cognitive impairment and timely diagnosis of AD can provide potential benefits for affected individuals and their care partners despite the lack of approved disease-modifying therapies (8). There is growing consensus that such benefits span a potential range of medical, social, emotional, financial, and planning advantages. There are, however, several important obstacles to receiving a timely diagnosis. For example, a recent study conducted by Alzheimer Europe has identified personal issues (e.g. family awareness and refusal to address symptoms) but also care pathway deficiencies (e.g. lack of knowledge/training of GPs or referring doctors) as well as system capacity constraints (e.g. waiting times for specialists, access to diagnostics) (33). Similarly, a recent survey conducted by Alzheimer’s Disease International found that almost 62% of healthcare providers worldwide think that dementia is part of normal aging (32). These findings suggest that strategies to enhance timely diagnosis of AD will need to be multifaceted, requiring increased awareness of the disease by the general public, enhanced training and knowledge of primary care physicians, and improved capacity for specialists and diagnostic equipment.
Conclusions
While there is a strong clinical and economic case for delaying the progression of AD, there is a need to factor in the perspectives of patients and care partners to paint a fulsome picture of the impact of the disease on those most affected, guide clinical research priorities, and assess the full value of interventions. Incorporating this critical lens is aligned with a growing emphasis on person-centred health care and will require policymakers, researchers, and clinicians together with the patient and care partner communities to explore novel approaches to gathering data, measuring outcomes, and assessing the value of interventions for AD.
Funding: This work was inspired by an educational symposium at the ISPOR EU 2020 Conference. The symposium included a panel with FJ, SBH, and MW and was sponsored by Biogen. The title of the panel discussion was “Insights into potential patient and caregiver benefits of changing disease course in AD: a discussion on the non-economic values of future potential early intervention”. Medical writing support for this paper was provided by Shift Health and was funded by Biogen. Shift Health consults with organizations across the health and life sciences sector, including Biogen. The authors (FJ, JG, MW and SBH) had full editorial control of the contents of the manuscript. The authors did not receive financial support or remuneration related to this work. Biogen conducted a courtesy review of this manuscript.
Conflict of interest: Dr. Jessen reports personal fees from Biogen, during the conduct of the study. Mr. Georges is the Executive Director of Alzheimer Europe which receives grants and support for its activities from the EU health and research programmes and from private and public organisations and institutions. Mr. Wortmann has nothing to disclose. Ms. Benham-Hermetz is the Director of Policy and Public Affairs at Alzheimer’s Research UK, which receives grants and support for its activities from private and public organisations and institutions. This work was inspired by an educational symposium at the ISPOR EU 2020 Conference. The symposium included a panel with FJ, SBH, and MW and was sponsored by Biogen. The title of the panel discussion was “Insights into potential patient and caregiver benefits of changing disease course in AD: a discussion on the non-economic values of future potential early intervention. Medical writing support for this paper was provided by Shift Health and was funded by Biogen. Shift Health consults with organizations across the health and life sciences sector, including Biogen. The authors (FJ, JG, MW and SBH) had full editorial control of the contents of the manuscript. Biogen conducted a courtesy review of this manuscript.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Patterson C. World Alzheimer Report 2018 – The State of the Art of Dementia Research: New Frontiers. 2018. https://www.alz.co.uk/research/WorldAlzheimerReport2018.pdf. Accessed May 28, 2021.
2. Wimo A, Guerchet M, Ali GC, et al. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimers Dement 2017;13:1-7.
3. El-Hayek YH, Wiley RE, Khoury CP, et al. Tip of the Iceberg: Assessing the Global Socioeconomic Costs of Alzheimer’s Disease and Related Dementias and Strategic Implications for Stakeholders. J Alzheimers Dis 2019;70:323-341.
4. Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer’s disease drug development pipeline: 2020. Alzheimers Dement (N Y) 2020;6:1-29.
5. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396:413-446.
6. Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement 2020;16:391-460.
7. Jack Jr CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018;14:535-562.
8. Liss JL, Seleri Marques Assunção S, Cummings J, et al. Practical Recommendations for Timely, Accurate Diagnosis of Symptomatic Alzheimer’s Disease (MCI and Dementia) in Primary Care: A Review and Synthesis. J Intern Med 2021;290:310-334.
9. Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: The Atherosclerosis Risk in Communities Neurocognitive Study. Alzheimers Dement (Amst) 2016;2:1-11.
10. Alzheimer’s Society. The progression of Alzheimer’s disease. Alzheimer’s Society. 2020. https://www.alzheimers.org.uk/about-dementia/symptoms-and-diagnosis/how-dementia-progresses/progression-alzheimers-disease. Accessed December 1, 2020.
11. Jones RW, Romeo R, Trigg R, et al. Dependence in Alzheimer’s disease and service use costs, quality of life, and caregiver burden: The DADE study. Alzheimers Dement 2015;11:280-290.
