R. Martins1,2, N. Kotsopoulos1,3, B. Michalowsky4, P. Pemberton-Ross5, M. Urbich5, M.P. Connolly1,2
1. Global Market Access Solutions, Health Economics Unit, St-Prex, Switzerland; 2. University Medical Centre Groningen (UMCG), University of Groningen, Groningen, The Netherlands; 3. Department of Economics, University of Athens, Athens, Greece; 4. German Center for Neurodegenerative Diseases (DZNE), Rostock/Greifswald, Greifswald, Germany; 5. Biogen International GmbH, Baar, Switzerland
Corresponding Author: Mark Connolly, Global Market Access Solutions, St-Prex, 1162, Switzerland, mark@gmasoln.com
J Prev Alz Dis 2022;4(9):758-768
Published online May 26, 2022, http://dx.doi.org/10.14283/jpad.2022.53
Abstract
Background: Alzheimer’s disease is a severe condition, impacting individual’s wellbeing and independence in daily activities. Informal care provision is common and of great value to societies but is not without negative externalities to households and the broader economy.
Objectives: Estimate the lifetime incremental fiscal consequences of Alzheimer’s disease in community-based individuals and their informal caregivers.
Setting: The fiscal consequences of Alzheimer’s disease was modeled using the German government and social security perspective.
Participants: Synthetic cohort containing 1,000 pairs of people with Alzheimer’s disease and their informal caregivers, compared to 1,000 demographically identical pairs from the general population.
Design: Disease progression was modeled using published equations and a state-transition microsimulation framework. Labor participation, financial support and paid taxes were estimated according to cognitive decline and caregiving responsibilities using German labor statistics and tax rates. Healthcare costs were sourced from several German publications. Costs and life-years were discounted at 3% annually.
Measurements: Results are reported as lifetime incremental differences in total tax revenue and transfer payments between the cohort affected by Alzheimer’s disease and their general population analogues.
Results: The Alzheimer’s disease-affected pair was associated with net incremental fiscal losses of €74,288 ($85,037) to the German government and social security over the lifetime of people with Alzheimer’s disease. Most costs were lost taxes on employment earnings (48.4%) due to caregivers working reduced hours. Caregivers were estimated to earn €56,967 ($65,209) less than their general population analogues. Financial support for informal and formal care accounted for 20.4%, and medical healthcare costs represented 24.0% of the incremental fiscal losses. Sensitivity analyses confirmed the robustness of the model results. In a cohort with early onset Alzheimer’s disease, incremental fiscal losses were predicted to be €118,533 ($114,209) over the lifetime of people with Alzheimer’s disease.
Conclusions: Alzheimer’s disease externalities profoundly impact public economics for governments and should be considered to inform policy making and healthcare planning.
Key words: Alzheimer’s disease, dementia, burden of disease, fiscal costs, microsimulation.
Introduction
Alzheimer’s disease (AD) is a severe neurodegenerative condition. At the early preclinical and mild cognitive impairment due to AD (MCI-AD) stages, the condition has little impact on everyday activities. Progression to AD-dementia eventually results in cognitive and memory impairment, and a profound loss of independence in daily activities (1). AD accounts for 60-80% of all forms of dementia (1, 2) and is one of the leading causes of mortality and disability worldwide (3). It mainly affects people over the age of 65, two-thirds being females (4).
Most people with AD (PwAD) are community-dwelling, and over 60% are cared for by informal caregivers, mainly family members (5). Informal care and supervision requirements increase with disease severity and can add to over 36 hours a week (6), representing the highest value of informal caregiving, followed by mental illness and multiple sclerosis (1). The economic value of unpaid informal care in Germany has been estimated to exceed €38,000 per PwAD annually (7). Additionally, informal care provision has a detrimental effect on caregiver’s physical and emotional wellbeing (1), limiting their ability to maintain a job and sustain earnings (7-12). Caregivers are also likely to increase out-of-pocket expenses, reducing their ability to save for their retirement (13).
The financial impacts of AD are not constrained to households but have wide-reaching effects on government public accounts i.e. public economics (13). Different sources financially cover the entire treatment and care pathway of people with dementia. Medical treatments, like diagnosis, outpatient medical and hospital care, medical aids, therapies, and medications, are covered by the statutory or private health insurances. If patients become functionally or cognitively impaired and need care, long-term care insurance covers professional home, nursing care and informal care costs. The degree of impairment determines the amount of the received non-monetary care benefits in kind or monetary care allowance as compensation for the informal care provided by caregivers. Also, further financial support could be made available by the long-term care insurance for all cognitively-impaired elderly to receive additional respite services. Thus, the high and increasing prevalence of AD increases national health and social care expenditure, affecting economic equity across generations (14). The number of people living with dementia in Europe is predicted to double by 2050 (2). Germany is expected to follow a similar trend (2). In 2016, AD was estimated to affect 1.2-1.7% of the entire German population, 1.0-1.4 million individuals (3, 15). From a German societal perspective, dementia-related healthcare costs were €54 billion in 2016 and have been estimated to increase to €145 billion by 2060 (16).
Burden of AD studies often take a health system or societal perspective, focusing on direct medical and non-medical costs, and unpaid caregiver costs (13). From a societal perspective, direct medical costs represent approximately 14% of total AD-related costs, far exceeded by indirect and social care costs (17). By definition, economic analyses conducted from a societal perspective can capture all costs incurred by the formal and informal healthcare sectors, and by non-healthcare sectors (18). Despite over 67% of studies utilizing a societal perspective, this would only account for the replacement costs of labor productivity losses, in addition to the direct costs of disease, disregarding other important economic consequences (19). In countries with publicly funded health and social care systems it makes intuitive sense to factor in important disease externalities that impact public entities. Using the government and social security perspective of costs, AD-related economic consequences such as financial support and foregone tax contributions, rarely considered in conventional cost-effectiveness analyses, can be quantified. This contributes to informing decision-makers of the full impact of AD on public budgets and allows healthcare interventions to be assessed within the policy arena
Therefore, we aim to estimate lifetime AD fiscal consequences in community-dwelling PwAD and primary informal caregivers compared to pairs with identical demographic characteristics unaffected by AD in the German general population. We use a government perspective (i.e., government and social security costs) to estimate the impact of AD progression on government public finances (20, 21), namely labor force participation, earnings, tax contributions, healthcare costs and financial support since MCI-AD to death in PwAD and caregivers.
Methods
Model overview
The analysis was developed as a microsimulation in Microsoft Excel, allowing individual-level modeling of PwAD/caregiver pair combinations and their likelihood of demographic and disease-related events. Care requirements and costs were modeled continuously, avoiding discrete memoryless Markov assumptions (22-24). The model simulates clinical progression from MCI-AD to AD-dementia (mild, moderate, severe) and death. It also estimates severity-related informal care needs and PwAD and caregivers’ ability of remaining at work. The model structure is shown in Supplemental Materials.
In general, simulation events were deemed to occur when a random probability, drawn using Excel Rand() function, was inferior or equal to the likelihood of that event (eg, death, institutionalization) at the decision node. We have followed the International Society for Pharmacoeconomics and Outcomes Research recommendations for state transition modeling (24). The model uses a 6-month cycle length, granular enough to capture the costs and consequences of AD. We have applied a 3% annual discount rate after year 1 to express future costs and life-years in preset values (25).
