M. Adesuyan1,2, Y.H. Jani1,2, D. Alsugeir1,3, E.C.L. Cheung4, C.S.L. Chui5-7, R. Howard8, I.C.K. Wong1,2,5, R. Brauer1
1. Research Department of Practice and Policy, University College London School of Pharmacy, London, UK; 2. Centre for Medicines Optimisation Research and Education, University College London Hospitals NHS Foundation Trust, London, UK; 3. Imam Abdulrahman Bin Faisal University, College of Clinical Pharmacy, Department of Pharmacy Practice; 4. Centre for Safe Medication Practice and Research, Department of Pharmacology and Pharmacy, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China; 5. School of Nursing, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China; 6. School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China; 7. Laboratory of Data Discovery for Health (D24H), Hong Kong Science and Technology Park, Sha Tin, Hong Kong SAR, China; 8. Division of Psychiatry, University College London, UK
Corresponding Author: Ruth Brauer, PhD, UCL School of Pharmacy, BMA House, Tavistock Square, London, WC1H 9JP, UK. Tel: (+44) 20 7874 1273, r.brauer@ucl.ac.uk
J Prev Alz Dis 2022;4(9):715-724
Published online September 28, 2022, http://dx.doi.org/10.14283/jpad.2022.77
Abstract
Background: Hypertension is a recognized risk factor for dementia. However, evidence for using antihypertensive agents to reduce the risk of Alzheimer’s disease in people with hypertension is inconclusive.
OBJECTIVE: To examine the association between antihypertensive agents and the incidence of Alzheimer’s disease in adults with hypertension and normal cognition.
DESIGN: We conducted a systemic review and performed meta-analyses using Ovid MEDLINE, Ovid Embase, Ovid PsycINFO, Web of science and Scopus, from inception to 18th February 2022.
SETTING: Cohort and case-control studies.
PARTICIPANTS: Adults ≥ 40 years with hypertension and normal cognition.
INTERVENTION: Antihypertensive agents.
MEASUREMENTS: We performed two separate meta-analyses, pooling the adjusted relative risk (RR) of non-antihypertensive comparator and antihypertensive comparator study design.
RESULTS: We included nine studies, totalling 1,527,410 individuals. Meta-analysis of non-antihypertensive user comparator studies found that the use of antihypertensive agents is associated with a reduced risk of incident Alzheimer’s disease (RR= 0.94, 95% CI 0.90-0.99; p=0.01). Meta-analysis of antihypertensive comparator studies found evidence that angiotensin II receptor blocker users are associated with a reduction in the risk of Alzheimer’s disease compared to other antihypertensive agents (RR= 0.78, 95% CI 0.68-0.88; p< 0.001).
CONCLUSION: Our review provides evidence that the use of antihypertensive agents is associated with a lower incidence of Alzheimer’s disease. The use of angiotensin II receptor blockers may provide the most benefit among antihypertensive agents. Lowering raised blood pressure may not be the only mechanism for cognitive protection and further investigation of the effects of angiotensin II on cognition is indicated.
Key words: Alzheimer Disease, angiotensin-converting enzyme inhibitors, angiotensin receptor antagonists, antihypertensive agents, hypertension.
Introduction
Dementia is a global epidemic, affecting over 50 million people and is one of the top 10 global causes of death (1). The number of people living with dementia worldwide is estimated to reach over 150 million in 2050 (2). Dementia can be a major cause of disability and dependence. The health and social care requirements of people living with dementia come with a significant emotional, physical, and financial cost to the affected individual and their loved ones. There is no cure for dementia or treatment that can slow neurodegeneration. Global population growth and ageing are the main drivers for the increase in projected cases (2). Therefore, a focus on interventions that can promote and extend healthy ageing is required in response to this global concern.
Hypertension is a recognised modifiable risk factor for all-cause dementia (3, 4). However, there are several dementia diagnoses that each have different pathophysiological mechanisms (5). The most common type is Alzheimer’s disease (AD), accounting for approximately 60-70% of cases, followed by vascular dementia (VaD) representing up to 20% of cases (4) and other less frequent subtypes making up the remaining cases. The association between VaD and hypertension is well understood and meta-analyses have demonstrated a strong risk between VaD and individuals with hypertension (6, 7). The pathophysiological mechanism for this association is largely through the impact of hypertension on atherosclerotic and cerebrovascular disease, which are leading causes of VaD (8). The use of blood pressure lowering agents to reduce the risk of incident and recurrent stroke and transient ischaemic attack (TIA) is recommended by several international guidelines (9-11). In 2019, a systematic review with meta-analysis by Lennon et al. (12) examined the association between the AD subtype and mid-life hypertension. Their review of observational studies found that midlife systolic hypertension was associated with an up to 25% increased risk of AD. The exploration of this pathogenic link has previously been investigated in a cross-sectional study, where amyloid plaques and neurofibrillary tangles were shown to be increased in the brains of people with a history of midlife hypertension (13). Real world evidence studies have demonstrated that hypertension plays a significant role in the pathogenesis of AD (14, 15). However, the body of evidence supporting the use of antihypertensive agents (AHA) to reduce the risk of incident AD is inconclusive (16, 17). If AHA reduce the risk of AD, it is unclear whether this association arises solely from the effects of systolic blood pressure reduction or through an alternative mechanism specific to an antihypertensive drug class.
Randomised controlled trials (RCT) remain the gold standard for measuring the effectiveness of an intervention (18). Two RCTs have reported the effect of AHA versus placebo on the incidence of AD. The Syst-Eur study (2002) (19) reported a 40% reduction in AD cases amongst those randomized to AHA, while the HYVET-COG trial (2008) (20) did not find a significant reduction in the incidence of AD. Neither study was powered to detect incidence of AD as a primary outcome. Furthermore, both studies were restricted by a relatively small sample size (2902, Sys-Eur and 3336, HYVET-COG) and short follow-up (3.9 years, Sys-Eur and 2 years, HYVET-COG); limiting the precision of both trials. This is a common challenge for RCTs that report outcomes of rare frequency or long latency. Moreover, there are no head-to-head clinical trials of AHAs to investigate which AHA offers the greatest AD risk reduction. In the absence of clinical trials designed to investigate the long-term impact of AHA on incident AD, observational studies can be a valuable source of evidence to address research questions (21). They allow for the inclusion of larger sample sizes, longer follow-up time and greater external validity by being more representative of the relevant population (22).
The rationale for investigating the association between AHA and AD is based on hypertension as a potential modifiable risk factor for AD (12). Previous reviews have included studies that may be at risk of selection bias as participants had no hypertension or the outcome of interest was not AD. For example, Peters et al. (2020) (23) found no difference between antihypertensive drug classes. However, their outcome was all-cause dementia and they did not distinguish studies specific to incident AD. Guan et al. (2011) (24) found no evidence of an association between antihypertensive users and non-users on incident AD. Their search strategy was limited to two databases, included studies of relatively small sample size (e.g. n<500) which may be unable to detect outcomes of rare frequency such as AD (25). Also, a decade has passed since they conducted their review and new observational studies have been published in this field. Larsson and Markus (2018) (26) reviewed prospective observational studies and found that antihypertensives use versus non-use was associated with a reduced incidence of AD. However, their review did not include analyses of head-to-head studies comparing antihypertensive drug classes. Also, a single investigator performed their data extraction and literature search in one database. Investigator error and the possibility of missing studies from other databases, may have affected the robustness of their methods and findings (27). Most recently, Scotti et al. (2021) (28) focused on the association between renin angiotensin-aldosterone system inhibitors (RAAS) and reported angiotensin II receptor blockers (ARB) reduce the risk of AD compared to other AHAs. This review included seven studies for the outcome of AD. Like previous reviews their meta-analyses included studies without baseline hypertension amongst antihypertensive users and their search strategy was limited to a single database. It is important to include studies with hypertension as the source population as it is a risk factor for AD and a primary indication for AHA (12). Antihypertensives can be used for other indications and including antihypertensive users without hypertension will not be an accurate reflection of the association in this high-risk group.
