A.E. Zülke1, S.G. Riedel-Heller1, F. Wittmann1, A. Pabst1, S. Röhr1,2,*, M. Luppa1,*
1. Institute of Social Medicine, Occupational Health and Public Health (ISAP), Medical Faculty, University of Leipzig, 04103 Leipzig, Germany; 2. Global Brain Health Institute (GBHI), Trinity College Dublin, Dublin, Ireland; * these authors share senior authorship
Corresponding Author: Andrea E. Zülke, Institute of Social Medicine, Occupational Health and Public Health (ISAP), Medical Faculty, University of Leipzig, Philipp-Rosenthal-Str. 55, 04103 Leipzig, Germany, E-Mail: andrea.zuelke@medizin.uni-leipzig.de
J Prev Alz Dis 2023;1(10):69-82
Published online November 22, 2022, http://dx.doi.org/10.14283/jpad.2022.80
Abstract
Background: The number of people living with dementia worldwide is increasing rapidly. Preventive approaches constitute a promising strategy to counter the dementia epidemic, and growing numbers of lifestyle interventions are conducted around the globe. Gender differences with respect to modifiable risk factors for dementia have been reported, however, little is known about gender-specific effectiveness of lifestyle trials against cognitive decline and dementia. A systematic review and meta-analysis was conducted to assess evidence on gender-specific design and effectiveness of randomized controlled trials against cognitive decline.
Methods: Systematic literature searches were conducted in MEDLINE, PsycINFO, Web of Science, Cochrane Central and ALOIS. Studies assessing global and/or domain-specific cognitive function in older adults free from dementia were eligible for the systematic review. We assessed between-group effect sizes using random-effects meta-analysis. Methodological quality of included studies was assessed using the Scottish Intercollegiate Guidelines Network (SIGN)-checklist.
Results: The systematic review and meta-analysis included 34 and 31 studies, respectively. Effects of lifestyle-interventions on global cognition were non-significant overall (g = .27; 95% CI: -.01; .56) and in male subsamples (g = -.05; 95% CI: -.55; .45), and small for female subsamples (g = .38; 95% CI: .05; .72). Small beneficial effects were found for memory (overall: g = .38; 95% CI = .17; .59). Stratified by gender, significant effects were observed only in women (g = .39; 95% CI = .13; .65; men: g = .37; 95% CI: .00; .73). Aspects of gender in study design and conduct were discussed in a small minority of studies. Comparable results were observed for executive function and verbal fluency. Methodological quality was deemed high in 17.6% of studies, acceptable and low quality in 52.9% and 29.4%, respectively.
Discussion: We found evidence for small differences in the effectiveness of lifestyle interventions on global cognition and memory in favor of women. However, small numbers of trials 1) targeting men and 2) reporting gender-specific results for older adults with mild cognitive impairment warrant further attention. Assessing differences in modifiable risk factors for dementia in men and women and systematically addressing aspects of gender in trial conduction and recruitment in future studies might increase knowledge on gender-specific effectiveness of lifestyle trials against cognitive decline.
Key words: dementia, cognition, gender, lifestyle, prevention, systematic review, meta-analysis.