Cohort general and sociodemographic characteristics
There is no publication reporting on Mini-Mental-State-Examination (MMSE) and activities of daily living (ADL) scores for all stages of AD in Germany. Therefore, we have created two paired synthetic cohorts using prior mean and variance parameters from the mild AD cohort of the German GERAS (9, 26), a multicountry prospective observational study following people with AD-dementia and their primary caregivers’ overtime. Each synthetic cohort contained 1,000 pairs of a PwAD and an informal caregiver and was cloned to identical pairs in the general population unaffected by AD. The cohort of PwAD was sampled from common distributions using age, gender, disease duration, MMSE and ADL scores. Age, gender, and relationship to PwAD were used to inform the cohort of caregivers. Mean demographic characteristics employed to generate the matched cohorts are reported in Supplemental Materials.
The GERAS study did not investigate age at MCI-AD onset, so we have back-calculated this value starting from the age at GERAS baseline reported by Boess and colleagues (74.7 years). We subtracted disease duration from the age at GERAS baseline and then subtracted a published average length of MCI-AD (3.4 years) from the calculated age at AD-dementia onset. The duration of MCI-AD was varied in sensitivity analysis.
Progression of Alzheimer’s disease
Clinical AD states were defined using MMSE scores of 27-29 for MCI-AD, 21-26 for mild AD-dementia, 10-20 for moderate AD-dementia and less than 10 for severe AD-dementia (27). Irrespective of age, all individuals enter the model at the MCI-AD stage. Progression through mild, moderate and severe AD dementia was modeled using equations derived from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) and seven clinical trials for donepezil data (28). The equations allowed for MMSE and total ADL scores to be updated at regular intervals, which were used to calculate individual care requirements. Because donepezil is known not to delay AD progression (1, 29), we have used the placebo equations to model overtime change in MMSE and total ADL scores. This assumption was varied in a scenario analysis. A detailed explanation of these equations and disease progression modeling is available from the online Supplemental Materials.
No MMSE or ADL data were available between the onset of mild AD and the age at enrollment in the GERAS study. Therefore, we have sampled disease duration from the GERAS reported mean and standard deviation assuming that over this period, MMSE and ADL scores were identical to those reported at GERAS baseline (Supplemental Materials).
Mortality
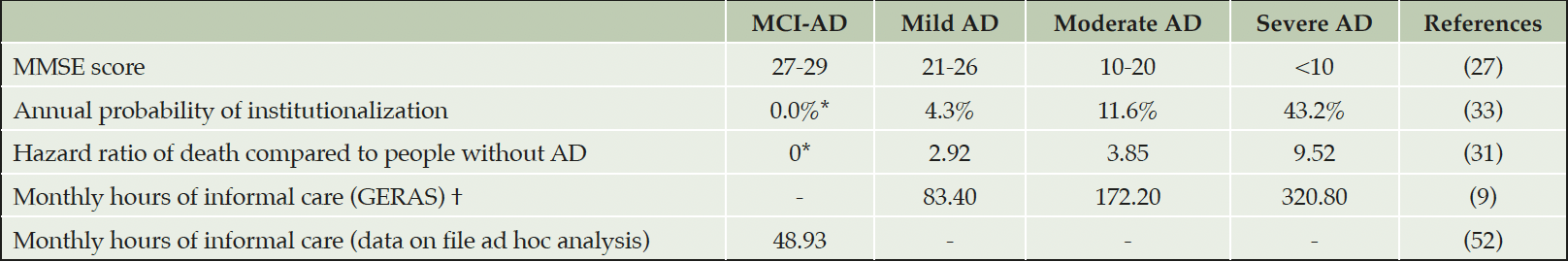
General mortality was implemented using cycle-adjusted probabilities based on published lifetables of the German Federal Statistical Office (DESTATIS) (30). The hazard ratios (HR) of excess mortality due to AD-dementia were sourced from a Danish population-based cohort (31) and applied to age-specific rates of death in the general population. The effect of excess mortality was explored in scenario analyses as it can lead to decreased government or social security transfers and caregiving requirements, therefore creating perverse incentives from premature AD-related deaths. We assumed that there was no excess mortality from MCI-AD. Parameters informing excess AD mortality are presented in Table 1. Transitions to death were allowed at any time. After PwAD death, caregivers were released from caring responsibilities, returned to employment, and no longer received AD-related financial support. Caregiver’s death was assumed not to affect PwAD’s probability of employment or receiving financial support. Caregiver’s death was also assumed to cause institutionalization in PwAD-dementia that had a MMSE score below 20.
AD, Alzheimer’s disease; MCI-AD, mild cognitive impairment due to AD; MMSE, Mini-Mental State Examination (Range from 30-0); *Assumption; †Average across AD severity categories was 209.4 hours per month.
Informal care utilization and institutionalization
Informal care utilization was modeled using an equation sourced from the GERAS study, which uses reported MMSE and total ADL scores to predict total hours of provided informal care per PwAD (32). A scenario was run where hours of informal care were informed by severity-specific estimates from the German GERAS study (9), (Table 1). Additionally, informal care utilization in people with MCI-AD was represented by an ad hoc analysis of data from the DelpHi-MV study (Michalowsky, data in file) (Table 1). More details about the dataset and analysis are available in Supplemental Materials.
The probabilities of institutionalization by AD severity were sourced from an analysis of data from the US National Alzheimer’s Coordinating Center (33) (Table 1). We assumed that working age caregivers of people becoming institutionalized could return to work due to reduced caregiving requirements. We have assumed that cognitive deficits associated with MCI-AD alone would not justify institutionalization (34).
Labor market participation
Age- and gender-specific probabilities of employment in the general population were sourced from German labor statistics (35). We assumed that individuals would not be able to carry on working from the age of 75 years. The likelihood of employment was assumed to be null in people with AD-dementia and MMSE scores below 25, or for those becoming institutionalized (36).
As supported by a recent systematic review (37), there is presently no publication linking MCI-AD to the likelihood of maintaining employment. Consequently, we used the prevalence of disability, defined as the inability to perform one or more ADL, as a proxy for employment discontinuation in people with impaired cognition due to MCI-AD. A French longitudinal study (N=368) estimated that the risk of disability in people over 65 years with MCI-AD (52.6%) was two times higher than in the general population (26.3%) (38). Age-specific rates of severe disability in the German population, were sourced from DESTATIS data (39).
The excess likelihood of leaving employment in people with AD-dementia before reaching the age of enrolment in the GERAS study was sourced from a matched Japanese cohort study of 143 employees and 77 family members diagnosed with early-onset dementia (EOD), defined as dementia occurring in individuals below the age of 65. Each participant was matched to 5 controls (people without EOD). Compared to the general population, people with mild AD-dementia under retirement age had an increased likelihood of not being employed HR 2.26, 95% CI 1.47 to 3.47), and so were their primary caregivers (HR 1.19, 95% CI 0.63 to 2.25) (40). Table 2 shows the probabilities of employment and disability used in the model.
The probability of employment and proportion of a full-time equivalent worked by employed caregivers were adjusted for the effect of daily care requirements using published equations (41). Equations from the same publication were used to adjust the employment hours in caregivers for people with MCI-AD using hours of care derived from the DelpHi-MV ad hoc analysis (Table 1). Further details are available in Supplemental Materials.
