A. Garnier-Crussard1,2, V. Dauphinot1, A. Zamudio-Rodriguez1, P. Krolak-Salmon1,2
1. Clinical and Research Memory Center of Lyon, Lyon Institute For Aging, Hospices Civils de Lyon, 69100 Villeurbanne, France; 2. Eduwell team, Lyon Neuroscience Research Center (CRNL), INSERM U1028, CNRS UMR5292, UCBL1, Lyon, France
Corresponding Author: Antoine Garnier-Crussard, MD MSc, Hôpital des Charpennes, Lyon Institute for Aging, Hospices Civils de Lyon, France, Clinical and Research Memory Centre of Lyon, 27 rue Gabriel Péri, 69100 Villeurbanne, France, tel: +33 4 72 43 20 50 ; Fax: +33 4 72 43 20 54, email: antoine.garnier-crussard@chu-lyon.fr
J Prev Alz Dis 2022;4(9):813-815
Published online September 14, 2022, http://dx.doi.org/10.14283/jpad.2022.75
Dear Editor,
Frailty, a state of increased vulnerability to poor resolution of homeostasis following a stress (1), is of increased interest in the field of Alzheimer’s disease (AD) and related diseases. The two dominant models of frailty, the phenotype model (2) and the cumulative deficit model (3), were first described in 2001. Twenty years later, the International Working Group added frailty in their recommendation for clinical diagnosis of AD as a “factor that can increase the risk of progression to Alzheimer’s disease” (4). Indeed, there is emerging literature showing relationships between frailty and clinical AD (5, 6), independently from neuropathology (7). Recently, Canevelli et al. hypothesized that frailty could explain the discrepancy between lesions and symptoms of AD (8).
Beyond the model of frailty, the World Health Organization introduced the new concept of intrinsic capacity (IC), defined as “the composite of all the physical and mental capacities of an individual” in the context of healthy ageing, including five key domains: locomotion, vitality, cognitive, psychological, and sensory functions (9). In this regard, higher IC has been associated with a lower risk of frailty onset; conversely, decline in IC has been already associated to neurocognitive disorders. Indeed, beyond cognition, which is intrinsically affected in AD, other components of IC, such as vitality (or nutrition) (10), locomotion (11), mood (12) or sensory loss (13), were previously associated with worse cognition or increased dementia risk in older adults. Thus, we assume that, beyond frailty, a capacity-centred model, with detection and management of IC declines in older adults with AD, will be an important step toward a better global and integrative approach to AD.
From a deficit-centred model to a capacity-centred model
Fan and Cheong recently described frailty as “the clinical viewpoint of a glass half empty, where it highlights functional deficits accumulated with aging and existing comorbidities, while IC represents a glass half full, accounting for the functional reserves of the patient” (14). A positive and integrated vision may lead to a better involvement of participants and healthcare professionals. Notably, even if frailty has not been conceived as a condition for excluding patients from interventions (15), it is usually viewed by clinicians in a negative, potentially exclusionary way. Moreover, to provide a comprehensive global and integrative approach to older adults beyond the “perimeter of geriatric medicine”, IC appears to be an important step forward in the field of AD (15).
An opportunity to promote the integrated care program in older adults, including those with AD
Integrated Care for Older PEople (ICOPE) corresponds to person-centred and integrated guidelines at the community-level to manage declines in IC (16, 17). ICOPE notably recommends to use tools (including self-administered tools) to screen for loss in IC in primary care. This also aims to support the “self-empowerment of each individual for his/her health status” (15). In the future, if ICOPE is developed on a large scale in older adults, cognition-specialized physicians (e.g., neurologists, geriatricians or psychiatrists) will see patients with a cognitive complaint, but perhaps also with declines in IC in other domains (i.e., sensory, psychological, vitality or locomotion), screened with ICOPE tools. As clinicians, it will be important to understand the relationships between these declines in IC and to propose personalized and tailored interventions in order to improve IC and functional abilities.
Decline in specific IC will lead to specific targeted interventions, centred on the individual’s values and priorities
A decline in IC will lead to targeted interventions; for example, a sensory loss could be managed, which could lead to improve the functional abilities. IC being independently associated with incident frailty (18), screening and management of IC loss (19) may lead to reduce frailty occurrence, and then improve functional abilities and prevent negative events in patients with AD. Therapeutic and preventive approaches based on IC by using competences of the people rather than their deficits (comparable to the neurocognitive rehabilitation approach in AD) may be proposed.
Interventions should not only focus on the individuals, but also focus on the environmental characteristics, and actions are needed against the social determinants of frailty and IC declines (20). The World Health Organization defines healthy ageing as “the process of developing and maintaining the functional ability that enables well-being in older age” (9), encompassing the relationships between IC and environmental characteristics. Environment comprises “all the factors in the extrinsic world that form the context of an individual’s life”, including factors from the micro level to the macro-level (home, communities and the broader society) (9). Thus, contrary to the concept of frailty, the concept of functional abilities has been developed in a more global and less individual way: functional abilities are made up of the IC (at the individual level) and relevant environmental characteristics (at the collective level) (9). This approach may lead to specific interventions at the individual and the collective level (Figure).
