S. Gauthier1, A. Boxer2, D. Knopman3, J. Sims4, R. Doody5, P. Aisen6, T. Iwatsubo7, R. Bateman8, B. Vellas9
Task Force members : Susan Abushakra (Framingham, USA); Sandrine Andrieu (Toulouse, France); Matthew Barton (Raleigh, USA); Randall Bateman (St Louis, USA); Monika Baudler (Basel, Switzerland); Joanne Bell (Willmington, USA); Tobias Bittner (Basel, Switzerland); Dawn Brooks (Indianapolis, USA); Mirek Brys (Indianapolis, USA); Szofia Bullain (South San Francisco, USA); Cherie Butts (Cambridge, USA); Maria Carrillo (Chicago, USA); Carmen Castrillo-Viguera (Cambridge, USA); Bill Chan (Beijing, China); Ivan Cheung (Woodcliff Lake, USA); Min Cho (Woodcliff Lake, USA); Suzanne Craft (Winston-Salem, USA); Jeffrey Cummings (Las Vegas, USA); Julien Delrieu (Toulouse, France); Michael Detke (San Francisco, USA); Shobha Dhadda (Woodcliff Lake, USA); Sanjay Dube (Viejo, USA); Billy Dunn (Beltsville, USA); Michael Egan (North Wales, USA); Rianne Esquivel (Malvern, USA); Colin Ewen (United Kingdom); Phyllis Ferrel (Indianapolis, USA); Michela Gallagher (Baltimore, USA); Wendy Galpern (New Jersey, USA); Hideki Garren (San Francisco, USA); Grönblad Anna-Kaija (Stockholm, Sweden); Juergen Haeussler (Titusville, USA); Harald Hampel (Woodcliff Lake, USA); Suzanne Hendrix (Salt Lake City, USA); Joseph Herring (North Wales, USA); Michael Irizarry (Woodcliff Lake, USA); Gene Kinney (San Francisco, USA); Hartmuth Kolb (Titusville, USA); Shailaja Korukonda (Woodcliff Lake, USA); Akihiko Koyama (Woodcliff Lake, USA); Lynn Kramer (Woodcliff Lake, USA); Luka Kulic (Basel, Switzerland); Ricky Kurzman (Woodcliff Lake, USA); Jaren Landen (Cambridge, USA); Lars Lannfelt (Uppsala, Sweden); John Lawson (Malvern, USA); Valérie Legrand (Nanterre, France); Jinhe Li (Gilbert, USA); Frank Longo (Stanford, USA); Manoj Malhotra (Woodcliff Lake, USA); William MEnard (Providence, USA); Mark Mintun (Indianapolis, USA); Cecilia Monteiro (South San Francisco, USA); Stacie O’Sullivan (Woodcliff Lake, USA); Tomas Odergren (Stockholm, Sweden); Gunilla Osswald (Stockholm, Sweden); Ronald Petersen (Rochester, USA); Michael Pontecorvo (Indianapolis, USA); Mary Ellen Quiceno (New Jersey, USA); Rema Raman (San Diego, USA); Larisa Reyderman (Woodcliff Lake, USA); Monica Rivera-Mindt (Bronx, USA); Sharon Rogers (Los Angeles, USA); Sharon Rosenzweig-Lipson (Baltimore, USA); Ivana Rubino (Cambridge, USA); Laurie Ryan (Bethesda, USA); Marwan Sabbagh (Phoenix, USA); Stephen Salloway (Providence, USA); Rachel Schindler (New York, USA); Lon Schneider (Los Angeles, USA); Peter Schüler (Langen, Germany); Hiroshi Sekiya (Malvern, USA); Dennis Selkoe (Boston, USA); Melanie Shulman (Cambridge, USA); Eric Siemers ((Zionsville, USA); Kaycee Sink (South San Francisco, USA); Reisa Sperling (Boston, USA); Joyce Suhy (San Mateo, USA); Chad Swanson (Woodcliff Lake, USA); Jina Swartz (London, United Kingdom); Pierre Tariot (Phoenix, USA); Edmond Teng (South San Francisco, USA); Jacques Touchon (Montpellier, France); Martin Traber (Basel, Switzerland); Dominic Walsh (Cambridge, USA); Michael Weiner (San Francisco, USA); Lisa Yarenis (Woodcliff Lake, USA); Wagner Zago (San Francisco, USA); Kenton Zavitz (Cambridge, United Kingdom)
1. McGill Center for Studies in Aging, Montreal, Canada; 2. Memory and Aging Center, Department of Neurology, University of California, San Francisco CA, USA; 3. Department of Neurology, Mayo Clinic, Rochester MN, USA; 4. Eli Lilly and Company, Indianapolis IN, USA; 5. Genentech and F. Hoffman LaRoche, Basel, Switzerland; 6. Keck Scholl of Medicine of USC San Diego CA, USA; 7. Department of Neuropathology, The University of Tokyo, Tokyo, Japan; 8. Department of Neurology, Washington University, St-Louis, MO, USA; 9. Toulouse University Hospital, Inserm 1295, University of Toulouse, UPS, France
Corresponding Author: Serge Gauthier, McGill Center for Studies in Aging, Montreal, Canada, serge.gauthier@mcgill.ca
J Prev Alz Dis 2022;
Published online March 15, 2022, http://dx.doi.org/10.14283/jpad.2022.29
Abstract
There was consensus that both amyloid and tau pathologies should be targeted in Alzheimer’s disease, as well as additional pathophysiological mechanisms such as neuroinflammation. The selection of one or both of these targets may depend upon a personalized approach that takes into account the genetic and acquired factors that cause AD in any given person as well as their stage of disease as reflected in a biomarker profile. The validation of this therapeutic approach will be made possible by new methodologies for subdividing into predominant pathology, by efficient methods for identifying people in the earliest stages of disease, and by combination studies.