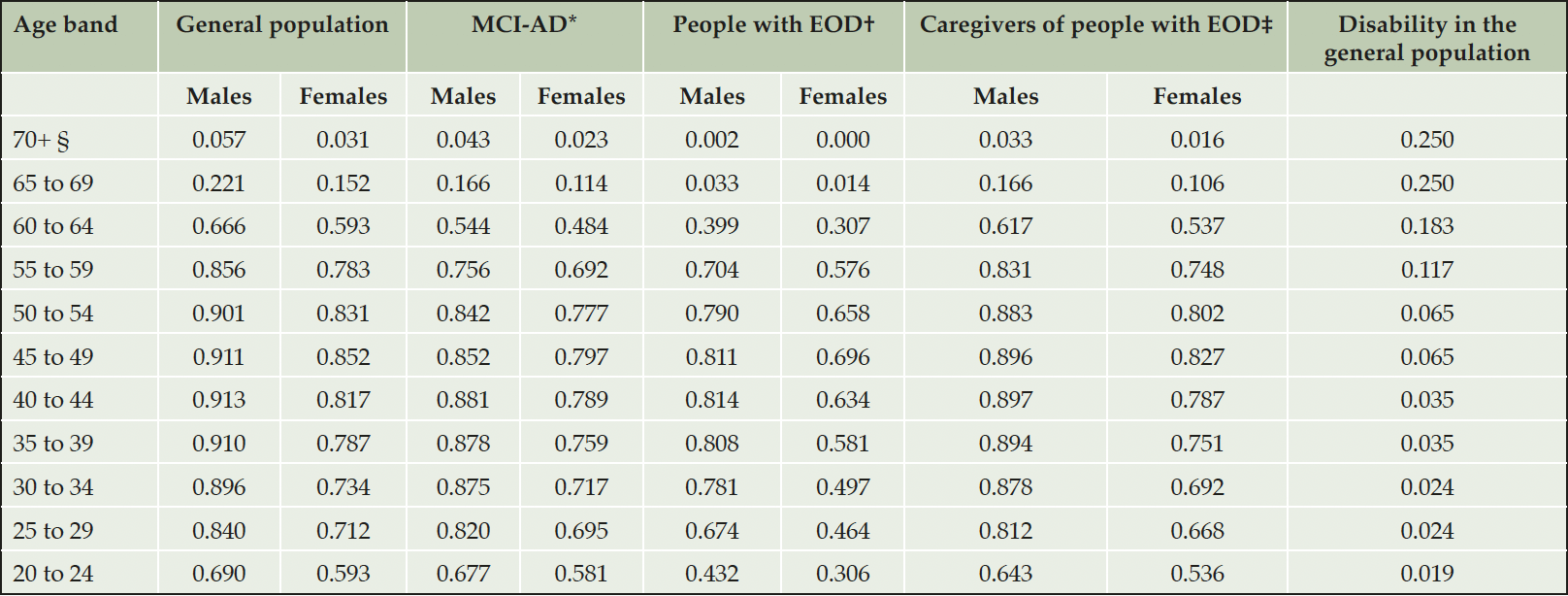
Table 2. Probabilities of employment and disability in the general population, adjusted probability of employment in people with AD and their informal caregivers
AD, Alzheimer’s disease; EOD, early onset dementia; MCI-AD, mild cognitive impairment due to AD; *Calculated as (1 – probability of disability in the general population) x gender specific employment in the general population; †Calculated by applying the HR 2.26 to the gender specific rate of employment in the general population; ‡Calculated by applying the HR 1.19 to the gender specific rate of employment in the general population. For equations, please refer to Supplemental Materials; §We assumed that individuals would only be able to carry on working until a maximum age of 75 years.
Fiscal consequences
The modeled fiscal outcomes comprise various sources of tax revenue and transfer payments that affect government and social security finances. Direct taxes paid on earnings and indirect taxes paid on consumer products were sources of revenue. Transfer payments were made of financial support provided to individuals carrying disability or their caregivers and medical healthcare costs.
Direct and indirect tax
To calculate the total taxes on labor income we have multiplied the age-specific gross income (42) by the tax wedge for Germany (49.40%) (43). The tax wedge represents the burden of direct tax paid by employees and the social security contributions paid by employees and employers. Indirect taxes paid by individuals result from daily consumption of products and services and were calculated by multiplying the value-added tax (VAT) by individuals’ disposable income from any source of revenue (earnings from employment or financial support). The disposable income is the amount individuals have available to spend or save after paying direct taxes and social security contributions on their income. The proportion of the gross salary representing the disposable income was calculated as the ratio between the average private consumption expenditure and the average gross income in Germany. The data used to derive the average disposable income and average VAT rate are shown in Supplemental Materials.
Sustained periods of absence from the labor market are likely to influence future earnings from employment. Consequently, we reduced the gross income of caregivers returning to work after 2 or more years caring for PwAD by a 7.2% rate (44).
Financial support for formal and informal care
Financial support was available as a relief («Entlastungsbetrag») granted to all cognitively-impaired elderly, care allowance («Pflegegeld») for people receiving informal care from relatives, care benefits in kind («Pflegesachleistung») for people receiving formal care by a health or social care professional, and AD-related support for caregivers (45). We used an ad hoc analysis of data from the DelpHi-MV study to assess the proportion of PwAD receiving each type of financial support (Michalowsky 2021- data in file). The amount of financial support was made conditional to care grade, as per the German Social Code (Book XI). Care grades range from one, indicating very mild functional impairment and low need for formal and informal care, to five, indicating severe functional impairment and high need for formal and informal care (46). The likelihood and value of financial support were informed by the German GERAS results (9). We assumed that people with MCI-AD would be as likely to receive financial support as the general population and that their caregivers would not be entitled to any AD-related financial support. Caregivers’ death prompted non-institutionalized PwAD to receive care benefits in kind.
In the cohort unaffected by AD, the number of individuals (analogous to PwAD) receiving financial support was informed by the proportion of persons in need of care in Germany (47). Level of care was informed by a World Health Organization report of German long-term care insurance beneficiaries (48).
Early retirement and absence from work due to disease are likely to influence income and the value of pensions (49). It is also known that low socio-economic status and education achievements increase the prevalence of chronic conditions such as cardiovascular disease, in itself a risk factor for AD (1). For simplicity and in face of limited evidence, we have chosen not to model the complex relationship between AD progression, the value of pensions and its interaction with government-funded financial support. Similarly, we excluded institutionalization costs as these are often not incurred by governments but paid out-of-pocket.
Medical healthcare costs
We sourced medical healthcare costs for people with MCI-AD and their analogues without AD from a cross-sectional study including 452 German individuals with or without MCI (10). Healthcare costs in PwAD dementia and analogous individuals without AD were sourced from another cross-sectional study matching 176 people with dementia to 173 healthy controls (8). We considered healthcare costs related to direct inpatient/outpatient medical care, medical aids, and drugs. The costs of formal nursing/professional home care and assisted living reported by Luppa et al. (10) and Leicht et al. (8) were excluded to avoid overlap with financial support and care benefits in kind received by PwAD. There is evidence supporting the caring for a PwAD is detrimental to caregiver’s physical and mental health but there is limited evidence of its monetary impact. We have therefore taken a conservative and simplistic approach and excluded healthcare costs for caregivers and their analogues from the model calculations. Inputs and calculations related to fiscal consequences are detailed in Supplemental Materials.