Aim
We aim to investigate if AHAs reduce the risk of AD. Therefore, a systematic review with meta-analysis was performed of observational studies, to determine the association between AHA and incident AD in patients with hypertension and normal cognition at baseline. As a secondary objective we set out to identify which antihypertensive drug class provides the greatest AD risk reduction.
Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (29) and is registered with the International Prospective Register of Adverse Events of Systematic Reviews, PROSPERO (CRD42021270065) (30).
Information Sources and Search strategy
A structured search strategy was conducted using Ovid MEDLINE, Ovid Embase, Ovid PsycINFO, Web of science and Scopus, from inception until 18/02/2022. Further literature retrieval was sourced through manual searching of references lists from shortlisted studies. The search strategy was limited to human studies and articles, but not English language. However, where no English translation was available for a shortlisted study, it was excluded. The full search strategy for each database is outlined in Appendix 1.
Eligibility criteria and Selection process
Included studies were limited to cohort or case-control study designs. The study population was adults with hypertension and normal cognition. We excluded studies where participants had cognitive impairment (measured by cognitive assessment tools or diagnosis) or dementia diagnosis at baseline. The intervention was individual or combined antihypertensive agents (angiotensin converting enzyme inhibitor, angiotensin II receptor blocker, beta-blockers, calcium channel blockers and diuretics) with a minimum exposure period of 12 months. The comparison group was either non-antihypertensive users or alternative antihypertensive agent(s). The outcome was AD defined by standard neurological diagnostic criteria (31) or diagnostic clinical codes/International Classification of Disease (ICD) codes. Lastly, only studies reporting effect estimates as hazard ratio (HR), relative risk (RR) or odds ratio (OR) with 95% confidence intervals (CI) were included. Two authors (M.A. and D.A.) independently assessed the eligibility of studies against the inclusion criteria, and any disagreements were resolved through discussion.
Data collection process
Data extraction was performed by M.A. and independently checked by D.A. using a pre-designed data extraction form; any disagreements were resolved by discussion. The following were extracted: first author’s name, publication year, country, study design, data source, number of participants, study period, mean follow-up, mean age, proportion of males, intervention/exposure, reference group/comparator, outcome measure, AD diagnostic tool, number of events, confounders and effect estimates with 95% confidence intervals. For studies that reported unadjusted and multiple adjusted effect estimates, we selected the effect that maximally adjusted for potential confounders. Original study investigators were contacted for missing and incomplete data or where similarities between articles indicated the possibility of multiple publications from the same cohort. Where studies reported results from the same population, data was extracted from the most recent publication with the longest follow-up.
Study risk of bias assessment
Two study authors (M.A. and E.C) independently assessed the risk of bias in included studies using The Risk of Bias in Non-randomised studies of Interventions (ROBINS-I) tool (32). The ROBINS-I tool consists of seven domains with signalling questions to help users judge the risk of bias in each domain. The judgments of risk for each domain are defined as ‘low,’ ‘moderate’, ‘serious’ and ‘critical’ risk of bias for the outcome assessed. A low risk corresponds to the risk of bias in a well conducted high quality RCT. Any study with a ‘critical risk’ of bias was not combined for meta-analysis as outlined in the ROBINS-I proforma (32).
Synthesis methods
The results of the identified studies were summarised using techniques of narrative synthesis and then critiqued, analysed and interpreted. Meta-analysis was conducted in two stages for the dichotomous outcome of incident AD. Firstly, to assess the effect of AHA on the risk of AD, pooled relative risk of non-antihypertensive user comparator design studies were estimated with 95% CI. Secondly, to assess which antihypertensive drug class had the lowest risk of AD, pooled RR of antihypertensive comparator design studies were estimated with 95% CI.
Clinical heterogeneity between studies was expected due to differences in participant characteristics, variation in follow-up period and differing covariates used for analysis. Therefore, we used a random effects model under the DerSimonian and Laird method (33) to calculate summary effects. We assessed the level of heterogeneity on the meta-analyses using Higgins I2 statistic (34) where I2 < 25% indicates low, 25-75% moderate and >75% high heterogeneity.
To assess the robustness of our results, two prespecified sensitivity analyses were undertaken. Firstly, based on the ROBINS-I assessment, the meta-analysis was restricted to studies of ‘low’ to ‘moderate’ risk of bias. Secondly, the meta-analysis was restricted to cohort studies. To explore the impact of heterogeneity amongst studies, subgroup analyses were performed based on follow-up time (<5 years and ≥5 years) and mean age of participants (<65 years and ≥65 years). Results were considered statistically significant when two-tailed p value was less than 0.05. All statistical analyses were conducted using STATA v17.1 (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC).