Key words: Alzheimer disease, amyloid, tau, therapeutic targets.
Introduction
We are in a stage of paradigm shift in the therapeutic field of Alzheimer`s disease (AD) from an era of symptomatic therapies to disease modification, now that a first monoclonal antibody against beta-amyloid has received Food and Drug Administration approval. There are major advances in our understanding of the interaction between amyloid and tau pathologies thanks to in vivo brain imaging using Positron Emission Tomography (PET) (1), as well as the interaction between tau pathology and microglial activation (2). On the other hand, there is awareness that while progressive beta-42 amyloid deposition and spreading of hyperphosphorylated tau fibrils are required findings in the current biological definition of AD (3), they may not sufficient for dementia to be manifest clinically, particularly in older persons, where other pathologies, such as vascular lesions, neocortical Lewy bodies and TDP-43/hippocampal sclerosis may interact with the primary AD pathologies (4).
Anti-amyloid treatments
Most of the randomized clinical trials (RCT) over the past decade aiming at disease modification in AD have targeted various components of the amyloid cascade. The most successful approach so far has been monoclonal antibodies targeting fibrillar Abeta, administered either intravenously or subcutaneously. There is cumulative evidence that high doses are required, despite the risk of Amyloid Related Imaging Abnormalities (ARIA), in order to normalize amyloid brain levels at least for the species measurable using PET imaging, and there is emerging evidence that the amyloid levels remain low for one to two years after the antibody therapy is stopped (5).
An encouraging observation is that anti-amyloid therapy using aducanumab lowers cerebro-spinal fluid (CSF) tau markers as well as plasma p-tau181 isoform (6), suggesting downstream effects on anti-amyloid therapy on tau pathophysiology. Similarly gantenerumab treatment in autosomal dominant AD led to a reduction in CSF Total Tau, p-tau 181 and attenuated increases in neurofilament light chain (7).
The magnitude of the benefit on clinical outcome measures over the 1.5 years of observation in clinical studies of amyloid-removing antibodies (e.g. aducanumab phase 3, and lecanemab and donanemab phase 2 trials) was relatively small and consisted of a reduction in the rate of clinical worsening (8). It is reasonable to speculate that the reduction of the decline trajectory in amyloid removal-treated patients could deviate further from the untreated state beyond 18 months, but it is possible that the antibody treated versus placebo differences in rate of decline will remain fixed. Thus, even if anti-amyloid therapy results can be replicated in ongoing phase 3 trials, the fact that some disease progression continues despite amyloid lowering justifies aggressive efforts to pursue other targets. In our review, the perspective on monoclonal anti-amyloid antibodies as of December 2021 is uncertain: the outcomes of the pivotal trials of donanemab, lecanemab and gantenerumab that will be available in 2022 and 2023 could paint a more or less favorable picture.
Anti-tau treatments
The conceptual logic of tau-directed approaches for symptomatic AD is considerably stronger than that of amyloid-directed approaches because tau accumulation occurs at the right time and the right place to account for clinical cognitive deficit. (9, 10). A large body of nonclinical studies in cell culture and animal models suggests that reduction of toxic tau gain of function (eg., accumulation, misfolding and/or posttranslational modifications) may be an effective therapeutic strategy for AD as well as other non-AD tauopathies (11). Tau protein exists in numerous aggregated states, some of which might be more amenable to treatment than others, and some might actually be central to the disease’s pathogenesis.