Model results calculations
Base case results were reported over the lifetime of the PwAD rounded to the closest decimal to accommodate for cycle length. Model results were expressed as incremental net consequence (INC) resulting from the difference between each cohort net present value (NPV).
Lifetime earnings from employment were reported for the cohort affected by AD and the general population but were not directly included in the NPV as these are incurred by individuals or employers in the form of lost earnings or reduced levels of production, respectively. The equations used in the calculations are shown below.

Where j is AD status, r is the annual discount rate of 3%, and t is time.
All costs were reported in 2021 euros. When required, costs were inflated to current values using the consumer price index (50).
Scenarios and sensitivity analysis
We implemented several scenarios to explore uncertainty in base case assumptions such as the intensity of formal and informal care, MCI-AD effect on employment, the amount of PwAD receiving financial support, the time horizon of the analysis and the rate of discounting. One-way sensitivity analyses (OWSA) were conducted using 95% confidence intervals (CI) bounds of all mean input values to identify the most influential parameters. These findings were summarized in a tornado diagram.
Results
The model predicted 10.1 years (standard deviation [SD] 2.2) were comprised from MCI-AD onset to death, corresponding to an average of 8.6 life-years (discounted). AD-dementia was estimated to last 6.7 years (SD 1.1) on average. Approximately 36.9% of this 10.1 year period was spent in MCI-AD (3.4 years), 21.5% in mild AD (2.2 years, SD 0.5), 26.3% in moderate AD (2.9 years, SD 1.2), and 15.3% in severe AD (1.7 years, SD 2.2). Model validation is discussed in the Supplemental Materials.
Base case
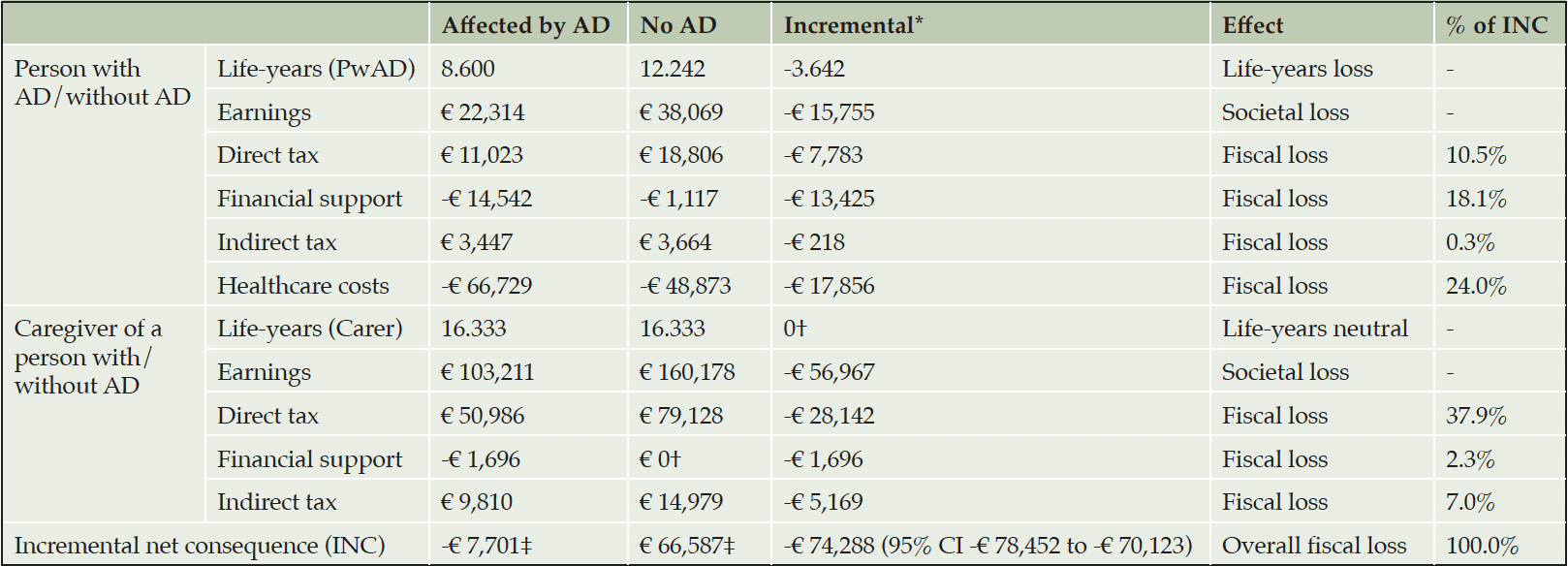
The model predicted that since AD onset and over the PwAD lifetime, a PwAD and his informal caregiver were associated with an INC totaling -€74,288 (95% CI -€ 78,452 to -€ 70,123) to the German government and social security compared to an identical pair unaffected by AD (Table 3). A PwAD was estimated to earn €15,755 less from employment leading to a 41.4% reduction in direct taxes paid. Additionally, PwAD were predicted to receive €13,425 more financial support and incur €17,856 more healthcare costs, than their analogues in the general population. Caregivers were predicted to earn €56,967 less from employment representing a 35.6% incremental reduction in direct tax (€28,142) and 34.5% (€5,169) in indirect tax contributions. Overall, the INC was primarily related to lost direct taxes on employment (48.4%), mostly caregiver employment. Financial support for formal and informal care and medical healthcare cost represents only 20.4% and 24.0% of the INC, respectively.
AD, Alzheimer’s disease; PwAD, person with AD; * Incremental results were calculated by subtracting the values in the column not affected by AD from the values in the column affected by AD; CI, confidence interval; †We have not modeled financial support required because of informal caregiving in the general population; ‡Calculated as the sum of direct taxes, financial support, indirect taxes and healthcare costs for a person with AD/without AD and a caregiver of a person with/without AD. Earnings were not included in the INC as they affect individuals directly, impacting the INC though direct and indirect tax contributions.