Results
Study selection
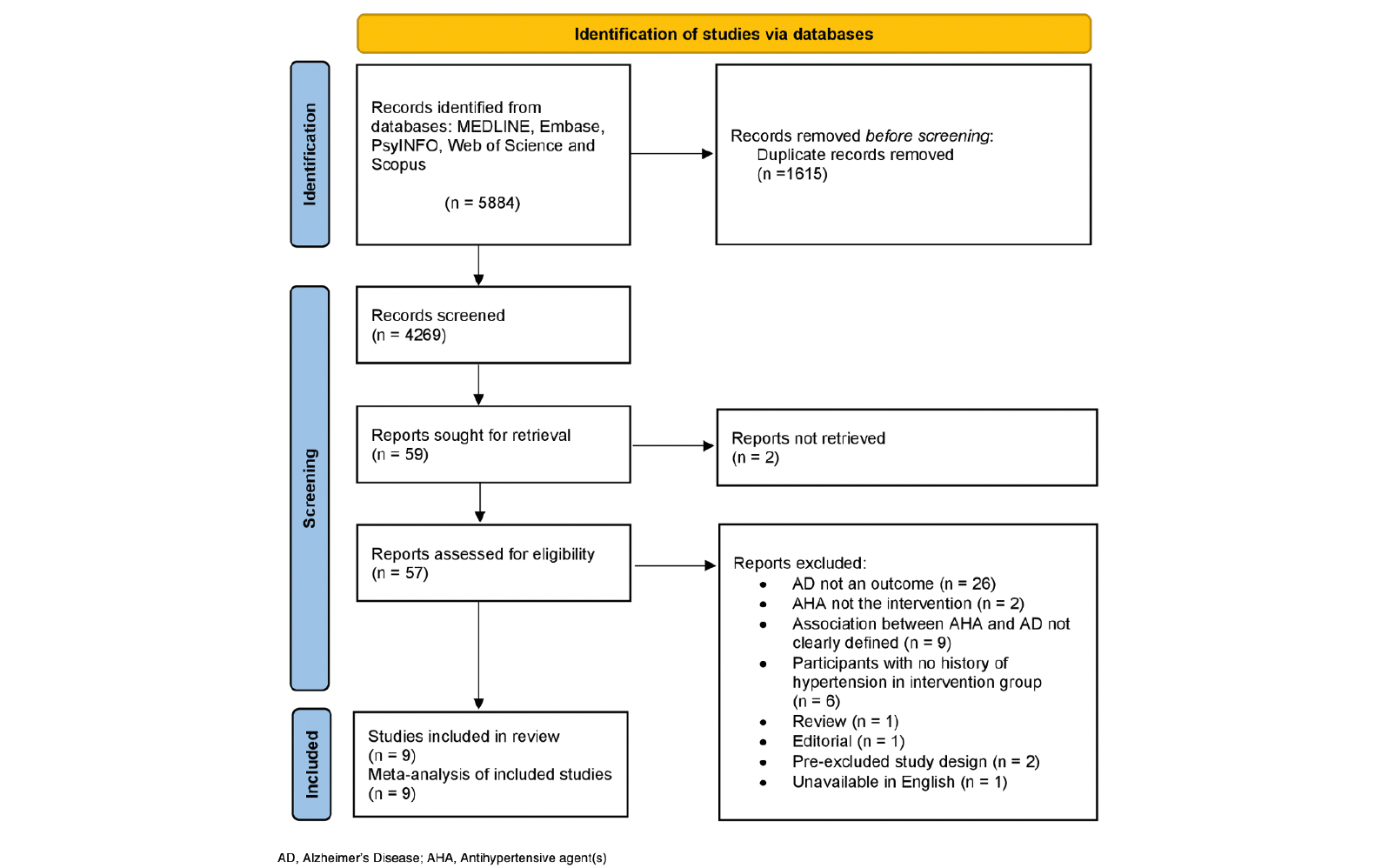
Our systematic search strategy identified 5,884 articles (Figure 1). After removing duplicates and screening the remaining titles and abstracts for relevance, 59 studies remained for full text retrieval and review. We were unable to retrieve the full text of two studies from correspondence authors. Therefore, 57 studies underwent full text eligibility assessment against the inclusion criteria. Nine studies met the inclusion criteria and were suitable for meta-analyses. Three studies were of the non-antihypertensive user comparator design (35-37), and six studies were of the antihypertensive user comparator design (17, 38-42). The most common reason for exclusion was due to AD not being assessed as an outcome (43-46). For the work by Tully et al. (37) we included the result from the largest exposure group, as the comparator group for each exposure was not independent (47).
Study characteristics
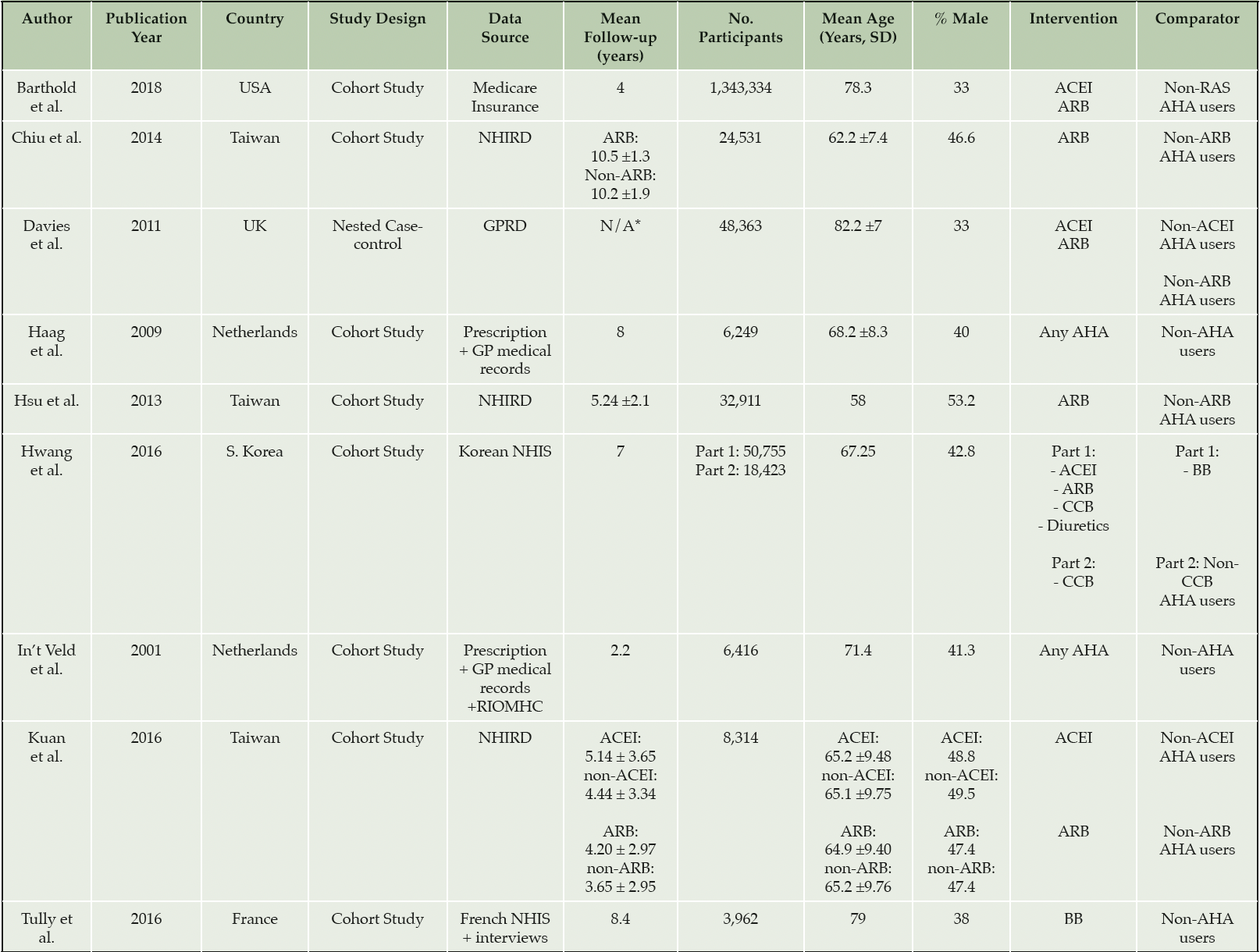
Eight cohort studies and one case-control study were included. The characteristics are summarised in Table 1. Study populations were from East Asia (n=4) and Europe (n=4) and the USA (n=1). Sample sizes of participants included in analysis ranged from 3,962 to 1,343,334. The overall median (range) duration of follow-up was 5.19 (2.2-10.5) years and the mean baseline age ranged from 58 to 82.2 years. The proportion of male participants ranged from 33 to 53%.
ACEI, angiotensin converting enzyme inhibitor; AChEI, anticholinesterase inhibitors; AHA, antihypertensive agent; ARB, angiotensin II receptor blocker; BB, beta blocker; CCB, calcium channel blocker; GPRD, General Practice Research Database; NHIRD, the Taiwanese National Health Insurance Research Database; NHIS, the National Health Insurance System; Non-RAS AHA, Non-renin angiotensin system antihypertensive agent; RIOMHC, the Regional Institute for Outpatient Mental Health Care. * Study period: 1997-2008
Risk of bias in studies
Risk of bias was assessed for each study and is summarised in Appendix 2. Five studies included in this review were judged to have an overall ‘moderate risk’ of bias. These studies provided satisfactory evidence for a non-randomised study across the seven domains of bias. Four studies were judged to be at a ‘serious risk’ of bias in at least one domain, but not at critical risk of bias in any domain and were therefore included in our meta-analyses.