Another factor complicating an understanding of the role of tau protein in tauopathies is the diversity of repeats (3R vs 4R) across diseases, and the fact that most of the current animal models of tauopathies utilize FTDP-17 or to MAPT mutations, which are more closely related to fronto-temporal dementia than to AD. AD is often considered a secondary tauopathy, since amyloid pathology precedes the accumulation of tau throughout the brain and spreading from the mesial temporal regions to the neocortex (12). Similarly, tau possesses numerous post-translational modifications (13), and currently it is not clear whether unmodified tau, C-terminal epitopes or N-terminal ones would be the best therapeutic targets. Recent studies using cryo-electron microscopy showed that the core of insoluble tau aggregates in AD and other tauopathies is primarily composed of peptides in the microtubule binding and C-terminal domains of tau, and that there are specific three dimensional protein conformations for each different tauopathy (14). These core regions of tau pathology are distinct from the extracellular soluble tau species that have been used as CSF and plasma biomarkers and that first generation anti-tau antibodies have targeted (15, 16). To date, four N-terminally targeted anti-tau monoclonal antibodies have failed to demonstrate signs of efficacy in Phase 2 clinical trials in prodromal-mild AD and/or progressive supranuclear palsy (PSP). These include gosuranemab, (17) tilavonemab, (18) semorinemab (19) and zagotenemab. All four antibodies target N-terminal regions of tau that are not central to the disease associated protein aggregates, suggesting that despite evidence that the antibodies bind N-terminal tau fragments in human patients’ CSF, (20, 21) one potential reason for lack of efficacy may be that N-terminal tau fragments are not central to tau toxicity. Even if N-terminal tau fragments are not central to AD related tau toxicity, they may have important physiological roles in the brain such as regulating neuronal excitability (22, 23). The recent report that Semorinemab seemed to slow cognitive decline (ADAS cog) at least in in mild-to-moderate AD remains to be understood, especially in light of the failure of treatment to show an effect on the co-primary outcome of functional ability (ADCS- IADL) (24), or earlier stages of disease, but if replicated, might conceivably be explained by effects on N-terminal tau fragments.
There are also tau antibodies now targeting the MTBR and C-terminal domain of tau, which is the region that deposits in tau aggregates in the brain. These antibodies still have the challenge of being extracellular and may or may not reach their intended target, but they have a potential advantage in targeting the tau species that make up tau pathology. Several programs are being developed (Eisai 2814, UCB0107, and others) and are now in clinical studies.
An alternate approach that may better recapitulate nonclinical studies of the role of tau in neurodegeneration is based on suppression of tau gene (MAPT) expression using antisense oligonucleotide (ASO) or other generic drugs in human patients. Recently, on such ASO was shown to be safe and well tolerated with AD showed a reduction in CSF total tau levels similar to that observed from heterozygous inactivation of MAPT in transgenic mice (25). While it remains to be seen whether this suppression will lead to a clinical benefit, such genetically targeted approaches are best situated to recapitulate nonclinical animal and cell culture experiments.
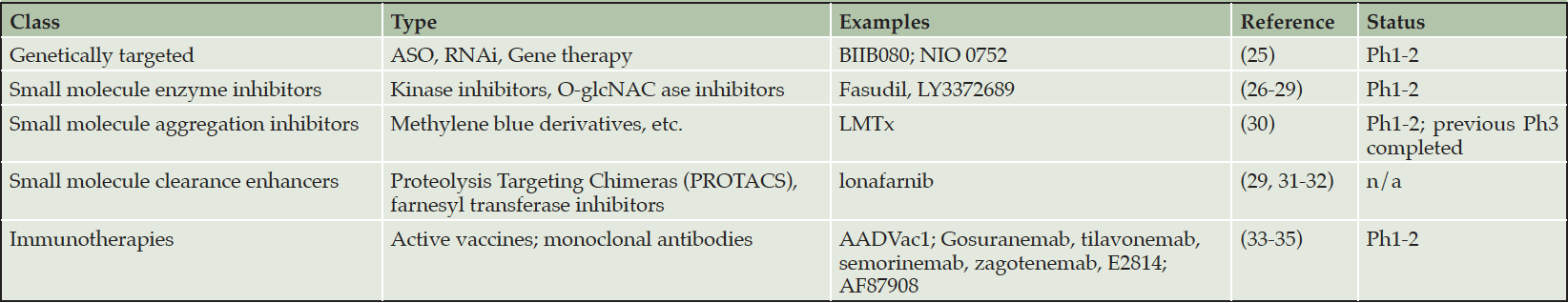
Five general approaches to anti-tau therapies are listed in Table 1.
The NIH funded Alzheimer’s Clinical Trial Consortium (ACTC) is planning a platform trial that would allow efficient testing of multiple tau therapies alone or in combination with an anti-amyloid drug. The ACTC Tau Platform (ATP) is currently under review for funding by the NIA and will look at key issues such as when in the course of disease can we measure tau treatment effects, who to enroll in terms of AT(N), and what effect size such as 30% SUVr reduction would correlate with a clinically meaningful response to treatment. Another issue is whether to use an active anti-amyloid comparator arm and a placebo arm in this new generation of randomized clinical trials. Platform trials offer many efficiencies over traditional clinical trial designs (36) and may reduce the amount of time necessary to determine proof of concept as well as the costs of clinical development of new tau therapies for AD.
The NIH funded DIAN-TU trials have launched a combination anti-amyloid and anti-tau program as part of the next generation platform (37). The design (NCT01760005) incorporates combined treatment with lecanemab and E2814, an antibody targeting the MTBR of tau in two populations of pre-symptomatic mutation carriers within 10 years of symptom onset and a symptomatic population up to CDR 1. The trial launched in December 2021 (38). The platform has two funded additional tau drug arms to launch which may or may not include anti-amyloid treatments and will focus on tau drugs with different targets.