Scenario analyses
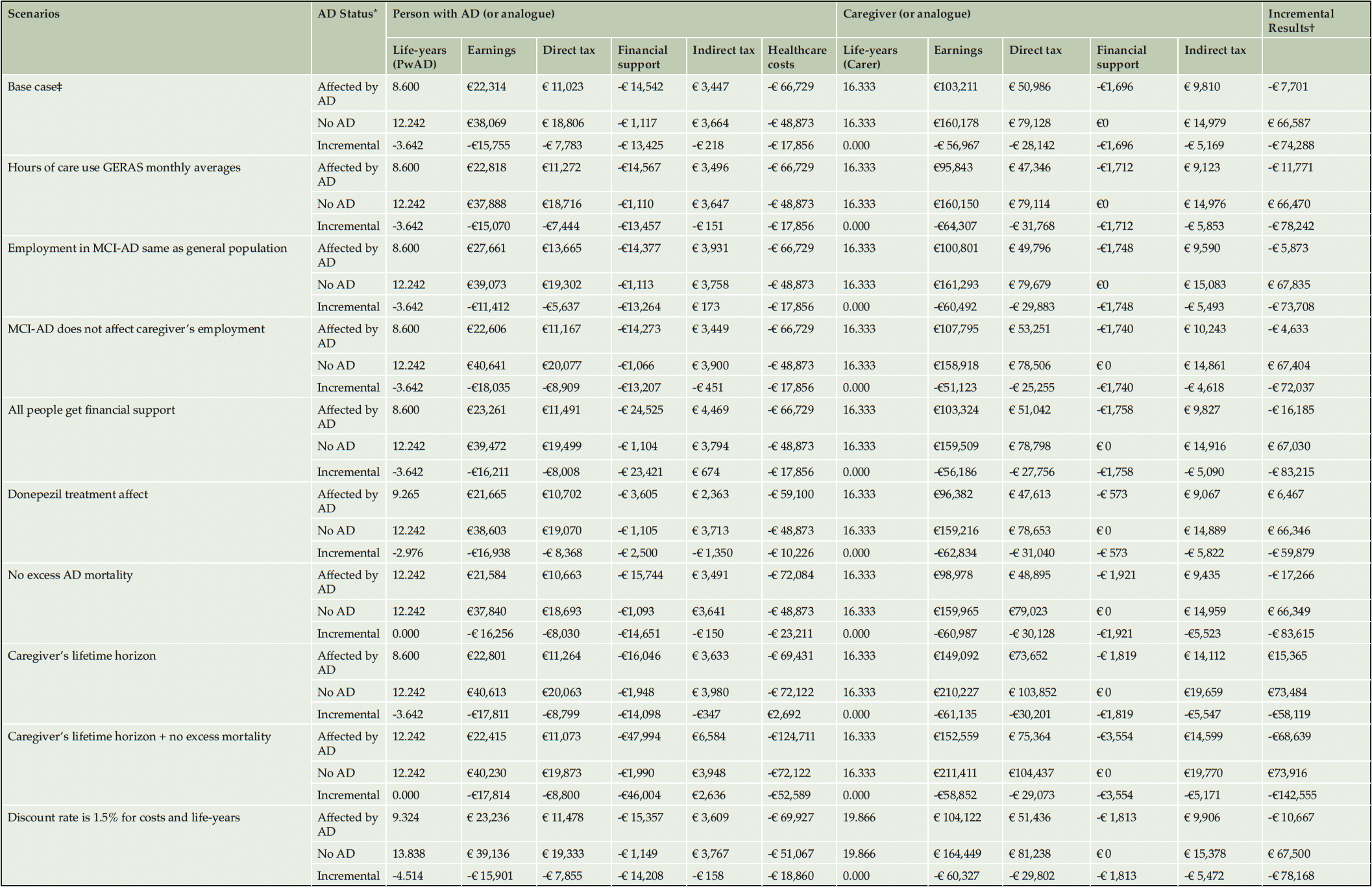
We have explored uncertainty around monthly caregiving requirements predicted by the fiscal analysis by utilizing estimates from the German GERAS cohort, implemented conditionally to AD severity (Table 4). This reduced INC by 5.3% (-€78,242) driven by a direct tax decrease in caregivers of PwAD. In the base case, the effect of MCI-AD on employment was implemented using disability as a proxy for inability to maintain employment. We run a scenario assuming that labor participation in people with MCI-AD was identical to that in the general population. Employment earnings and direct tax in PwAD were both increased by 24.0%, leading to less than 1% increasing in INC (-€73,708). We explored the effect of caregiving for a person with MCI-AD on caregiver’s employment, as PwAD remain reasonably independent at this stage. This had minimal impact on model results, increasing INC by 3.0% (-€71,037). Base case results assumed only people with a care assessment received financial support. All PwAD were assigned to financial support in an alternative scenario, leading to INC of -€83,215, a 12.0% increase in public losses. In the base case we assumed PwAD received the same treatment as the placebo arm population of the trials informing the Getsios equations (28). We have run a scenario using the donepezil treatment effect which led to a 19.4% increase in INC (-€59,879).
We have extended the time horizon of the analysis to the lifetime of the caregivers in one scenario which resulted in INC of -€58,119, a 21.8% increase from base case. The change was mostly due to increased direct and indirect tax revenue resulting from caregivers returning to work which off-set transfers required by PwAD.
Premature AD-mortality may lead to fewer individuals requiring transfer payments and can release informal caregivers back to employment. Because these can constitute a perverse incentive, we have run scenarios without the effect of excess AD mortality, using both the base case time horizon and the caregiver’s lifetime time horizon, respectively. Over the PwAD lifetime time horizon removing excess AD mortality led to a 12.6% reduction in INC (-€83,615). Over the caregiver’s lifetime time horizon, averaging 16.3 life-years, INC reduced by 145.3%% (-€142,555) due to 3.3 times increase in financial support and 1.9 times increase in healthcare spending by PwAD.
A scenario was also run using an 1.5% discount rate, reflecting the German annual growth consumption value of health, applied to costs and life-years (51). The resulting INC was -€78,168, a 5.2% decrease from baseline.Overall, the results of the model were very robust to the scenario analyses.
Alzheimer’s disease; MCI-AD, mild cognitive impairment due to AD.;*Incremental results calculated by subtracting values from people not affected by AD from the values for people affected by AD.;†Calculated as the row total sum for direct tax, financial support, indirect tax and healthcare costs.;‡Model predicted a 10.1-year average duration of AD, scenario used the time horizon value rounded up to the closest multiple of the model’s cycle length (10.5 years).
One-way sensitivity analyses
The mean age of PwAD and caregivers influenced results the most. At the lower and upper ends of the 95% CIs, the INCs were reduced by 59.6% (-€118,533) or 49.2% (-€110,844) and increased by 58.7%% (-€30,712) or 45.0% (-€40,824), respectively. The HR of early onset dementia-related loss of employment in caregivers caused the third-largest change from base case, a 12.3% reduction in INC (-€86,592) at the lower end of the 95% CI and a 16.6% increase (-€65,138) at the upper end. The remaining parameters increased the INC by a maximum of 5.0% or decreased it by a maximum of 9.2%, not dramatically affecting results. The tornado diagram summarizing the OWSA involving the ten most influential model inputs is shown in Figure 1.