Abbreviations: AD: Alzheimer’s disease; APOE: Apolipoprotein; AVLT: Auditory Verbal Learning Test; BDNF: Brain-derived neurotrophic factor; BL: baseline; CANTAB: Cambridge neuropsychological test automated Subscale; CERAD: Consortium to Establish a Registry for Alzheimer’s Disease; CI: confidence interval; Coeff: coefficient; CPT: Continuous Performance Test; DKEFS: Delis-Kaplan Executive Function System; DSC: Digit Symbol Coding; DSST: Digit Symbol Substitution Test; FCRST: Free and Cued Selective Reminding Test; FINGER: Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability; GMLT: Groton Maze Learning Test; HVLT: Hopkins Verbal Learning Test; LNS: Letter-Number Sequencing; MCI: mild cognitive impairment; MCSA: Mayo Clinic Study of Aging; MMSE: Mini Mental State Examination; MoCA: Montreal Cognitive Assessment; NTB: neuropsychological test battery; OCL: One-card learning; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RBMT: Rivermead Behavioural Memory Test; RAVLT: Rey Auditory Verbal Learning Test; RCT: randomized controlled trial; Ref: reference; REML: restricted maximum likelihood; RPM: Raven’s progressive matrices; SCD: subjective cognitive decline; SCWT: Stroop Colour Word Test; SE: standard error; SES: socioeconomic status; SIGN: Scottish Intercollegiate Guidelines Network; TMT: Trail Making Test; WAIS-III: Wechsler Adult Intelligence Scale-Third Edition; WCST: Wisconsin Card Sorting Test; WW-FINGERS: World-Wide FINGERS
Introduction
Today, 55 million people worldwide are living with dementia, with projected increases to 78 million by 2030 due to growth of the ageing population (1). Available treatments are able to improve cognitive function and neuropsychiatric symptoms to a small extent and over limited periods of time, but still provide no cure (3). Evidence on potentially modifiable risk factors has been accumulating rapidly, pointing out the possibility of prevention (4). Twelve potentially modifiable risk factors have been established to date: low education in early life, hypertension and obesity in midlife, diabetes mellitus, smoking, excessive alcohol consumption, physical inactivity, depression, social isolation, hearing loss, traumatic brain injury and air pollution (5). Together, these risk factors are estimated to account for 40% of dementia cases. Declining incidence rates of dementia observed in several high-income countries have been linked to increased levels of education and improved management of cardiovascular diseases, indicating the potential of dementia prevention by targeting modifiable risk factors (6, 7).
Incidence and prevalence of dementia is higher in women, owing in part to differences in life expectancy (8), although this gap has been declining in developed countries. Certain lifestyle risk factors for dementia are distributed unequally by gender, however, it is not fully understood whether these risk factors impact dementia risk differently in men and women. In women, but not men, hypertension was found to increase risk for memory decline (9), incident mild cognitive impairment (MCI) (10) and dementia (11–13). Depression is associated with increased risk for incident MCI (14) and conversion from MCI to dementia (14, 15) more strongly for women than for men. Studies reported that diabetes mellitus is linked to greater cognitive decline (16), incident MCI (10) and risk for dementia (13, 17) for women than for men. Associations between history of stroke and higher risk for dementia (13) and cognitive decline (9) were observed only in men in some studies, while others reported higher risk for incident MCI (10) and dementia (18) after stroke for both men and women. Low education has been reported to increase memory decline (19) and risk for dementia (13) especially in women and incident MCI for both men and women (10). Smoking increased memory decline only in men in some studies (9, 20), while others reported higher risk for dementia for both genders (18). Growing evidence on prevention potential for dementia has resulted in a large number of randomized controlled trials (RCTs) targeting modifiable risk factors, with the most promising results observed in multi-domain trials which simultaneously target multiple risk factors (22–25). Recent meta-analyses reported small but significant beneficial effects of multi-domain interventions on risk for dementia (23) and cognitive composite scores (22). The pioneer multi-domain intervention trial, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER)-study, is currently being adapted in numerous countries and settings worldwide, resulting in the global WORLD-WIDE FINGERs (WW-FINGERS) network (26).
Regarding potential benefits of interventions to protect cognitive function in older age, little is known about whether men and women respond differently to interventions (13, 27). Knowledge on possible differential effects of interventions between genders might benefit the design of future studies targeting older men and women, but also aide the implementation of the respective interventions in real world-settings (27). Recent reviews have highlighted the need for more targeted intervention designs in dementia prevention which take into account different needs of specific subgroups of older people, depending on cultural or geographic background and further personal characteristics and dementia risk profiles (25, 28, 29). In the face of many trials reporting small or non-significant effects on cognitive function and risk for dementia, consideration of these differences might increase intervention effectiveness (27). This includes the consideration of possible differences in intervention effectiveness according to gender. Against this background, we conducted a systematic review and meta-analysis to assess aspects of gender in design and effectiveness of non-pharmacological RCTs targeting modifiable risk factors for cognitive decline and dementia.