Therapeutic perspectives beyond reduction of amyloid and tau pathologies
Working within the framework of a pathogenic sequence of AD that includes the over- or aberrant- production of the APP and the Aβ peptide, and then proceeds eventually to 3R/4R tau deposition in the entorhinal cortex with eventual expansion of tauopathy into the temporal and parietal neocortices, there are numerous unanswered questions about the controls at each step (39). If it turns out that either Aβ accumulation or tau accumulation are not the only direct causes of neurodegeneration that produces cognitive impairment, targets along this mechanistic chain other than Aβ or tau reductions might prove to be more effective points of intervention. Or, even if Aβ and tau accumulation are indeed directly in the key mechanistic chain, there could be treatable rate-limiting steps with non-Aβ, non-tau targets.
Several general domains suggest themselves from what is known about the biology of AD. One approach is to understand synaptic homeostasis and how to recover from dyshomeostasis. Treatment with low-dose levetiracetam is one such example (40). Second, understanding the early steps of protein misfolding and how to prevent misfolded proteins from remaining in the cytoplasm is another strategy. Epichaperome inhibitors are being tested in pursuit of this approach (41). Third, dysregulation of neuronal autophagy and the complex machinery of proteostasis may have druggable targets (42). Blocking GTP-Rab5 overactivation with neflamapimod is one example (43). Fourth, understanding dysfunctional inflammatory or cytotoxic responses that are generated by upstream process such as the generation of toxic Aβ or tau species could lead to targeting kinase inhibitors that are specific to activated microglia (44) or to stimulating microglial activity with a proinflammatory cytokine granulocyte macrophage colony-stimulating factor to promote clearance of misfolded proteins (45). Attempting to intervene in the interaction of cellular senescence and neurodegeneration is yet a different approach (46, 47). These examples are but a few of the promising targets that should be pursued.
One aspect of the etiology of the disease that has been largely neglected until recent years is the role of aging (48). The National Institute of Aging, highlighted geroscience for AD drug discovery. Importantly, the paper also outlines specific areas where attention to the pillars of aging might be fruitful in our efforts against Alzheimer’s. A better understanding of the biology of aging paves the way to pharmacological compounds linked to the hallmarks of aging, including senolytics, inflammasome inhibitors, metformin, rapamycin, resveratrol mesenchymal stem cells, mitochondria agents (49)
The general lack of specific pharmacodynamic markers for non-Aβ, non-tau targets is perhaps the greatest conceptual and practical barrier to the discovery of therapeutics for this class of putative therapies. A pharmacodynamic biomarker is of critical importance for determining dosing and for obtaining early-stage evidence to justify moving from phase 2 to phase 3. To be sure the amyloid and tau therapeutic approaches were also stymied for some time by this same limitation. A sponsor seeking to move a therapeutic agent into the clinic should be simultaneously developing a fluid or imaging pharmacodynamic biomarker which would support the mechanism of action of the therapy. This is hardly a trivial matter considering the long gestation and multiple laboratories involved in the development of amyloid or tau biomarkers, and the ongoing frustration of lacking a biomarker for TDP43, for example. Investments in new pharmacodynamic biomarkers must be a major focus of funding and research in AD therapeutics. Geroscience will also probably help to determine biological age in the future (50)
From a RCT perspective limitations for testing these approaches are the inability of smaller and shorter trials to identify relevant disease-modification and impact on clinical symptoms emergence or progression. Fortunately, biological characterisation of potential participants using biomarkers will help recruit homogeneous populations for proof-of-concept studies.
Conclusions
There is strong epidemiologic, genetic, and experimental support for both amyloid and tau as therapeutic targets in the prevention and treatment of AD. There may be a ceiling limiting how much anti-amyloid and anti-tau monotherapies independently slow down clinical progression, despite clear target engagement, especially if initiated at the symptomatic stage of dis. Combination studies where distinct pathologies could be targeted by precise timing of administration ease are critical and inevitable, looking for synergistic effects. Immune system modulation at a strategic time in the course of disease may further modify disease progression.
Though beyond the scope of this year’s EU/US Task Force meeting, the treatment options for persons with an Alzheimer-like phenotype but that are amyloid negative deserves further discussion beyond exclusion from anti-amyloid RCT.