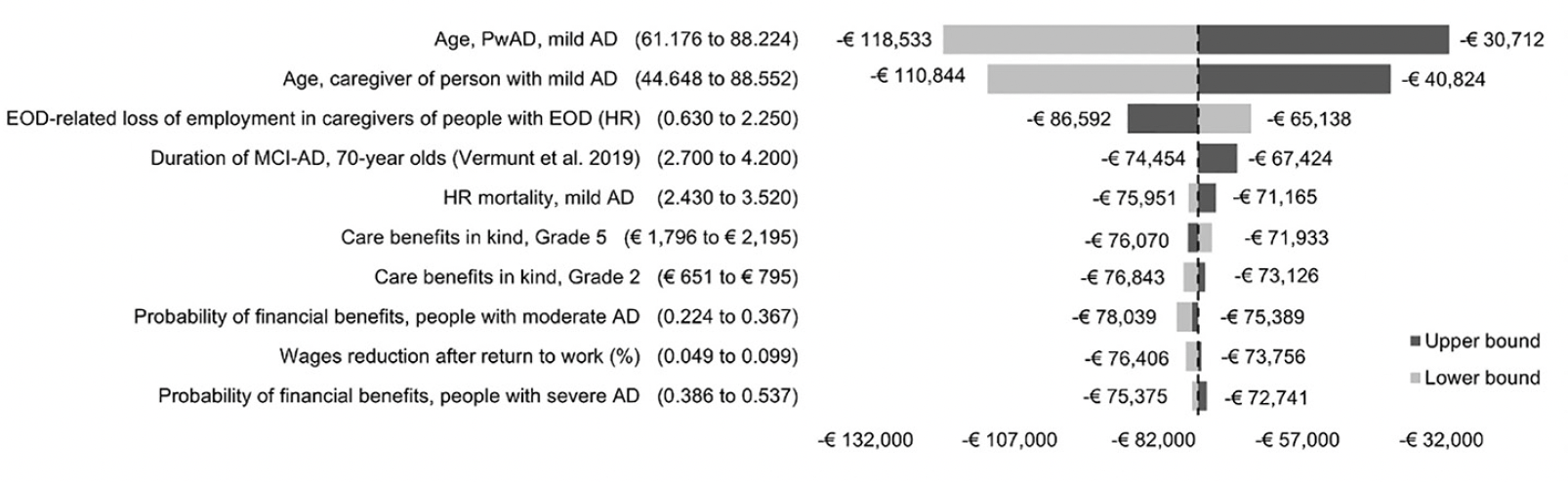
Figure 1. One-way sensitivity analyses based on 95% confidence interval for 10 most sensitive input parameters
AD, Alzheimer’s disease; ADL, activities of daily living; EOD, early onset dementia; HR, hazard ratio; MCI-AD, mild cognitive impairment due to AD; MMSE, Mini-Mental State Examination; PwAD, person with Alzheimer’s disease.
Discussion
To the best of our knowledge, this is the first study analyzing the economic consequences of AD from the German government and social security perspective. The analysis revealed that AD has tremendous spillover effects beyond direct medical care costs and impacts multiple economic sectors. Our model predicted an AD-related fiscal loss of €74,288, most being incurred within ten years of AD onset. Most costs were due to employment-related direct tax losses, representing 48.4% (€35,925) of INC. This was primarily caused by caregivers who had to reduce or quit their work to provide informal care. Over 10.5 years, caregivers were predicted to have their earnings reduced by €56,967 compared to their analogues not caring for PwAD. This underlines the importance of including caregiver’s perspective in such analyses. Also, the German government is likely to provide thirteen times more financial support for home-based formal and informal care to a PwAD than to a person without AD. This financial support represented 18.1% (€13,425) of the total fiscal losses. Medical health care spending represents 24.0% of the incremental fiscal loss, approximately the same as incremental financial support to households affected by AD (20.4%).
Assuming an AD-dementia prevalence of 1.2 million German individuals (3, 15), we could use our results to predict that over a 10-year period AD could cost the public purse €89 billion, approximately €8.5 billion per annum. Published cost-of-illness studies report informal care costs ranging from 27.8% (12) to 70.8% (52) and healthcare costs ranging from 21.1% (52) to 60.0% (12) of total AD costs in community-based PwAD in Germany. We predicted that home-based care costs represented 79.6% of total incremental care costs with 20.4% being attributed to other forms of healthcare. Productivity costs for PwAD have been reported to range from 5.1% (52) to 11.2% (12) of total AD costs, similar to the 10.5% estimation by this fiscal analysis. Our approach is innovative and expands on published cost-of-illness studies as it includes the effect of disease on the labor activity and subsequent direct and indirect tax consequences. Therefore, future cost-of-illness studies and cost-effectiveness analyses should consider the government and social security perspective to inform health policy more precisely, which would be important to improve national healthcare resource allocation.
The model was particularly sensitive to the mean age of PwAD and caregivers. Unsurprisingly, the earlier the onset of AD, the greater the burden of the disease in reducing one’s ability to remain at work, with earlier spillover effects to caregivers. We estimate that if the population of focus in this analysis was that with early-onset AD, total fiscal losses would likely exceed €110,000 per PwAD and caregiver pair. Despite constituting only 1-2% of the entire AD population (53) these individuals experience AD burden much earlier in life, significantly augmenting indirect costs burden.
There are several strengths to this analysis. Our methodology allowed capturing economic consequences of AD that are often missed by conventional burden of disease studies. We have clearly defined the fiscal consequences and the government and social security perspective taken, and have made significant efforts to explore and validate model results..
There are nonetheless limitations to our analysis. We have used German-specific inputs whenever available, including demographics, labor statistics, and the value of fiscal consequences. However, we have used several inputs that are not specific to Germany and can contribute to the overall uncertainty of the analysis. Such inputs include the equations derived from Canadian data estimating the likelihood of employment in caregivers and the assumptions required to model the impact of MCI-AD and mild AD on employment. Despite the evidence of an overall effect of chronic diseases such as AD on employment, it is likely that country specific resources and labor characteristics would affect the intensity of this effect (54). Despite recent publications on this field, we encourage further research expanding our knowledge of early AD.
The equations published by Getsios and colleagues (28) have faced criticism related to the appropriateness of using CERAD data, and the possibility of double counting treatment effects owed to MMSE being included as an explanatory variable when predicting other clinical outcomes (55). We believe these limitations are less important to the present analysis because we do not use donepezil treatment effect in our base case analysis. We present average results for 1,000 PwAD and informal caregivers but we recognize the multiplicity of pathways occurring in real world settings. Finally, our model predicted informal care requirements in people with moderate and severe AD, which differ from other publications (9, 32). Exploring this source in a scenario did not significantly impact results. It is likely that after reaching 5-6 daily hours of care, consequences to labor participation become marginal.
We have demonstrated that AD economic burden is considerable. Nonetheless, additional sources of uncertainty were not explored and may impact the real economic costs of AD. Firstly, caregivers re-entering employment may face obstacles related to fixed hiring costs and obsolescence (56). Secondly, we have not considered the fact that some PwAD have more than one informal caregiver. Finally, we did not consider the detrimental effect of caregiving on one’s physical, emotional and mental health, which could translate in more healthcare resources use and government transfers being required (1, 57). We anticipate all the above would contribute to an increased fiscal loss.
Conclusions
We have demonstrated the AD fiscal burden from the German government and social security perspective, using a framework allowing decision-makers to compare across public economic sectors. We do so by estimating losses due to forgone earnings, tax contributions, increased need for financial support for formal and informal care, and medical care costs in people affected by AD compared to the general population. While cost-of-illness analyses or cost-effectiveness analyses commonly focus on health sector expenses, healthcare costs represented a small proportion of total fiscal consequences. Caregiver forgone taxes on employment contributed the most to these estimated total fiscal losses. It is therefore urgent that these broader fiscal consequences and the government and social security perspectives are considered to inform policy making and health care planning.
Funding: This work was funded by Biogen. RM, NK and MPC are employees for Global Market Access Solutions, who were paid consultants to Biogen. BM was a paid consultant contributing to the analysis. PPR is an employee and stockholder of Biogen. MU is a paid consultant for Biogen. The authors hold no financial interests in the sponsoring company.
Disclosures: During the peer review process, Biogen had the opportunity to review and comment on the manuscript. The authors retained full editorial control of the manuscript and provided their final approval on all content to be published. The work described here will be used towards the doctoral requirements for R. Martins.
Acknowledgements: The authors would like to acknowledge Dominik Prager for his contributions to this work.