Materials and methods
Eligibility criteria
Studies were eligible for the systematic review if they fulfilled the following criteria: 1) the study design was a randomized-controlled trial; pilot studies were eligible if a randomized allocation of participants was conducted; 2) a lifestyle-intervention was applied, targeting either diet, physical activity, social activity, cognitive activity, smoking, alcohol consumption, or psychoeducation; 3) participants were free from dementia at baseline; 4) a standardized measure of cognitive function (global or domain-specific) was applied at baseline and post-intervention; 5) the study reported gender-specific outcomes or indicated that gender-specific outcomes were assessed. Where the retrieved article did not report gender-specific outcomes but indicated to have conducted respective analyses, authors were contacted to obtain the additional data; 6) the article was published in English or German. Where necessary data for the meta-analysis could not be obtained, the respective studies were reported narratively but were excluded from the meta-analysis.
Exclusion criteria
Studies were excluded if 1) the interventions were targeted at people with dementia; 2) the study did not address age-related cognitive decline, e.g. studies on post-operative cognitive function; 3) a pharmacological intervention was applied; 4) the study focused on subjects with severe pre-existing conditions affecting risk for cognitive decline and dementia, e.g. myocardial infarction, cancer, stroke, or major depression.
Registration, protocol and guidelines
Our review was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (30). The review was registered prospectively in PROSPERO (registration number: CRD42021235281) and the review protocol outlining the rationale, eligibility criteria and strategy for data analysis has been published (31).
Search strategy and study selection
We conducted a database search using a comprehensive search strategy for MEDLINE (PubMed interface), Cochrane Central Register of Controlled Trials, PsycINFO, Web of Science (Web of Science interface) and ALOIS without restriction on date or publication status. The full search strategy is provided in Additional File 1.
In a first step, titles and abstracts were screened of all database returns by two researchers independently (AZ, FW). Thereafter, studies were checked according to the eligibility criteria outlined above by full-text analysis.
Data extraction
Data from included studies were extracted independently by two investigators (FW, AZ) using a standardized data extraction form. Discrepancies at each stage of the selection process were resolved by discussion with inclusion of a third researcher (ML). The following data were extracted: study characteristics: first author, year of publication, country, numbers of participants randomized to intervention(s) and control group, cognitive outcome(s), type of intervention, type of control condition, intervention duration.; participant’s characteristics: mean age, gender (distribution), cognitive function at baseline (cognitively healthy/SCD/MCI).
Quality assessment
Quality of included studies was assessed by two reviewers (AZ, FW) independently, using the SIGN (Scottish Intercollegiate Guidelines Network)-checklist for RCTs. This instrument covers information on internal validity (randomization, blinding, allocation concealment, drop-out rates, use of intention-to-treat-analysis) and general criteria for rating the quality of the study. As blinding of participants is hardly feasible in lifestyle intervention trials, a point for “blinding of subjects and investigators” was given if studies indicated that statisticians or other researchers involved in the study had been blinded. Disagreements between reviewers were resolved by discussion. Studies were judged as high quality if none of the aspects covered in the quality criteria was addressed insufficiently. If one to three criteria were not fulfilled or not adequately addressed, the study was considered to be of acceptable quality. Studies not fulfilling more than three quality criteria were scored as low-quality (32).
Effect sizes and meta-analytical procedures
Effect sizes of included studies, measuring change in cognitive function between baseline/pre-intervention and post-intervention (i.e. treatment effects) were obtained using sample sizes, means and standard deviations of included trials. We calculated effect sizes as between-group effect sizes per outcome, using data from intention-to-treat-analyses, where possible. If no intention-to-treat-data were available, we used estimators of per-protocol-analyses. Studies testing more than one intervention against a control group contributed data from all relevant intervention groups. Standardized mean group differences within included studies and a pooled overall effect size for the respective outcomes across studies were estimated using Hedge’s g to account for heterogeneity in sample sizes across studies. Values of Hedge’s g should be interpreted as follows: g < 0.5: small effect; g = 0.5-0.8: moderate effect; g >0.8: strong effect (33). Further, we assessed heterogeneity by inspecting forest plots and by applying I²-statistics. In addition, we used Egger’s regression test to evaluate publication bias, and conducted a non-parametric trim-and-fill analysis to investigate the possible impact of publication bias (34). In order to assess potential determinants of the pooled effect sizes, we conducted random effects meta-regression analyses including the variables cognitive function at baseline, mean age at baseline, type of intervention, gender, number of intervention sessions/intensity, duration of intervention, and outcome assessment. The restricted maximum likelihood (REML) estimator was used to model the between-study variance in both the pooled effect size analyses and the meta-regression analyses. All analyses were conducted using Stata/SE 16.0 (StataCorp, College Station/TX).