Results of individual studies
The outcome result of each study is summarised in Appendix 4.
Results of synthesis
Part 1: Non-antihypertensive user comparator studies
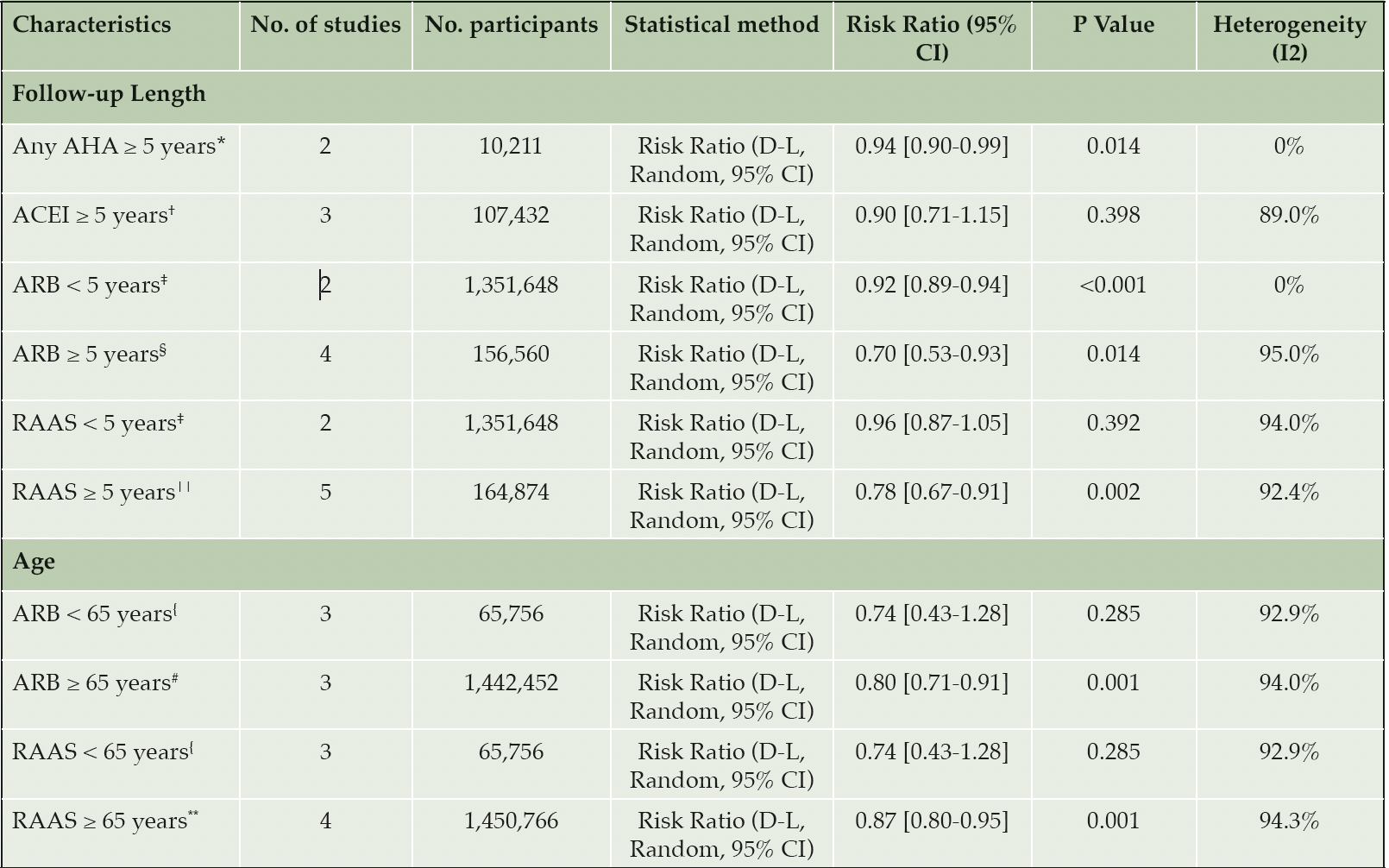
Results of the meta-analysis of the three non-antihypertensive user comparator cohort studies suggested evidence of an association between antihypertensive drug use and a reduced risk of AD (RR= 0.94, 95% CI 0.90-0.99; p= 0.01, Figure 2). Our results show that the use of any antihypertensive drug is associated with a 6% reduction in the risk of incident AD compared to non-antihypertensive drug users. Higgins I2 statistical test suggested there was no heterogeneity of results across the studies (I2= 0%). However, the pooled estimate was heavily weighted towards a single study by Haag et al. (35). Sensitivity analysis of studies restricted to ‘moderate’ risk of bias was not performed, as only one of the three non-antihypertensive comparator studies were deemed at moderate risk (37). Subgroup analysis of studies with a follow-up period ≥ 5 years also showed risk reduction (RR= 0.94, 95% CI 0.90-0.99; p< 0.05, Table 3 – row 3). Further subgroup analysis by age categories was not performed as all participants were older than 65 years.
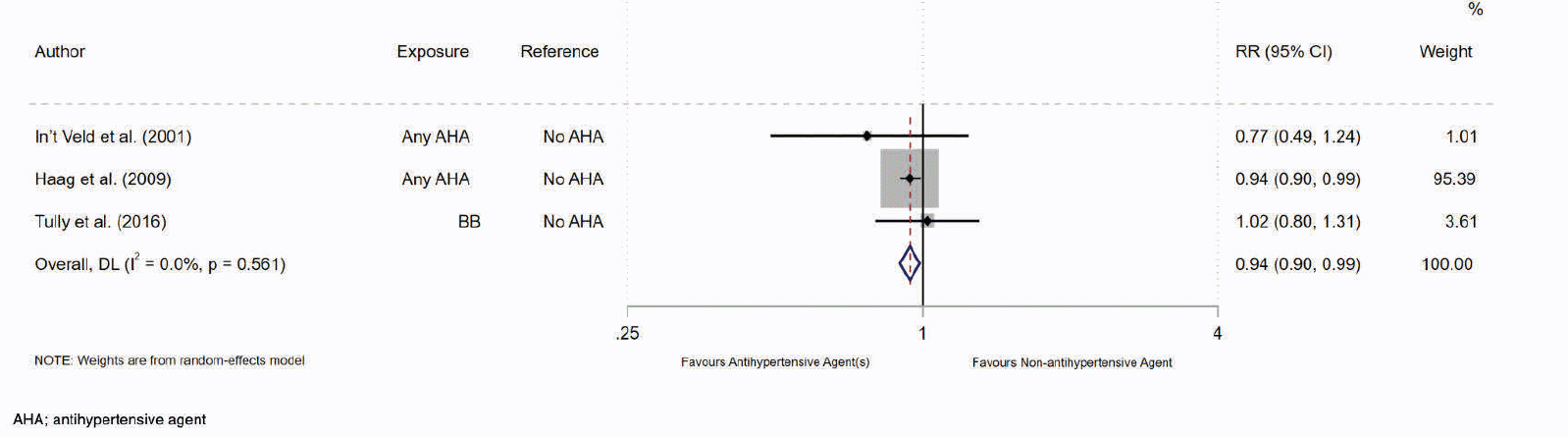
Figure 2. Forest Plot of the Association between Antihypertensive drug use compared to No Antihypertensive drug use and the Relative Risk of Incident Alzheimer’s Disease
Part 2: Active comparator studies
There was an insufficient number of eligible studies for appropriate pooling in meta-analysis, to compare the relative treatment effects of beta-blockers, calcium channel blockers and diuretics. The antihypertensive drug classes suitable for combination in meta-analysis were studies of ACEI or ARB use compared to other antihypertensives.