Ethical standards: The economic analysis reported here is based on previously reported literature. No individual patient data has been collected for this study and no ethics approval was required.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Alzheimer’s Association. 2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020 DOI: 10.1002/alz.12068.
2. Alzheimer Europe. Dementia in Europe Yearbook 2019: Estimating the prevalence of dementia in Europe 2020 [Available from: file:///C:/Users/ruima/Downloads/FINAL%2005707%20Alzheimer%20Europe%20yearbook%202019%20(1).pdf.
3. Nichols E, Szoeke CEI, Vollset SE, Abbasi N. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet Neurology. 2019;18(1):88-106 DOI: 10.1016/S1474-4422(18)30403-4.
4. Niu H, Álvarez-Álvarez I, Guillén-Grima F, Aguinaga-Ontoso I. Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurología (English Edition). 2017;32(8):523-32 DOI: https://doi.org/10.1016/j.nrleng.2016.02.009.
5. Robert Koch Institute. Altersdemenz 2005 [Available from: https://kcgeriatrie.de/Info-Service_Geriatrie/Documents/gbe_altersdemenz_2005.pdf.
6. Oliva-Moreno J, Trapero-Bertran M, Peña-Longobardo LM, Del Pozo-Rubio R. The Valuation of Informal Care in Cost-of-Illness Studies: A Systematic Review. Pharmacoeconomics. 2017;35(3):331-45 DOI: 10.1007/s40273-016-0468-y.
7. Michalowsky B, Thyrian JR, Eichler T, Hertel J, Wucherer D, Flessa S, et al. Economic Analysis of Formal Care, Informal Care, and Productivity Losses in Primary Care Patients who Screened Positive for Dementia in Germany. J Alzheimers Dis. 2016;50(1):47-59 DOI: 10.3233/jad-150600.
8. Leicht H, Heinrich S, Heider D, Bachmann C, Bickel H, van den Bussche H, et al. Net costs of dementia by disease stage. Acta Psychiatr Scand. 2011;124(5):384-95 DOI: 10.1111/j.1600-0447.2011.01741.x.
9. Boess F, Lieb M, Schneider E, Zimmermann T, Dodel R, Belger M. Kosten der Alzheimer-Erkrankung in Deutschland – aktuelle Ergebnisse der GERAS-Beobachtungsstudie. Gesundheitsökonomie & Qualitätsmanagement. 2016;21(05):232-41 DOI: 10.1055/s-0042-100956.
10. Luppa M, Heinrich S, Matschinger H, Hensel A, Luck T, Riedel-Heller SG, et al. Direct costs associated with mild cognitive impairment in primary care. Int J Geriatr Psychiatry. 2008;23(9):963-71 DOI: 10.1002/gps.2018.
11. Schwarzkopf L, Menn P, Kunz S, Holle R, Lauterberg J, Marx P, et al. Costs of care for dementia patients in community setting: an analysis for mild and moderate disease stage. Value Health. 2011;14(6):827-35 DOI: 10.1016/j.jval.2011.04.005.
12. Reese JP, Heßmann P, Seeberg G, Henkel D, Hirzmann P, Rieke J, et al. Cost and Care of Patients with Alzheimer’s Disease: Clinical Predictors in German Health Care Settings. Journal of Alzheimer’s Disease. 2011;27:723-36 DOI: 10.3233/JAD-2011-110539.
13. El-Hayek YH, Wiley RE, Khoury CP, Daya RP, Ballard C, Evans AR, et al. Tip of the Iceberg: Assessing the Global Socioeconomic Costs of Alzheimer’s Disease and Related Dementias and Strategic Implications for Stakeholders. J Alzheimers Dis. 2019;70(2):323-41 DOI: 10.3233/jad-190426.
14. Binstock RH. From compassionate ageism to intergenerational conflict? The Gerontologist. 2010;50(5):574-85.
15. DESTATIS. Population by sex and citizenship in 2016 2016 [Available from: https://www.destatis.de/EN/Themes/Society-Environment/Population/Current-Population/Tables/liste-current-population.html;jsessionid=0D4C08D6D69D86246F72768512952718.live741
16. Michalowsky B, Kaczynski A, Hoffmann W. [The economic and social burden of dementia diseases in Germany-A meta-analysis]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2019;62(8):981-92 DOI: 10.1007/s00103-019-02985-z.
17. Kließ MK, Martins R, Connolly MP. Major Cost Drivers in Assessing the Economic Burden of Alzheimer’s Disease: A Structured, Rapid Review. The Journal of Prevention of Alzheimer’s Disease. 2021 DOI: 10.14283/jpad.2021.17.
18. Garrison LP, Jr., Pauly MV, Willke RJ, Neumann PJ. An Overview of Value, Perspective, and Decision Context—A Health Economics Approach: An ISPOR Special Task Force Report [2]. Value in Health. 2018;21(2):124-30 DOI: 10.1016/j.jval.2017.12.006.
19. Drost R, van der Putten IM, Ruwaard D, Evers S, Paulus ATG. Conceptualizations of the societal perspective within economic evaluation: a systematic review. Int J Technol Assess Health Care. 2017;33(2):251-60 DOI: 10.1017/s0266462317000526.
20. Connolly MP, Kotsopoulos N, Postma MJ, Bhatt A. The Fiscal Consequences Attributed to Changes in Morbidity and Mortality Linked to Investments in Health Care: A Government Perspective Analytic Framework. Value in Health. 2017;20(2):273-7 DOI: https://doi.org/10.1016/j.jval.2016.11.018.
21. Kotsopoulos N, Connolly MP. Is the gap between micro- and macroeconomic assessments in health care well understood? The case of vaccination and potential remedies. Journal of Market Access & Health Policy. 2014;2(1):23897 DOI: 10.3402/jmahp.v2.23897.
22. Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Fourth ed. Great Clarendon Street, Oxford, OX2 6DP2015.
23. DSU. NICE DSU Technical support document 15: Cost-effectiveness modelling using patient-level simulation. 2014.
24. Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, et al. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force–3. Value Health. 2012;15(6):812-20 DOI: 10.1016/j.jval.2012.06.014.
25. IQWIG IfQuWiG. General Methods – Version 6.0. In: Care IfQaEiH, editor. Cologne2006.
26. Hager K, Henneges C, Schneider E, Lieb M, Kraemer S. [Alzheimer dementia: course and burden on caregivers : Data over 18 months from German participants of the GERAS study]. Nervenarzt. 2017;89(4):431-42 DOI: 10.1007/s00115-017-0371-6.
27. Green C, Zhang S. Predicting the progression of Alzheimer’s disease dementia: A multidomain health policy model. Alzheimer’s & Dementia. 2016;12(7):776-85 DOI: https://doi.org/10.1016/j.jalz.2016.01.011.
28. Getsios D, Blume S, Ishak KJ, Maclaine GD. Cost effectiveness of donepezil in the treatment of mild to moderate Alzheimer’s disease: a UK evaluation using discrete-event simulation. Pharmacoeconomics. 2010;28(5):411-27 DOI: 10.2165/11531870-000000000-00000.
29. NICE NIfHaCE. Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease. Technology appraisal guidance [TA217]. 2018.
30. DESTATIS. Life table (period life table): Germany, years, gender, completed age 2020 [Available from: https://www-genesis.destatis.de/genesis/online?operation=table&code=12621-0001&bypass=true&levelindex=1&levelid=1602068072032#abreadcrumb.