Results
Study selection
We identified 12.492 studies through literature searches in the databases PubMed, Web of Science, Cochrane, PsycInfo and ALOIS, of which 4.439 were duplicates and therefore removed. Two additional studies were identified screening the reference lists of current reviews. After screening titles and abstracts, 7.010 of articles were removed, the most common reasons being: study investigated no cognitive outcome (n=2.987), study was no RCT (n=2.211) or tested a pharmacological intervention (n=712), leaving 1.043 full-texts which were screened for eligibility. No studies were excluded due to language of publication. At this stage, the most common reasons for exclusion were trials not reporting gender-specific results (n=655), studies reporting secondary outcomes of trials already screened (n=260) or interventions not aimed at prevention (n=78). Finally, n=34 studies were included in the systematic review. If an article did not report pre- and post-measures (mean, SD) of cognitive outcomes, corresponding authors were contacted to obtain the respective values. In cases were no information on pre- and post-intervention values of cognitive outcomes could not be obtained (n=3), the article was included in the systematic review but was not considered for the meta-analysis. The process of study selection is described in Figure 1.
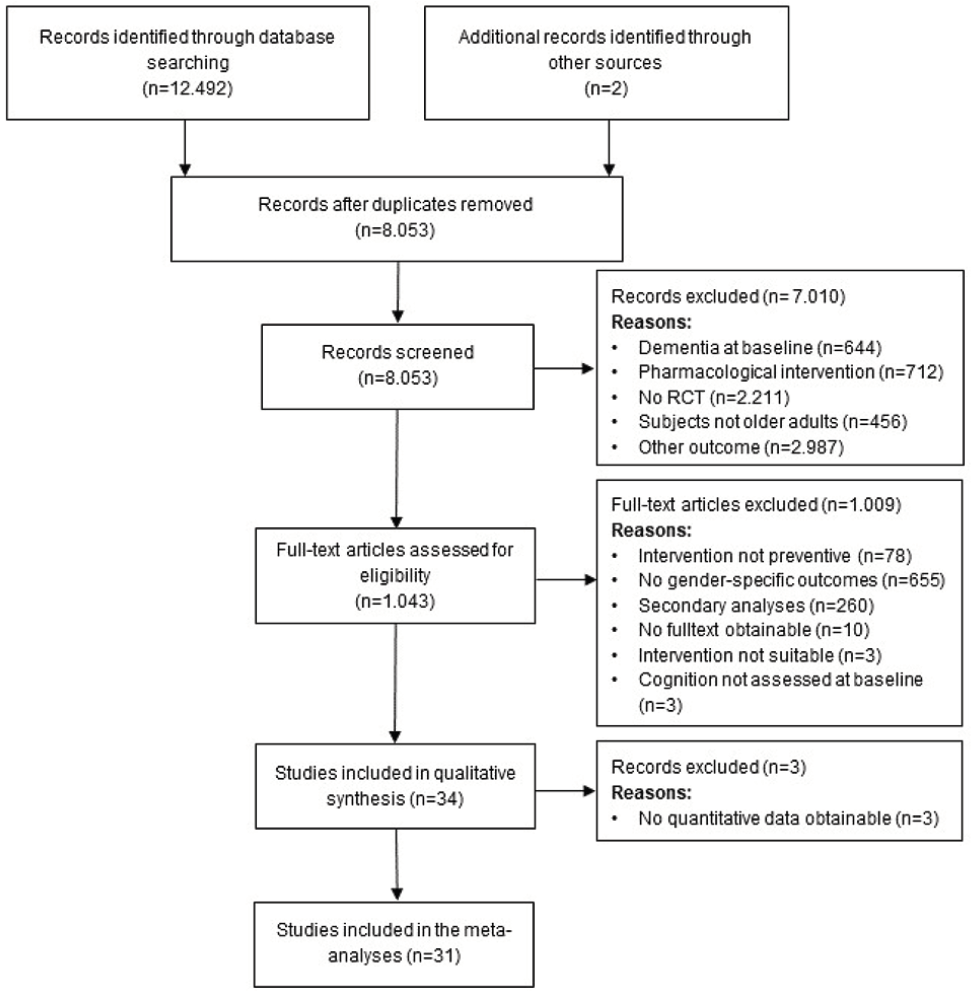
Figure 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram of the study selection process
Description of included articles
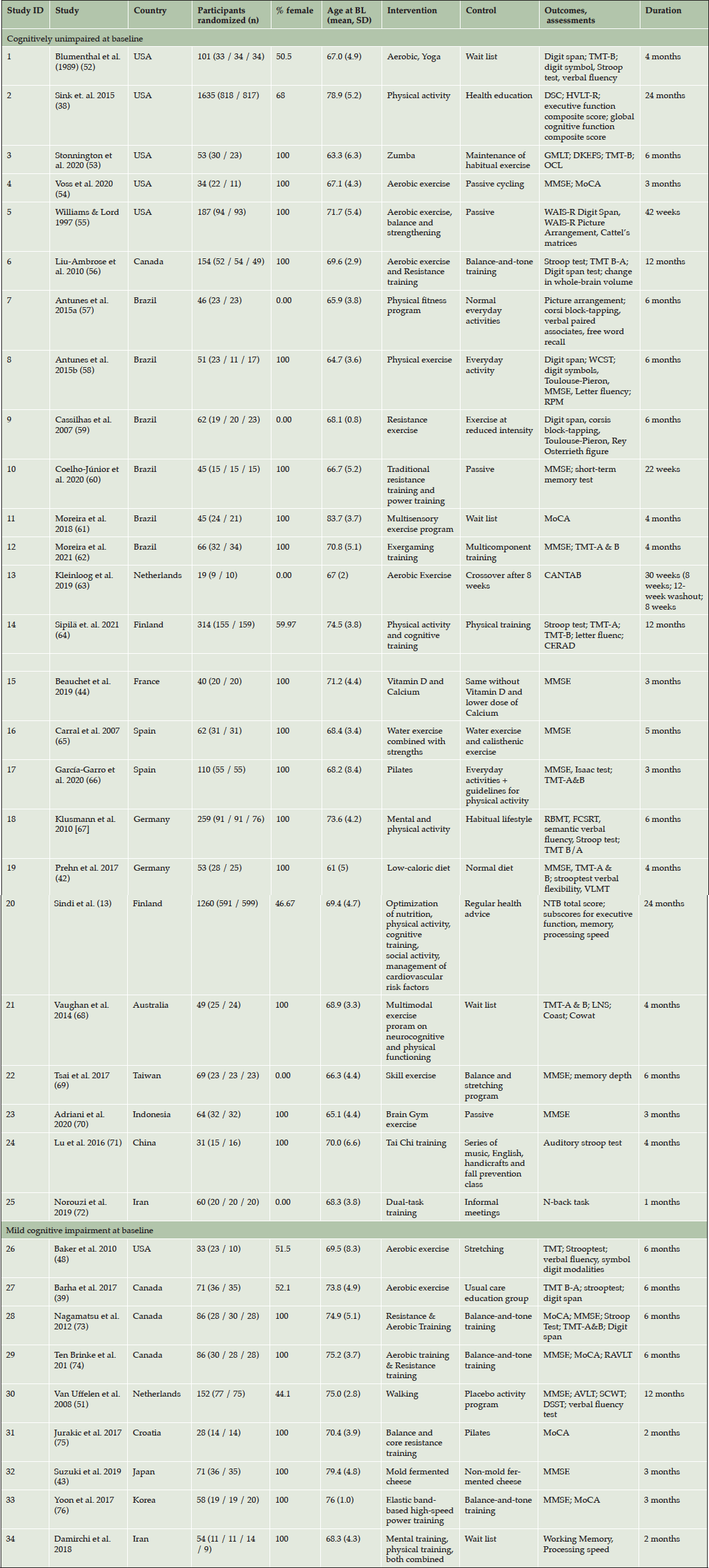
A full description of study characteristics is provided in Table 1.Studies included in the systematic review were conducted across a range of geographical regions, including the USA and Canada (1-6; 26-29), Brazil (7-12), Europe (13-20, 30, 31), Australia (21), Asia (22-24, 32, 33) and Iran (25, 34).