12. Benoit M, Berrut G, Doussaint J, et al. Apathy and depression in mild Alzheimer’s disease: A Cross-sectional study using diagnostic criteria. J Alzheimers Dis 2012;31:325-334.
13. Galasko D, Schmitt F, Thomas R, Jin S, Bennett D, Ferris S. Detailed assessment of activities of daily living in moderate to severe Alzheimer’s disease. J Int Neuropsychol Soc 2005;11:446-453.
14. Bowling A, Rowe G, Adams S, et al. Quality of life in dementia: A systematically conducted narrative review of dementia-specific measurement scales. Aging Ment Health 2015;19:13-31.
15. Missotten P, Squelard G, Ylieff M, et al. Quality of life in older Belgian people: Comparison between people with dementia, mild cognitive impairment, and controls. Int J Geriatr Psychiatry 2008;23:1103-1109.
16. Perren S, Schmid R, Wettstein A. Caregivers’ adaptation to change: The impact of increasing impairment of persons suffering from dementia on their caregivers’ subjective well-being. Aging Ment Health 2006;10:539-548.
17. Mhaoláin AMN, Gallagher D, Crosby L, et al. Frailty and quality of life for people with Alzheimer’s dementia and mild cognitive impairment. Am J Alzheimers Dis Other Demen 2012;27:48-54.
18. Tochel C, Smith M, Baldwin H, et al. What outcomes are important to patients with mild cognitive impairment or Alzheimer’s disease, their caregivers, and health-care professionals? A systematic review. Alzheimers Dement (Amst) 2019;11:231-247.
19. Dibenedetti DB, Slota C, Wronski SL, et al. Assessing what matters most to patients with or at risk for Alzheimer’s and care partners: A qualitative study evaluating symptoms, impacts, and outcomes. Alzheimers Res Ther 2020;12:1-15.
20. Harding AJE, Morbey H, Ahmed F, et al. A Core Outcome Set for Nonpharmacological Community-Based Interventions for People Living With Dementia at Home: A Systematic Review of Outcome Measurement Instruments. Gerontologist 2020;1-14.
21. Hartry A, Aldhouse NVJ, Al-Zubeidi T, Sanon M, Stefanacci RG, Knight SL. The conceptual relevance of assessment measures in patients with mild/mild-moderate Alzheimer’s disease. Alzheimers Dement (Amst) 2018;10:498-508.
22. Janssen O, Vos SJB, García-Negredo G, et al. Real-world evidence in Alzheimer’s disease: The ROADMAP Data Cube. Alzheimers Dement 2020;16:461-471.
23. Harrison JK, Noel-Storr AH, Demeyere N, Reynish EL, Quinn TJ. Outcomes measures in a decade of dementia and mild cognitive impairment trials. Alzheimers Res Ther 2016;8:1-10.
24. Couch E, Lawrence V, Co M, Prina M. Outcomes tested in non-pharmacological interventions in mild cognitive impairment and mild dementia: A scoping review. BMJ Open 2020;10:1-15.
25. Reilly ST, Harding AJE, Morbey H, et al. What is important to people with dementia living at home? A set of core outcome items for use in the evaluation of non-pharmacological community-based health and social care interventions. Age Ageing 2020;49:664-671.
26. Clarke C, Woods B, Moniz-Cook E, Mountain G, Øksnebjerg L, Chattat R, et al. Measuring the well-being of people with dementia: A conceptual scoping review. Health Qual Life Outcomes 2020;18:1-14.
27. Jessen F. What are we trying to prevent in Alzheimer disease? Dialogues Clin Neurosci 2019;21:27-34.
28. Jennings LA, Ramirez KD, Hays RD, Wenger NS, Reuben DB. Personalized Goal Attainment in Dementia Care: Measuring what persons with dementia and their caregivers want. J Am Geriatr Soc 2018;66:2120-2127.
29. van Overbeeke E, Forrester V, Simoens S, Huys I. Use of Patient Preferences in Health Technology Assessment: Perspectives of Canadian, Belgian and German HTA Representatives. Patient 2021;14:119-128.
30. Pennington BM. Inclusion of Carer Health-Related Quality of Life in National Institute for Health and Care Excellence Appraisals. Value Health 2020;23:1349-1357.
31. Lakdawalla DN, Doshi JA, Garrison LP, Phelps CE, Basu A, Danzon PM. Defining Elements of Value in Health Care-A Health Economics Approach: An ISPOR Special Task Force Report [3]. Value Health 2018;21:131-139.
32. Alzheimer’s Disease International. World Alzheimer Report 2019, Attitudes to Dementia. Alzheimer’s Disease International. 2019. https://www.alzint.org/u/WorldAlzheimerReport2019.pdf. Accessed December 6, 2020.
33. Alzheimer Europe. European Carers’ Report 2018: Carers’ experiences of diagnosis in five European countries. Alzheimer Europe. 2018. https://www.alzheimer-europe.org/Beta/E-Shop/Carers-report/European-Carers-Report-2018. Accessed March 18, 2020.