31. Andersen K, Lolk A, Martinussen T, Kragh-Sorensen P. Very mild to severe dementia and mortality: A 14-year follow-up – The Odense study. Dement Geriatr Cogn Disord. 2010;29(1):61-7 DOI: 10.1159/000265553.
32. Reed C, Belger M, Vellas B, Andrews JS, Argimon JM, Bruno G, et al. Identifying factors of activities of daily living important for cost and caregiver outcomes in Alzheimer’s disease. Int Psychogeriatr. 2016;28(2):247-59 DOI: 10.1017/S1041610215001349.
33. Davis M, T OC, Johnson S, Cline S, Merikle E, Martenyi F, et al. Estimating Alzheimer’s Disease Progression Rates from Normal Cognition Through Mild Cognitive Impairment and Stages of Dementia. Curr Alzheimer Res. 2018;15(8):777-88 DOI: 10.2174/1567205015666180119092427.
34. Green C, Handels R, Gustavsson A, Wimo A, Winblad B, Sköldunger A, et al. Assessing cost-effectiveness of early intervention in Alzheimer’s disease: An open-source modeling framework. Alzheimers Dement. 2019;15(10):1309-21 DOI: 10.1016/j.jalz.2019.05.004.
35. DESTATIS. Population, employed persons, unemployed persons, economically active persons, inactive persons : Germany, years, age groups 2020 [Available from: https://www-genesis.destatis.de/genesis/online?sequenz=tabelleErgebnis&selectionname=12211-0002#abreadcrumb.
36. OECD. Pensionists at a Glance 2019: Country profiles – Germany 2019 [Available from: https://www.oecd.org/els/public-pensions/PAG2019-country-profile-Germany.pdf.
37. Silvaggi F, Leonardi M, Tiraboschi P, Muscio C, Toppo C, Raggi A. Keeping People with Dementia or Mild Cognitive Impairment in Employment: A Literature Review on Its Determinants. Int J Environ Res Public Health. 2020;17(3):842 DOI: 10.3390/ijerph17030842.
38. Artero S, Touchon J, Ritchie K. Disability and mild cognitive impairment: a longitudinal population-based study. International Journal of Geriatric Psychiatry. 2001;16(11):1092-7 DOI: https://doi.org/10.1002/gps.477.
39. DESTATIS. Severely disabled people by sex, age and severe-disability rate 2021 [Available from: https://www.destatis.de/EN/Themes/Society-Environment/Health/Disabled-People/Tables/disabled-people-gender-age.html.
40. Sakata N, Okumura Y. Job Loss After Diagnosis of Early-Onset Dementia: A Matched Cohort Study. Journal of Alzheimer’s Disease. 2017;60:1231-5 DOI: 10.3233/JAD-170478.
41. Lilly MB, Laporte A, Coyte PC. Do they care too much to work? The influence of caregiving intensity on the labour force participation of unpaid caregivers in Canada. J Health Econ. 2010;29(6):895-903 DOI: 10.1016/j.jhealeco.2010.08.007.
42. DESTATIS. Workers, salaried employees, gross monthly earnings (prod. Trade and service sector): Former federal territory / new states, reference month (up to 10/2005), age groups, gender 2021 [Available from: https://www-genesis.destatis.de/genesis/online?operation= statistic&levelindex=0&levelid=1624460641504&code=62111#abreadcrumb
43. OECD. Taxing wages – Germany 2020 [Available from: http://www.oecd.org/tax/tax-policy/taxing-wages-germany.pdf.
44. Speiser A. Back to work: the effect of a long-term career interruption on subsequent wages in Switzerland. Swiss Journal of Economics and Statistics. 2021;157(1) DOI: 10.1186/s41937-020-00068-4.
45. Federal Ministry of Health. Ratgeber Demenz: Informationen für die häusliche Pflege von Menschen mit Demenz 2019 [14th updated edition: October 2019:[Available from: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/5_Publikationen/Pflege/Broschueren/BMG_Ratgeber_Demenz_Oktober_2019_barr.pdf.
46. German Social Code. Social code – Book XI- Sozialgesetzbuch 2021 [Available from: https://www.sozialgesetzbuch-sgb.de/sgbxi/1.html.
47. Federal Health Monitoring Autority. Persons in need of care (number and quota). Classification: years, region, age, sex 2019 [Available from: https://www.gbe-bund.de/gbe/pkg_olap_tables.prc_set_hierlevel?p_uid=gast&p_aid=7007243&p_sprache=E&p_help=2&p_indnr=510&p_ansnr=51636598&p_version=3&p_dim=D.002&p_dw=1000002&p_direction=drill.
48. WHO. Germany: Country case study on the integrated delivery of long-term care 2020 [Available from: https://www.euro.who.int/__data/assets/pdf_file/0011/426386/05_DEU-LTC_web.pdf.
49. Comission E. Germany – Pensions and other old age benefits 2021 [Available from: https://ec.europa.eu/social/main.jsp?catId=1111&langId=en&intPageId=4554.
50. DESTATIS. Consumer price index (including rates of change): Germany, years 2021 [Available from: https://www-genesis.destatis.de/genesis/online?operation=table&code=61111-0001&bypass=true&levelindex=1&levelid=1619038422420#abreadcrumb.
51. John J, Koerber F, Schad M. Differential discounting in the economic evaluation of healthcare programs. Cost Effectiveness and Resource Allocation. 2019;17(1):29 DOI: 10.1186/s12962-019-0196-1.
52. Michalowsky B, Flessa S, Eichler T, Hertel J, Dreier A, Zwingmann I, et al. Healthcare utilization and costs in primary care patients with dementia: baseline results of the DelpHi-trial. The European Journal of Health Economics. 2018;19(1):87-102 DOI: 10.1007/s10198-017-0869-7.
53. Blauwendraat C, Wilke C, Jansen IE, Schulte C, Simón-Sánchez J, Metzger FG, et al. Pilot whole-exome sequencing of a German early-onset Alzheimer’s disease cohort reveals a substantial frequency of PSEN2 variants. Neurobiology of Aging. 2016;37:208.e11-.e17 DOI: https://doi.org/10.1016/j.neurobiolaging.2015.09.016.
54. OECD/European Union. The labour market impacts of ill-health OECD/European Union (2016), “The labour market impacts of ill-health”, in Health at a Glance: Europe 2016: State of Health in the EU Cycle, OECD Publishing, Paris. Health at a Glance: Europe 2016: State of Health in the EU Cycle: OECD Publishing; 2016. p. 17-36.
55. Bond M, Rogers G, Peters J, Anderson R, Hoyle M, Miners A, et al. The effectiveness and cost-effectiveness of donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer’s disease (review of Technology Appraisal No. 111): a systematic review and economic model. Health Technol Assess. 2012;16(21):1-470 DOI: 10.3310/hta16210.
56. Heger D, Korfhage T. Short- and Medium-Term Effects of Informal Eldercare on Labor Market Outcomes. Feminist Economics. 2020:1-23 DOI: 10.1080/13545701.2020.1786594.
57. Chiao CY, Wu HS, Hsiao CY. Caregiver burden for informal caregivers of patients with dementia: A systematic review. International Nursing Review. 2015;62(3):340-50 DOI: https://doi.org/10.1111/inr.12194.