Disclosure: The Task Force was partially funded by registration fees from industrial participants. These corporations placed no restrictions on this work. Dr. Gauthier is a member of scientific advisory boards with Advantage Therapeutics, Alzheon, Amyriad Therapeutics, Biogen Canada, Cerveau Technologies, Lundbeck, TauRx, Dr. Knopman serves on a Data Safety Monitoring Board for the DIAN study. He served on a Data Safety monitoring Board for a tau therapeutic for Biogen but received no personal compensation. He is an investigator in clinical trials sponsored by Biogen, Lilly Pharmaceuticals and the University of Southern California. He has served as a consultant for Roche, Samus Therapeutics, Magellan Health and Alzeca Biosciences but receives no personal compensation. He receives funding from the NIH. Dr. Boxer has served as a consultant and/or SAB member for Alector, Arkuda, Arvinas, AZTherapeutics, Denali, GSK, Humana, Oligomerix, Ono, Oscotec, Roche, Stealth, Transposon, True Binding and Wave. He receives research support from Biogen, Eisai, Regeneron, the NIH, Tau Consortium, Bluefield Project, Association for Frontotemporal Degeneration, Alzheimer’s Association and Alzheimer’s Drug Discovery Foundation. * Grant Support: NIH U19AG063911, U54NS092089, R01AG038791, U01AG045390, Tau Research Consortium, Bluefield Project to Cure FTD, University of California Cures AD Program, Association for Frontotemporal Degeneration, CBD Solutions, Alzheimer’s Drug Discovery Foundation, Alzheimer’s Association, UCSF Parkinson’s Spectrum Disorders Center; * Industry research support (funding): Biogen, Eisai, Regeneron; Industry research support (unfunded collaborations); Biogen, Eli Lilly, Novartis. – Industry consulting: AGTC, Alector, Arkuda, Arvinas, AZTherapeutics, GSK, Lundbeck, Oligomerix, Ono, Roche, Samumed, Stealth, Third Rock, Transposon, Wave; Dr. Aisen reports grants from Janssen, Eli Lilly, Eisai, NIA, the Alzheimer’s Association, and FNIH; and consulting fees from Biogen, Roche, Merck, Abbvie, Lundbeck, Proclara, and Immunobrain Checkpoint. He is one of JPAD EiC with no personal compensation. Dr. Sims is employee of Eli Lilly. Dr. Doody is a full-time employee of Genentech, Inc. and F. Hoffmann-La Roche Ltd. Dr. Iwatsubo has nothing to disclose related to this article. Dr. Bateman is Director of DIAN–TU and Principal Investigator of DIAN–TU001. He receives research support from the NIA of the NIH, DIAN–TU trial pharmaceutical partners (Eli Lilly and Company, F. HoffmanLa Roche Ltd and Avid Radiopharmaceuticals), Alzheimer’s Association, GHR Foundation, Anonymous Organization, DIAN–TU Pharma Consortium (active: Biogen, Eisai, Eli Lilly and Company, Janssen, F. HoffmannLa Roche Ltd/Genentech; previous: AbbVie, Amgen, AstraZeneca, Forum, Mithridion, Novartis, Pfizer, Sanofi, United Neuroscience). He has been an invited speaker and consultant for AC Immune, F. HoffmanLa Roche Ltd and Janssen and a consultant for Amgen and Eisai. D.H., the Department Head of Neurology where the research is being conducted, is an inventor on patents for solanezumab, currently being tested in the DIAN–TU clinical trials. If solanezumab is approved as a treatment for AD or dominantly inherited AD, Washington University and D.H. will receive part of the net sales of solanezumab from Eli Lilly and Company, which has licensed patents related to solanezumab from Washington University. Dr. Vellas is an investigator in clinical trials sponsored by Biogen, Lilly, Roche, Eisai Pharmaceuticals and the Toulouse University Hospital. He has served as SAB member for Biogen , Alzheon, Green Valey, Norvo Nordisk, Longeveron, but received no personal compensation. He has served as consultant and/or SAB member for Roche, Lilly, Eisai, TauX with personal compensation. He is member of the Editorial Board of JPAD with no personal compensation; and did not have a role in the editorial process/review for this manuscript.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Therriault J, Pascoal TA, Sefranek S, Mathotaarachchi S, Benedet AL, Chamoun M et al. Amyloid-dependent and amyloid-independent effects of tau in individuals without dementia. Ann Clin Transl Neurol 2021 Oct;8(10): 2083-2092.
2. Pascoal TA, Benedet AL, Ashton NJ, Kang MS, Therriault J, Chamoun M et al. Microglial activation and tau propagate jointly across Braak stages. Nat Med 2021 Sep;27(9): 1592-1599.
3. Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB et al. NIA-AA research framework: toward a biological definition of Alzheimer`s disease. Alzheimer Dement. 2018 Apr;14(4): 535-562.
4. Power MC, Mormino E, Soldan A, James BD, Yu L, Armstrong NM et al. Combined neuropathological pathways account for age-related risk of dementia. Ann Neurol 2018 Jul;84(1): 10-22.
5. Cohen S, van Dyck CH, Mummery CJ, Porsteinsson A, Kong J, Miller R et al. Baseline EMBARK data from EMERGE, ENGAGE and PRIME participants in the EMBARK re-dosing study. JPAD 2021 Nov:8(4): S63-64. Presented at the 14th CTAD Conference, Boston, abstract LBR02.
6. Hansson O, Nisenbaum L, Chen T, Rajagovindan R, Tian Y, Muralidharan KK et al. Dose- and time- dependant changes in plasma p-tau181 in patients treated with aducanumab in the ENGAGE and EMERGE trials. Presented at the 14th CTAD Conference, Boston, Late Braking News Round Table 8.
7. Salloway S, Farlow M, McDade E, Clifford DB, Wang G, Llibre-Guerra JJ et al. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease. Nature Medicine 2021 Jun;27: 1187-1196.
8. Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW et al. Donanemab in early Alzheimer’s disease. N Engl J Med 2021 May; 384(18);1691-1704.