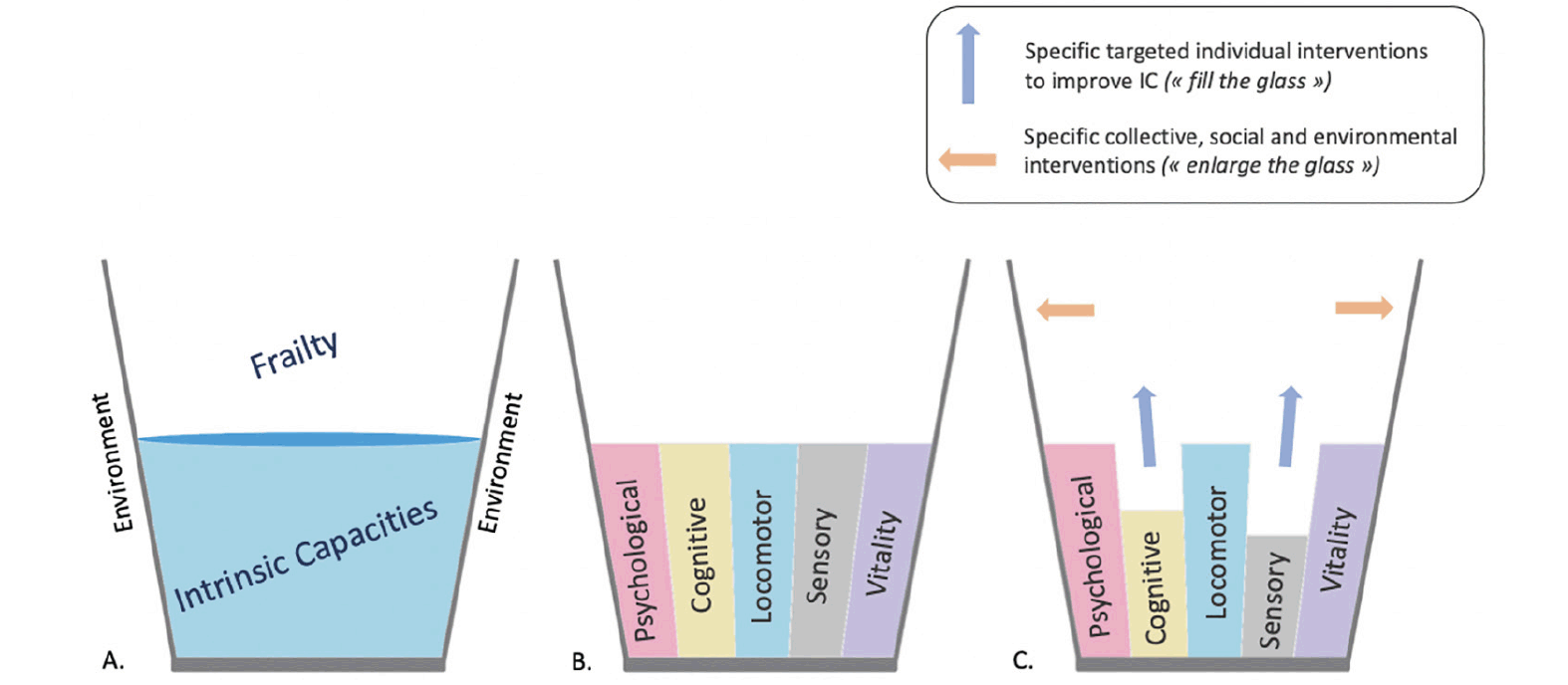
Figure 1. Intrinsic capacities (i.e., psychological, cognitive, locomotor, sensory and vitality) as the “glass half full” and opportunity for individual and collective interventions
The more the glass is filled (i.e., individual with high intrinsic capacities) the more the frailty decreases (A. and B.). In addition, the size of the glass may correspond to the individual’s environment. This approach may open window to develop specific targeted interventions to improve functional abilities in AD at the individual level (“fill the glass”, blue arrows) and at the collective level (“enlarge the glass”, orange arrows) (C.). This schematic representation highlights the individual heterogeneity in IC and environmental characteristics, that could lead to personalized interventions.
To conclude, if the concept of IC has been developed in the context of healthy ageing, we assume that it may be a major advance in the field of AD and related diseases. IC is not the opposite of frailty, which corresponds to a clinically relevant reduction of IC (15) previously associated with AD (4–7). The monitoring of IC in older adults, including those with AD will help to detect patient’s “fragilization” (15), to adapt the personalized care, to better motivate the people regarding therapeutic approaches and finally to delay cognitive and functional decline, with specific cognitive (21) and non-cognitive (19) interventions. Future research is needed to confirm that IC could represent prognostic factors in AD, to better understand the inter-relationship between cognition and other IC declines, as well as to confirm that the IC monitoring in AD is efficient to improve clinical outcomes.