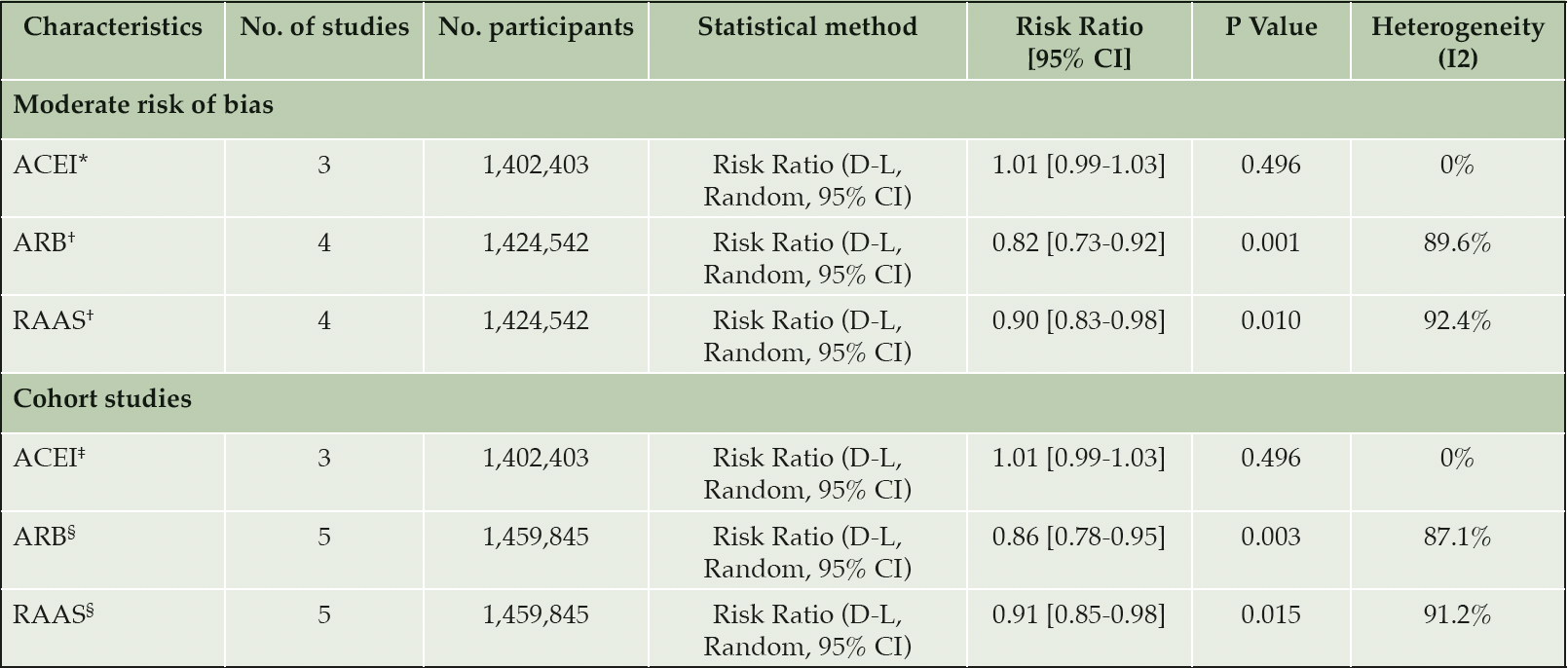
A total of four studies reported the association between ACEI drug use and the incidence of AD. There was no evidence of an association between ACEI users and AD compared to other antihypertensive drug users (RR= 0.93, 95% CI 0.81-1.06; p= 0.282, Figure 3). Heterogeneity was observed to be at a high level across these studies (I2 = 90.5%). Several sensitivity analyses were performed. The results of meta-analysis restricted to studies of ‘moderate’ risk of bias and studies of cohort design are outlined in Table 2. There was no difference in the associated AD risk between ACEI users and other antihypertensives in studies restricted to ‘moderate’ risk of bias and cohort study design (RR= 1.01, 95% CI 0.99-1.03; p= 0.496). Subgroup analysis as detailed in Table 3 (row 4) also showed no evidence in the association between ACEI and AD for follow-up ≥ 5 years (RR= 0.90, 95% CI 0.71-1.15; p= 0.398). Subgroup analyses by < 5 years’ follow-up and age could not be conducted for ACEI due to an insufficient number of studies with < 5 years follow-up and participants aged < 65 years.
The pooled estimates from six studies reported the association between ARB drug use and the incidence of AD. There was strong evidence that ARB use was associated with a reduction in the risk of AD compared to other antihypertensives (RR= 0.78, 95% CI 0.68-0.88; p< 0.05, AHA; antihypertensive agent (Figure 3). High heterogeneity was observed across studies (I2 = 92.2%). Sensitivity analyses was performed restricting studies to those with a ‘moderate’ risk of bias (RR= 0.82, 95% CI 0.73-0.92; p= 0.001, Table 2 – row 4) and cohort study design (RR= 0.86, 95% CI 0.78-0.95; p= 0.003, Table 2 – row 8). These results demonstrate the robustness of our primary finding with continued evidence for the reduction in the risk of AD from use of ARBs. Subgroup analysis of studies with a follow-up period < 5 years also reported risk reduction (RR= 0.92, 95% CI 0.89-0.94; p< 0.05, Table 3 – row 5). Stronger evidence was observed for follow-up ≥ 5 years (RR= 0.70, 95% CI 0.53-0.93; p= 0.014, Table 3 – row 6). Further subgroup analysis by age identified that ARB users aged ≥ 65 years are associated with risk reduction (RR= 0.80, 95% CI 0.71-0.91; p= 0.001), but the association was weaker for those aged < 65 years (RR= 0.74, 95% CI 0.43-1.28; p= 0.285).
Our results of the antihypertensive user comparator studies focused on ACEI and ARB use as the intervention. Both drugs are renin-angiotensin-aldosterone system (RAAS) antihypertensives, therefore we were able to combine the results from these studies for post-hoc comparison of the risk of AD between users of RAAS antihypertensives and other antihypertensives. There was evidence that RAAS antihypertensive were associated with a reduced risk of AD (RR= 0.85, 95% CI 0.78-0.92). Sensitivity analyses limited to ‘moderate’ risk of bias and cohort studies consistently showed a protective effect from RAAS drug use (Table 2 – row 5 and 9). Subgroup analysis of ≥ 5 years follow-up also showed RAAS users are associated with a reduced risk of AD compared to other antihypertensive users (RR= 0.78, 95% CI 0.67-0.91; p= 0.002). There was no difference in risk associated with < 5 years follow-up (Table 3 – row 7). Similar to ARB users, RAAS users aged ≥ 65 years demonstrated evidence for AD risk reduction, but this was not seen in RAAS users < 65 years (Table 3 – row 12).