9. Sanchez, J. S., J. A. Becker, H. I. L. Jacobs, B. J. Hanseeuw, S. Jiang, A. P. Schultz, M. J. Properzi, S. R. Katz, A. Beiser, C. L. Satizabal, A. O’Donnell, C. DeCarli, R. Killiany, G. El Fakhri, M. D. Normandin, T. Gomez-Isla, Y. T. Quiroz, D. M. Rentz, R. A. Sperling, S. Seshadri, J. Augustinack, J. C. Price, and K. A. Johnson. ‘The cortical origin and initial spread of medial temporal tauopathy in Alzheimer’s disease assessed with positron emission tomography’, Sci Transl Med, 2021;13.
10. Ghetti, B., A. L. Oblak, B. F. Boeve, K. A. Johnson, B. C. Dickerson, and M. Goedert. ‘Invited review: Frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: a chameleon for neuropathology and neuroimaging’, Neuropathology and applied neurobiology, 2015;41: 24-46.
11. Chang, C. W., E. Shao, and L. Mucke. ‘Tau: Enabler of diverse brain disorders and target of rapidly evolving therapeutic strategies’, Science, 2021;371.
12 VandeVrede, L., A. L. Boxer, and M. Polydoro. ‘Targeting tau: Clinical trials and novel therapeutic approaches’, Neurosci Lett, 2020;731: 134919.
13. Wesseling H et al. Tau PTM profiles indentify patient heterogeneity and stages of Alzheimer’s disease. Cell 2020;183: 1699-1713.
14. Shi, Y., W. Zhang, Y. Yang, A. G. Murzin, B. Falcon, A. Kotecha, M. van Beers, A. Tarutani, F. Kametani, H. J. Garringer, R. Vidal, G. I. Hallinan, T. Lashley, Y. Saito, S. Murayama, M. Yoshida, H. Tanaka, A. Kakita, T. Ikeuchi, A. C. Robinson, D. M. A. Mann, G. G. Kovacs, T. Revesz, B. Ghetti, M. Hasegawa, M. Goedert, and S. H. W. Scheres. ‘Structure-based classification of tauopathies’, Nature, 2021;598: 359-63.
15. Horie, Kanta, Nicolas R Barthélemy, Chihiro Sato, and Randall J Bateman. “CSF Tau Microtubule Binding Region Identifies Tau Tangle and Clinical Stages of Alzheimer’s Disease.” Brain, no. awaa373 (December 7, 2020). https://doi.org/10.1093/brain/awaa373.
16. Horie, Kanta, Nicolas R. Barthélemy, Nipun Mallipeddi, Yan Li, Erin E. Franklin, Richard J. Perrin, Randall J. Bateman, and Chihiro Sato. “Regional Correlation of Biochemical Measures of Amyloid and Tau Phosphorylation in the Brain.” Acta Neuropathologica Communications 8, no. 1 (August 27, 2020): 149. https://doi.org/10.1186/s40478-020-01019-z.
17. Dam, T., A. L. Boxer, L. I. Golbe, G. U. Hoglinger, H. R. Morris, I. Litvan, A. E. Lang, J. C. Corvol, I. Aiba, M. Grundman, L. Yang, B. Tidemann-Miller, J. Kupferman, K. Harper, K. Kamisoglu, M. J. Wald, D. L. Graham, L. Gedney, J. O’Gorman, S. B. Haeberlein, and Passport Study Group. ‘Safety and efficacy of anti-tau monoclonal antibody gosuranemab in progressive supranuclear palsy: a phase 2, randomized, placebo-controlled trial’, Nat Med, 2021;27: 1451-57.
18. Hoglinger, G. U., I. Litvan, N. Mendonca, D. Wang, H. Zheng, B. Rendenbach-Mueller, H. K. Lon, Z. Jin, N. Fisseha, K. Budur, M. Gold, D. Ryman, H. Florian, and Investigators Arise. ‘Safety and efficacy of tilavonemab in progressive supranuclear palsy: a phase 2, randomised, placebo-controlled trial’, Lancet Neurol, 2021;20: 182-92.
19. Ayalon, G., S. H. Lee, O. Adolfsson, C. Foo-Atkins, J. K. Atwal, M. Blendstrup, H. Booler, J. Bravo, R. Brendza, F. Brunstein, R. Chan, P. Chandra, J. A. Couch, A. Datwani, B. Demeule, D. DiCara, R. Erickson, J. A. Ernst, O. Foreman, D. He, I. Hotzel, M. Keeley, M. C. M. Kwok, J. Lafrance-Vanasse, H. Lin, Y. Lu, W. Luk, P. Manser, A. Muhs, H. Ngu, A. Pfeifer, M. Pihlgren, G. K. Rao, K. Scearce-Levie, S. P. Schauer, W. B. Smith, H. Solanoy, E. Teng, K. R. Wildsmith, D. Bumbaca Yadav, Y. Ying, R. N. Fuji, and G. A. Kerchner. ‘Antibody semorinemab reduces tau pathology in a transgenic mouse model and engages tau in patients with Alzheimer’s disease’, Sci Transl Med, 2021;13.