Conflict of interets: None.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752–62. https://doi.org/10.1016/S0140-67312)62167-9.
2. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in Older Adults: Evidence for a Phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–57. https://doi.org/10.1093/gerona/56.3.M146.
3. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of Deficits as a Proxy Measure of Aging. ScientificWorldJournal 2001;1:323–36. https://doi.org/10.1100/tsw.2001.58.
4. Dubois B, Villain N, Frisoni GB, Rabinovici GD, Sabbagh M, Cappa S, et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group. Lancet Neurol 2021;20:484–96. https://doi.org/10.1016/S1474-442221)00066-1.
5. Borges MK, Canevelli M, Cesari M, Aprahamian I. Frailty as a Predictor of Cognitive Disorders: A Systematic Review and Meta-Analysis. Front Med Lausanne) 2019;6. https://doi.org/10.3389/fmed.2019.00026.
6. Fabrício D de M, Chagas MHN, Diniz BS. Frailty and cognitive decline. Transl Res 2020;221:58–64. https://doi.org/10.1016/j.trsl.2020.01.002.
7. Wallace LMK, Theou O, Darvesh S, Bennett DA, Buchman AS, Andrew MK, et al. Neuropathologic burden and the degree of frailty in relation to global cognition and dementia. Neurology 2020;95:e3269–79. https://doi.org/10.1212/WNL.0000000000010944.
8. Canevelli M, Bruno G, Valletta M, Cesari M. Could there Be Frailty in the Discrepancy between Lesions and Symptoms of Alzheimer’s Disease? J Frailty Aging 2022. https://doi.org/10.14283/jfa.2022.43.
9. World Health Organization. World report on ageing and health 2015.
10. Tombini M, Sicari M, Pellegrino G, Ursini F, Insardá P, Di Lazzaro V. Nutritional Status of Patients with Alzheimer’s Disease and Their Caregivers. J Alzheimers Dis 2016;54:1619–27. https://doi.org/10.3233/JAD-160261.
11. Sverdrup K, Selbæk G, Bergh S, Strand BH, Thingstad P, Skjellegrind HK, et al. Physical performance across the cognitive spectrum and between dementia subtypes in a population-based sample of older adults: The HUNT study. Arch Gerontol Geriatr 2021;95:104400. https://doi.org/10.1016/j.archger.2021.104400.
12. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020;396:413–46. https://doi.org/10.1016/S0140-673620)30367-6.
13. Kuo P-L, Huang AR, Ehrlich JR, Kasper J, Lin FR, McKee MM, et al. Prevalence of Concurrent Functional Vision and Hearing Impairment and Association With Dementia in Community-Dwelling Medicare Beneficiaries. JAMA Netw Open 2021;4:e211558. https://doi.org/10.1001/jamanetworkopen.2021.1558.
14. Fan BE, Cheong CY. Enhancing Geriatric Assessment for Non-Hodgkin Lymphoma with Intrinsic Capacity: Can we move beyond existing models of Frailty? J Geriatr Oncol 2022. https://doi.org/10.1016/j.jgo.2022.04.007.
15. Belloni G, Cesari M. Frailty and Intrinsic Capacity: Two Distinct but Related Constructs. Front Med 2019;6:133. https://doi.org/10.3389/fmed.2019.00133.
16. Banerjee A, Sadana R. Integrated Care For Older People ICOPE): From Guidelines To Demonstrating Feasibility. J Frailty Aging 2021;10:84–5. https://doi.org/10.14283/jfa.2020.40.
17. World Health Organization. Integrated care for older people: Guidelines on community-level interventions to manage declines in intrinsic capacity 2017.
18. Yu R, Leung J, Leung G, Woo J. Towards Healthy Ageing: Using the Concept of Intrinsic Capacity in Frailty Prevention. J Nutr Health Aging 2022;26:30–6. https://doi.org/10.1007/s12603-021-1715-2.
19. Blancafort Alias S, Cuevas-Lara C, Martínez-Velilla N, Zambom-Ferraresi F, Soto ME, Tavassoli N, et al. A Multi-Domain Group-Based Intervention to Promote Physical Activity, Healthy Nutrition, and Psychological Wellbeing in Older People with Losses in Intrinsic Capacity: AMICOPE Development Study. Int J Environ Res Public Health 2021;18:5979. https://doi.org/10.3390/ijerph18115979.
20. Daly T. The vital need for action against the social determinants of frailty. Aging Med 2022;5:73–73. https://doi.org/10.1002/agm2.12195.
21. Amieva H, Robert PH, Grandoulier A-S, Meillon C, De Rotrou J, Andrieu S, et al. Group and individual cognitive therapies in Alzheimer’s disease: the ETNA3 randomized trial. Int Psychogeriatr 2016;28:707–17. https://doi.org/10.1017/S1041610215001830.