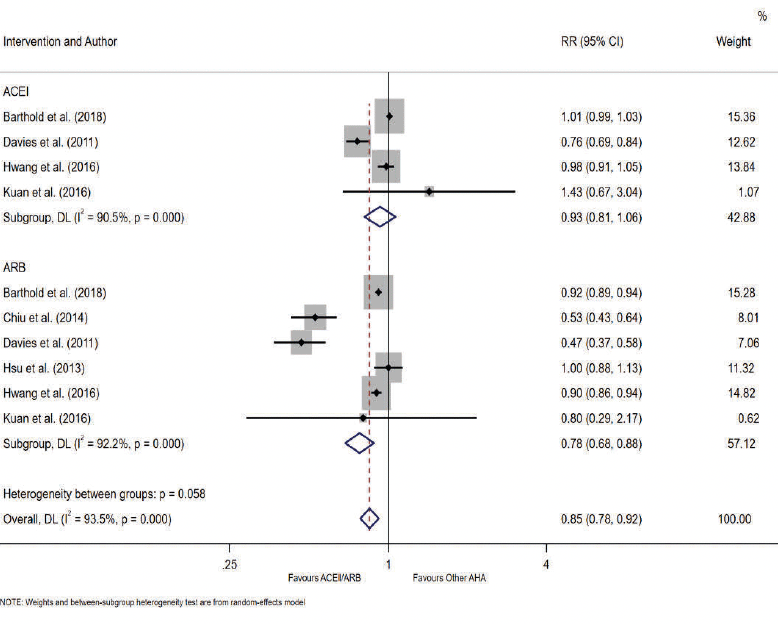
Figure 3. Forest Plot of the Association between ACEI, ARB and RAAS use compared to other antihypertensive agents (AHA) drug use and the Relative Risk of Incident Alzheimer’s Disease
*Moderate risk of bias studies: Barthold et al. Hwang et al. Kuan et al.; †Moderate risk of bias studies: Barthold et al. Chiu et al. Davies et al. Kuan et al.; ‡Cohort studies: Barthold et al. Hwang et al. Kuan et al.; §Cohort studies : Barthold et al. Chiu et al. Hsu et al. Hwang et al. Kuan et al.
Studies included in each length of follow-up subgroup: *Haag et al. Tully et al. †Davies et al. Hwang et al. Kuan et al. ; ‡Barthold et al. Kuan et al. §Chiu et al. Davies et al. Hsu et al. Hwang et al.; ||Chiu et al. Davies et al. Hsu et al. Hwang et al. Kuan et al.. Studies included in each age subgroup: {Chiu et al. Hsu et al. Kuan et al.; #Barthold et al. Davies et al. Hwang et al.; **Barthold et al. Davies et al. Hwang et al. Kuan et al.
Discussion
The results of this systematic review and meta-analysis suggest that in individuals with hypertension, use of any antihypertensive agent compared to non-antihypertensive users is associated with a reduced risk of incident AD. These results were heavily weighted by Haag et al. (35) due to the level of precision the authors reported in their effect estimate. In subgroup analysis, Haag et al. was excluded and a significant risk reduction in AHA use with low heterogeneity was still observed. However, there are some concerns over the certainty of the primary finding as sensitivity analysis to assess the robustness of this result could not be tested due to an insufficient number of studies deemed to be at moderate risk of bias. The non-user comparator design is at risk of selection biases and specifically prevalent user bias (48). This type of bias occurs when antihypertensive users initiate therapy before the start of follow-up and have therefore survived the occurrence of the outcome. As a result, the beneficial effect of treatment compared to non-users may be overestimated. Our assessment of bias identified selection bias (Appendix 3) as the main domain contributing to bias within these studies.
Our review also found that compared to other antihypertensive agents, the use of ARBs is associated with a reduced risk of incident AD, however considerable heterogeneity between studies was observed. Conversely, there was no evidence for an association between ACEI and incident AD relative to other antihypertensive agents. We planned to identify which of the licensed antihypertensive drug classes provide the greatest risk reduction in AD. However, there was an insufficient number of eligible studies of BB, CCB and diuretic use for appropriate pooling in meta-analysis. Therefore, we were only able to conduct individual analyses of ACEI and ARB drug classes.
The results of antihypertensive comparator studies are limited to relative treatment effects but can be interpreted as we have shown that any antihypertensive agent is associated with a reduced risk of AD. The risk reduction in ARB use was maintained when we removed studies potentially affected by ‘serious’ bias. This lends confidence to the protective effect of ARB use from moderately well conducted observational studies. Moreover, our findings are supported by the suggested pharmacological mechanism that ARB exert their protective effect on incident AD (49-51). The inhibition of angiotensin II type 1 (ATR1) receptors reduces blood pressure. However, it has been suggested and observed in animal models (52, 53) that this blockade results in upregulation of angiotensin II type 2 (ATR2) receptors. Activation of this receptor, results in reduced oxidative stress, neuroinflammation and improved cerebral blood flow (51) which can lead to a reduction in amyloid-βeta and incidence of AD.
The robustness of our ACEI user comparator result (Figure 3) was confirmed with sensitivity analyses in Table 2 – rows 3 and 7. Showing no difference compared to other AHA when we restricted synthesis to moderate risk of bias and cohort studies by excluding the case-control study (40). This restriction resulted in improved precision and reduced statistical heterogeneity compared to the primary ACEI effect estimate. This demonstrates the limitations of combining different study methodologies and is a possible source of heterogeneity observed amongst the antihypertensive comparator studies. Our subgroup analyses (Table 3) showed that follow-up time and age of participants were not major factors contributing to the high level of heterogeneity between antihypertensive comparator studies. However, the absence of a significant change in variation among our subgroups may be due to the relatively small number of included studies. We therefore recommend these results be interpreted with caution, as a relatively small number of studies has low power to detect a difference in subgroup analyses (54). The large variation may also be the result of different definitions of ACEI or ARB exposure. Post-hoc subgroup analysis to explore this could not be performed due to the low number of studies.
Comparison with other studies
Our finding that any AHA reduces the risk of incident AD compared to non-antihypertensive users is supported by Larsson et al. (2018) (26) who reported a 22% risk reduction. They found a greater risk reduction than our analysis but included studies of non-hypertensive populations that we had excluded based on our research question (55, 56). Work by Chang-Quan et al. (2011) (57) found no significant difference between users of AHA and non-antihypertensive users, however their review included studies of small size (<500 participants) and participants without hypertension at baseline which can introduce a form of confounding by indication and limit the overall validity of their findings. Work by Scotti et al. (2021) supports our positive ARB risk reduction finding. However, our review differs by the systematic approach to identifying studies from searched databases and our assessment of bias within studies for inclusion in meta-analysis. Based on the ROBINS-I tool we did not include studies which Scotti et al. deemed appropriate based on the Newcastle Ottawa quality score scale (56). An individual patient data review by Ding et al. (2020) (58) included unpublished results from studies not included in this review. They reported a non-significant reduction in the association between ARB and other AHA. However, this was based on two studies with a small number of participants, which made it difficult to estimate a reliable association as per the authors of this review. Additional results by Ding et al. supports our ACEI finding where they report no difference in association between ACEI and AD compared to other AHA. This observed finding is also supported by a recent mendelian randomization study, which benefits from overcoming selection and indication bias (59).