20. Boxer, A. L., I. Qureshi, M. Ahlijanian, M. Grundman, L. I. Golbe, I. Litvan, L. S. Honig, P. Tuite, N. R. McFarland, P. O’Suilleabhain, T. Xie, G. S. Tirucherai, C. Bechtold, Y. Bordelon, D. S. Geldmacher, M. Grossman, S. Isaacson, T. Zesiewicz, T. Olsson, K. K. Muralidharan, D. L. Graham, J. O’Gorman, S. B. Haeberlein, and T. Dam. ‘Safety of the tau-directed monoclonal antibody BIIB092 in progressive supranuclear palsy: a randomised, placebo- controlled, multiple ascending dose phase 1b trial’, Lancet Neurol, 2019;18: 549-58.
21. Sopko, R., O. Golonzhka, J. Arndt, C. Quan, J. Czerkowicz, A. Cameron, B. Smith, Y. Murugesan, G. Gibbons, S. J. Kim, J. Q. Trojanowski, V. M. Y. Lee, K. R. Brunden, D. L. Graham, P. H. Weinreb, and H. Hering. 2020. ‘Characterization of tau binding by gosuranemab’, Neurobiol Dis, 146: 105120.
22. Bright, J., S. Hussain, V. Dang, S. Wright, B. Cooper, T. Byun, C. Ramos, A. Singh, G. Parry, N. Stagliano, and I. Griswold-Prenner. ‘Human secreted tau increases amyloid-beta production’, Neurobiology of aging, 2015;36: 693-709.
23. Chang, C. W., M. D. Evans, X. Yu, G. Q. Yu, and L. Mucke. ‘Tau reduction affects excitatory and inhibitory neurons differently, reduces excitation/inhibition ratios, and counteracts network hypersynchrony’, Cell Rep, 2021;37: 109855.
24. Monteiro C. Presented at the 14th Conference Clinical Trials Alzheimer’s Disease, November 9–12, 2021, Boston,
25. Mummery, C., C Junge, H Kordasiweicz, L. Mignong, K. Moore, C. Yun, T. Baumann, D. Li, D.A. Norris, R. Crean, D. Graham, E. Huang, E. Ratti, and R.M. Lane. 2021. “Results of the First-in-Human, Randomized, Double-Blind, Placebo-Controlled Phase 1bStudy of Lumbar Intrathecal Bolus Administrations of Antisense Oligonucleotide (ISIS 814907; BIIB080) Targeting Tau mRNA in Patients with Mild Alzheimer’s Disease.” In Alzheimer’s Association International Conference. Denver.
26. Bartolome-Nebreda, J. M., A. A. Trabanco, A. I. Velter, and P. Buijnsters. ‘O-GlcNAcase inhibitors as potential therapeutics for the treatment of Alzheimer’s disease and related tauopathies: analysis of the patent literature’, Expert Opin Ther Pat, 2021;31: 1117-54.
27. Gentry, E. G., B. W. Henderson, A. E. Arrant, M. Gearing, Y. Feng, N. C. Riddle, and J. H. Herskowitz. ‘Rho Kinase Inhibition as a Therapeutic for Progressive Supranuclear Palsy and Corticobasal Degeneration’, J Neurosci, 2016;36: 1316-23.
28. Hamano, T., N. Shirafuji, S. H. Yen, H. Yoshida, N. M. Kanaan, K. Hayashi, M. Ikawa, O. Yamamura, Y. Fujita, M. Kuriyama, and Y. Nakamoto. ‘Rho-kinase ROCK inhibitors reduce oligomeric tau protein’, Neurobiol Aging, 2020;89: 41-54.
29. Alquezar, C. S. Arya and A.W. Kao. ‘Tau Post-translational Modifications: Dynamic Transformers of Tau Function, Degradation, and Aggregation’, Front Neurol, 2020;11:595532
30. Gauthier, S., H. H. Feldman, L. S. Schneider, G. K. Wilcock, G. B. Frisoni, J. H. Hardlund, H. J. Moebius, P. Bentham, K. A. Kook, D. J. Wischik, B. O. Schelter, C. S. Davis, R. T. Staff, L. Bracoud, K. Shamsi, J. M. Storey, C. R. Harrington, and C. M. Wischik. ‘Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial’, Lancet, 2016;388: 2873-84.
31. Hyun, S., and D. Shin. ‘Chemical-Mediated Targeted Protein Degradation in Neurodegenerative Diseases’, Life (Basel), 2021;11.
32. Hernandez, I., G. Luna, J. N. Rauch, S. A. Reis, M. Giroux, C. M. Karch, D. Boctor, Y. E. Sibih, N. J. Storm, A. Dithe az, S. Kaushik, C. Zekanowski, A. A. Kang, C. R. Hinman, V. Cerovac, E. Guzman, H. Zhou, S. J. Haggarty, A. M. Goate, S. K. Fisher, A. M. Cuervo, and K. S. Kosik. 2019. ‘A farnesyltransferase inhibitor activates lysosomes and reduces tau pathology in mice with tauopathy’, Sci Transl Med, 2019;11.
34. Novak, P., B. Kovacech, S. Katina, R. Schmid, P. Scheltens, E. Kontsekova, S. Ropele, L. Fialova, M. Kramberger, N. Paulenka-Ivanovova, M. Smisek, J. Hanes, E. Stevens, A. Kovac, S. Sutovsky, V. Parrak, P. Koson, M. Prcina, J. Galba, M. Cente, T. Hromadka, P. Filipcik, J. Piestansky, M. Samcova, C. Prenn-Gologranc, R. Sivak, L. Frolich, M. Fresser, M. Rakusa, J. Harrison, J. Hort, M. Otto, D. Tosun, M. Ondrus, B. Winblad, M. Novak, and N. Zilka. ‘ADAMANT: a placebo-controlled randomized phase 2 study of AADvac1, an active immunotherapy against pathological tau in Alzheimer’s disease’, Nat Aging, 2021;1: 521-34.
34. Andersson, C. R., J. Falsig, J. B. Stavenhagen, S. Christensen, F. Kartberg, N. Rosenqvist, B. Finsen, and J. T. Pedersen. ‘Antibody-mediated clearance of tau in primary mouse microglial cultures requires Fcgamma-receptor binding and functional lysosomes’, Sci Rep, 2019;9: 4658.
35. Roberts, M., I. Sevastou, Y. Imaizumi, K. Mistry, S. Talma, M. Dey, J. Gartlon, H. Ochiai, Z. Zhou, S. Akasofu, N. Tokuhara, M. Ogo, M. Aoyama, H. Aoyagi, K. Strand, E. Sajedi, K. L. Agarwala, J. Spidel, E. Albone, K. Horie, J. M. Staddon, and R. de Silva. ‘Pre-clinical characterisation of E2814, a high-affinity antibody targeting the microtubule-binding repeat domain of tau for passive immunotherapy in Alzheimer’s disease’, Acta Neuropathol Commun, 2020;8: 13.
36. Woodcock, J., and L. M. LaVange. ‘Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both’, N Engl J Med, 2017;377: 62-70.
37. Bateman, Randall J., Tammie L. Benzinger, Scott Berry, David B. Clifford, Cynthia Duggan, Anne M. Fagan, Kathleen Fanning, et al. “The DIAN-TU Next Generation Alzheimer’s Prevention Trial: Adaptive Design and Disease Progression Model.” Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 13, no. 1,2017: 8–19. https://doi.org/10.1016/j.jalz.2016.07.005.
38. https://www.prnewswire.com/news-releases/first-subject-enrolled-in-phase-iiiii-study-of-eisais-anti-mtbr-tau-antibody-e2814-for-dominantly-inherited-alzheimers-disease-diad-conducted-by-dian-tu-301463234.html
39. Knopman et al. Alzheimer disease. Nature Reviews. Disease primers. 2021; 7;33.
40. Haberman RP, Branch A, Gallagher M. Targeting neural hyperactivity as a treatment to stem progression of late-onset Alzheimer’s disease. Neurotherapeutics 2017;14; 662-676.
41. Ginsberg SD et al. Disease-specific interactome alterations via epichaperomics: the case for Alzheimer’s disease. FEBS 2021
42. Small SA, Petsko GA. Endosomal recycling reconciles the Alzheimer’s disease paradox. Sci Transl Med 2020;12.
43. Pensalfini A et al. Endosomal dysfunction induced by directly overactivating Rab5 recapitulates prodromal and neurodegenerative features of Alzheimer’s disease. Cell Rep 2020; 33:108420.
44. Morganti JM, Goulding DS, Van Eldik LJ. Deletion of p38α MAPK in microgla blunts trauma-induced inflammatory responses in mice. Journal of Neuroinflammation 2020;16:98.
45. Potter H et al. Safety and efficacy of sargramostin (GM-CSF) in the treatment of Alzheimer’s disease. Alzheimer’s & Dementia 2021;7:e12158.
46. Baker DJ & Petersen RC. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J Clin Invest 2018;128: 1208-1216.
47. Gonzales, M.M., Garbarino, V.R., Zilli, E.M. et al. Senolytic Therapy to Modulate the Progression of Alzheimer’s Disease (SToMP-AD): A Pilot Clinical Trial. J Prev Alzheimers Dis,2021. https://doi.org/10.14283/jpad.2021.62
48. Sierra, F. Geroscience and the Role of Aging in the Etiology and Management of Alzheimer’s Disease. J Prev Alzheimers Dis 2020;7,2–3. https://doi.org/10.14283/jpad.2019.49
49. Guerville, F., De Souto Barreto, P., Ader, I. et al. Revisiting the Hallmarks of Aging to Identify Markers of Biological Age. J Prev Alzheimers Dis 2020;7, 56–64. https://doi.org/10.14283/jpad.2019.50
50. Sayed, N., Huang, Y., Nguyen, K. et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat Aging 2021;1, 598–615. https://doi.org/10.1038/s43587-021-00082-y