Strengths and Limitations
This review has some limitations. Firstly, all studies included in our meta-analyses are susceptible to confounding. Although each study adjusted for baseline covariates, they differed between each study, and we cannot rule out residual and unmeasured confounders. Despite this, we applied a thorough risk of bias assessment tool that compared each study to a target randomized control trial and did not include any study at ‘critical’ risk of bias in this review. Secondly, it is possible publication bias may be impacting our findings and results should be interpreted cautiously. We planned to generate funnel plots from meta-analysis and apply the Egger’s statistical test (60). However, less than 10 studies were included in meta-analyses and these tests are underpowered to detect publication bias from chance, in a small number of included studies (61). Thirdly, all studies except Hsu et al. (41) reported a mean baseline age of participants over 60 years. This review is unable to infer the association between antihypertensive agents and adults with mid-life hypertension (< 60 years), who may be at the greatest risk of AD (12). We were also unable to identify if the associated risk reduction in AHA use arises from blood pressure reduction or an alternative mechanism specific to an antihypertensive drug class. Our included studies employed an intention to treat method similar to RCTs and measured baseline blood pressure, but did not adjust for it as a time varying confounder. Yet, a recent trial (SPRINT-MIND, 2019) (62) showed there was no evidence to suggest that an intensive systolic BP target of 120 mm/Hg was more beneficial than a target of 140 mm/Hg in reducing the risk of all-cause dementia. Although the outcome was not specific to AD, this lends credence to the hypothesis that the preventative effects of antihypertensives such as ARBs, may be from a repurposed effect not linked solely to blood pressure reduction. This review does come with some strengths. We were able to pool a large sample of participants, which improves the precision of our findings. The average follow-up of over 5 years allows us to begin to infer the association of long term AHA exposure and AD. Also, our meta-analyses were conducted including studies from diverse ethnic backgrounds which gives strength to the generalisability of our findings. Lastly, our meta-analyses of participants with hypertension demonstrates that blood pressure lowering medications may be beneficial in reducing the risk of AD.
Conclusion
Our systematic review found evidence that antihypertensive drug use may lower the incidence of AD, with support for the use of ARBs over other AHA providing the most benefit. Future research should include a well conducted head-to-head randomized controlled trial of ARBs versus another antihypertensive drug class to investigate a causal effect in the reduction of incident AD.
Funding: This review did not receive any specific grant funding. Matthew Adesuyan’s PhD is funded by the Centre for Medicines Optimisation Research and Education, UCLH NHS Foundation Trust and University College London and by the UCLH NIHR Biomedical Research Centre.
Ethical standards: Not applicable.
Conflict of interest: The authors have no conflict of interest to report.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made
References
1. World Health Organization. Global Health Estimates: Life expectancy and leading causes of death and disability. 2019. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates Accessed 16 February 2022.
2. Nichols E, Steinmetz JD, Vollset SE, et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. The Lancet Public Health. 2022;7(2):e105-e25. doi: 10.1016/S2468-2667(21)00249-8
3. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-46. doi: 10.1016/S0140-6736(20)30367-6
4. Organization WH. Risk reduction of cognitive decline and dementia: WHO guidelines. 2019 [Available from: https://www.who.int/publications/i/item/risk-reduction-of-cognitive-decline-and-dementia.
5. Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12(10):383-8. doi: 10.1016/0165-6147(91)90609-v
6. Sharp SI, Aarsland D, Day S, et al. Hypertension is a potential risk factor for vascular dementia: systematic review. Int J Geriatr Psychiatr. 2011;26(7):661-9. doi: 10.1002/gps.2572
7. de Heus RAA, Tzourio C, Lee EJL, et al. Association Between Blood Pressure Variability With Dementia and Cognitive Impairment: A Systematic Review and Meta-Analysis. Hypertension. 2021;78(5):1478-89. doi: 10.1161/HYPERTENSIONAHA.121.17797
8. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8(11):1006-18. doi: 10.1016/S1474-4422(09)70236-4
9. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75(6):1334-57. doi: 10.1161/HYPERTENSIONAHA.120.15026
10. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021-104.
11. National Institute for Health and Care Excellence: Guidelines. Hypertension in adults: diagnosis and management. London: National Institute for Health and Care Excellence (NICE). Copyright © NICE 2022.; 2022.
12. Lennon MJ, Makkar SR, Crawford JD, Sachdev PS. Midlife Hypertension and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2019;71(1):307-16. doi: 10.3233/JAD-190474
13. Rodrigue KM, Rieck JR, Kennedy KM, et al. Risk factors for β-amyloid deposition in healthy aging: vascular and genetic effects. JAMA Neurol. 2013;70(5):600-6. doi: 10.1001/jamaneurol.2013.1342
14. Yoo JE, Shin DW, Han K, et al. Blood Pressure Variability and the Risk of Dementia: A Nationwide Cohort Study. Hypertension. 2020;75(4):982-90. doi: 10.1161/HYPERTENSIONAHA.119.14033
15. Shah NS, Vidal JS, Masaki K, et al. Midlife blood pressure, plasma β-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension. 2012;59(4):780-6. doi: 10.1161/HYPERTENSIONAHA.111.178962
16. Yasar S, Corrada M, Brookmeyer R, Kawas C. Calcium channel blockers and risk of AD: the Baltimore Longitudinal Study of Aging. Neurobiol Aging. 2005;26(2):157-63. doi: 10.1016/j.neurobiolaging.2004.03.009
17. Hwang D, Kim S, Choi H, et al. Calcium-Channel Blockers and Dementia Risk in Older Adults – National Health Insurance Service – Senior Cohort (2002-2013). Circ J. 2016;80(11):2336-42. doi: 10.1253/circj.CJ-16-0692
18. Hariton E, Locascio JJ. Randomised controlled trials – the gold standard for effectiveness research: Study design: randomised controlled trials. BJOG. 2018;125(13):1716-. doi: 10.1111/1471-0528.15199
19. Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352(9137):1347-51. doi: 10.1016/s0140-6736(98)03086-4
20. Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7(8):683-9. doi: 10.1016/s1474-4422(08)70143-1
21. Chodankar D. Introduction to real-world evidence studies. Perspect Clin Res. 2021;12(3):171-4. doi: 10.4103/picr.picr_62_21
22. Schneeweiss S, Eichler HG, Garcia-Altes A, et al. Real World Data in Adaptive Biomedical Innovation: A Framework for Generating Evidence Fit for Decision-Making. Clin Pharmacol Ther. 2016;100(6):633-46. doi: 10.1002/cpt.512
23. Peters R, Yasar S, Anderson CS, et al. Investigation of antihypertensive class, dementia, and cognitive decline: A meta-analysis. Neurology. 2020;94(3):e267-e81. doi: 10.1212/wnl.0000000000008732
24. Guan JW, Huang CQ, Li YH, et al. No association between hypertension and risk for Alzheimer’s disease: a meta-analysis of longitudinal studies. J Alzheimers Dis. 2011;27(4):799-807. doi: 10.1212/wnl.0000000000008732
25. Hajian-Tilaki K. Sample size estimation in epidemiologic studies. Caspian J Intern Med. 2011;2(4):289-98.
26. Larsson SC, Markus HS. Does Treating Vascular Risk Factors Prevent Dementia and Alzheimer’s Disease? A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2018;64(2):657-68. doi: 10.3233/jad-180288
27. Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP. Single data extraction generated more errors than double data extraction in systematic reviews. J Clin Epidemiol. 2006;59(7):697-703. doi: 10.1016/j.jclinepi.2005.11.010
28. Scotti L, Bassi L, Soranna D, et al. Association between renin-angiotensin-aldosterone system inhibitors and risk of dementia: A meta-analysis. Pharmacological Research. 2021;166:105515. doi: 10.1016/j.phrs.2021.105515
29. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. 2021;372:n71. doi: 10.1016/j.neurobiolaging.2020.12.011. doi: 10.1136/bmj.n71
30. Adesuyan M, Brauer R, Yogini J. PROSPERO: International prospective register of systematic reviews2021. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021270065.
31. National Institute for Health and Care Excellence. Dementia: assessment, management and support for people living with dementia and their carers 2018 [Available from: https://www.nice.org.uk/guidance/ng97.
32. Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Bmj. 2016;355:i4919. doi: 10.1136/bmj.i4919
33. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-88. doi: 10.1016/0197-2456(86)90046-2
34. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327(7414):557-60. doi: 10.1136/bmj.327.7414.557
35. Haag MDM, Hofman A, Koudstaal PJ, Breteler MMB, Stricker BHC. Duration of antihypertensive drug use and risk of dementia A prospective cohort study. Neurology. 2009;72(20):1727-34. doi: 10.1212/01.wnl.0000345062.86148.3f
36. in’t Veld BA, Ruitenberg A, Hofman A, Stricker BHC, Breteler MMB. Antihypertensive drugs and incidence of dementia: the Rotterdam Study. Neurobiol Aging. 2001;22(3):407-12. doi: 10.1016/s0197-4580(00)00241-4
37. Tully PJ, Dartigues JF, Debette S, et al. Dementia risk with antihypertensive use and blood pressure variability. Neurology. 2016;87(6):601-8. doi: 10.1212/WNL.0000000000002946
38. Berthold D, Joyce G, Wharton W, Kehoe P, Zissimopoulos J. The association of multiple anti-hypertensive medication classes with Alzheimer’s disease incidence across sex, race, and ethnicity. PLoS One. 2018;13(11):18. doi: 10.1371/journal.pone.0206705
39. Chiu WC, Ho WC, Lin MH, et al. Angiotension receptor blockers reduce the risk of dementia. J Hypertens. 2014;32(4):938-47. doi: 10.1097/hjh.0000000000000086
40. Davies NM, Kehoe PG, Ben-Shlomo Y, Martin RM. Associations of Anti-Hypertensive Treatments with Alzheimer’s Disease, Vascular Dementia, and Other Dementias. J Alzheimers Dis. 2011;26(4):699-708. doi: 10.3233/jad-2011-110347
41. Hsu CY, Huang CC, Chan WL, et al. Angiotensin-Receptor Blockers and Risk of Alzheimer’s Disease in Hypertension Population – A Nationwide Cohort Study. Circ J. 2013;77(2):405-10. doi: 10.1253/circj.CJ-12-0658
42. Kuan YC, Huang KW, Yen DJ, et al. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers reduced dementia risk in patients with diabetes mellitus and hypertension. Int J Cardiol. 2016;220:462-6. doi: 10.1016/j.ijcard.2016.06.215
43. Bohlken J, Jacob L, Kostev K. The Relationship Between the Use of Antihypertensive Drugs and the Incidence of Dementia in General Practices in Germany. J Alzheimers Dis. 2019;70(1):91-7. doi: 10.3233/JAD-190362
44. Goh KL, Bhaskaran K, Minassian C, et al. Angiotensin receptor blockers and risk of dementia: Cohort study in UK Clinical Practice Research Datalink. Br J Clin Pharmacol. 2015;79(2):337-50. doi: 10.1111/bcp.12511
45. Sink KM, Leng X, Williamson J, et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med. 2009;169(13):1195-202. doi: 10.1001/archinternmed.2009.175
46. van Middelaar T, van Vught LA, van Charante EPM, et al. Lower dementia risk with different classes of antihypertensive medication in older patients. J Hypertens. 2017;35(10):2095-101. doi: 10.1097/hjh.0000000000001411
47. Deeks JJ HJ, Altman DG (editors),. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022) 2022. Available from: www.training.cochrane.org/handbook.
48. Strom BL, Melmon tlKL. The Use of Pharmacoepidemiology to Study Beneficial Drug Effects. Pharmacoepidemiology2019. p. 813-36. doi: 10.1002/9781119959946.ch37
49. Ouk M, Wu C-Y, Rabin JS, et al. Associations between brain amyloid accumulation and the use of angiotensin-converting enzyme inhibitors versus angiotensin receptor blockers. Neurobiol Aging. 2021;100:22-31. doi: 10.1016/j.neurobiolaging.2020.12.011
50. Kehoe PG, Wilcock GK. Is inhibition of the renin–angiotensin system a new treatment option for Alzheimer’s disease? The Lancet Neurology. 2007;6(4):373-8. doi: 10.1016/S1474-4422(07)70077-7
51. Gebre AK, Altaye BM, Atey TM, Tuem KB, Berhe DF. Targeting Renin–Angiotensin System Against Alzheimer’s Disease. Frontiers in Pharmacology. 2018;9(440). doi: 10.3389/fphar.2018.00440
52. Kehoe PG, Wong S, Al Mulhim N, Palmer LE, Miners JS. Angiotensin-converting enzyme 2 is reduced in Alzheimer’s disease in association with increasing amyloid-β and tau pathology. Alzheimers Res Ther. 2016;8(1):50. doi: 10.1186/s13195-016-0217-7
53. Jing F, Mogi M, Sakata A, et al. Direct stimulation of angiotensin II type 2 receptor enhances spatial memory. J Cereb Blood Flow Metab. 2012;32(2):248-55. doi: 10.1038/jcbfm.2011.133
54. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to meta-analysis: John Wiley & Sons; 2021.
55. Lindsay J, Laurin D, Verreault R, et al. Risk Factors for Alzheimer’s Disease: A Prospective Analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156(5):445-53. doi: 10.1093/aje/kwf074
56. Li N-C, Lee A, Whitmer RA, et al. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. Bmj. 2010;340:b5465. doi: 10.1136/bmj.b5465
57. Chang-Quan H, Hui W, Chao-Min W, et al. The association of antihypertensive medication use with risk of cognitive decline and dementia: a meta-analysis of longitudinal studies. Int J Clin Pract. 2011;65(12):1295-305.
58. Ding J, Davis-Plourde KL, Sedaghat S, et al. Antihypertensive medications and risk for incident dementia and Alzheimer’s disease: a meta-analysis of individual participant data from prospective cohort studies. The Lancet Neurology. 2020;19(1):61-70. doi: 10.1111/j.1742-1241.2011.02810.x
59. Walker VM, Kehoe PG, Martin RM, Davies NM. Repurposing antihypertensive drugs for the prevention of Alzheimer’s disease: a Mendelian randomization study. Int J Epidemiol. 2020;49(4):1132-40. doi: 10.1093/ije/dyz155
60. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629-34. doi: 10.1136/bmj.315.7109.629
61. Sterne JAC, Higgins JP, Page MJ. Assessing risk of bias due to missing results in a synthesis. 2020. In: Cochrane Handbook for Systematic Reviews of Interventions [Internet]. 6.1. Available from: https://training.cochrane.org/handbook/archive/v6.1/chapter-13#section-13-3-5-4.
62. Williamson JD, Pajewski NM, Auchus AP, et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial (SPRINT MIND TRIAL). JAMA. 2019;321(6):553-61. doi: 10.1001/jama.2018.21442