Control conditions varied between passive, e.g. waitlist control design or no intervention (1, 3, 5, 7, 8, 10, 11, 13, 18, 19, 21, 23, 34), health education (2, 17, 20, 27), placebo or versions of the interventions at reduced intensity (4, 6, 9, 12, 14-16, 22, 26, 28-33) and alternative activities (crafting (24); informal social gatherings (25)).
AVLT, Auditory-Verbal Learning Test; BDNF, Brain-derived neurotrophic factor; BL: baseline; CANTAB, Cambridge neuropsychological test automated Subscale; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; CPT, Continuous Performance Test ; DKEFS, Delis-Kaplan Executive Function System; DSC, Digit Symbol Coding; DSST, Digit Symbol Substitution Test; FCRST, Free and Cued Selective Remind¬ing Test; GMLT, Groton Maze Learning Test; HVLT-R, Hopkins Verbal Learning Test–Revised; LNS, Letter-Number Sequencing; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; NTB: neuropsychological test battery; OCL, One-card learning; RAVLT, Rey Auditory Verbal Learning Test; RBMT, Rivermead Behavioural Memory Test; RPM, Raven’s progressive matrices; SCWT, Stroop Colour Word Test; TMT, Trail Making Test; WCST, WAIS-R: Wechsler Adult Intelligence Scale, revised version; Wisconsin Card Sorting Test
Studies including men and women with unimpaired cognitive function
Among the included trials, n = 25 were aimed at older men and women with unimpaired cognitive function (1-25). Of these studies, five (1, 2, 4, 14, 20) included both men and women; five trials (7, 9, 13, 22, 25) targeted men only, while 15 trials (3-6, 8, 10, 12, 15, 16, 18, 19, 21, 23, 24) included exclusively female samples.
Studies including men and women with unimpaired cognitive function mainly tested physical activity-interventions (1-11, 13, 16, 17, 21, 22), while three trials combined physical and cognitive training (12, 14, 25). Study (23) applied an intervention to increase cognitive activity. In study (18), participants took part in either a physical or cognitive training. Two studies were single-domain interventions targeting nutrition (15, 19). Study (20) described gender-specific effects of the FINGER-multi-domain-lifestyle intervention, comprising physical, cognitive and social activity, optimization of nutrition and management of cardiovascular risk factors.
Sample sizes of trials investigating men and women with unimpaired cognitive function ranged from n = 19 (13) to n = 1.635 (2), with a mean of n = 195 participants. Duration of trials ranged from 4 weeks (25) to 24 months (2; 20).
Studies targeted at older individuals with MCI
A total of n = 9 studies were targeted at men or women with MCI (26). Of these, three trials included both men and women (26, 27, 30), while the remaining trials were conducted with female samples (28, 29, 31-34). No trials targeting older adults with MCI were conducted in exclusively male samples. We found no trial targeting men or women with subjective cognitive decline at baseline. Two studies (28, 29) covered the same trial and sample, but reported different cognitive outcomes and were, therefore, both included in the review.
Trials targeted at older men and women with MCI mostly applied physical activity interventions, e.g. aerobic or resistance training or combinations thereof (26-31, 33). Study (34) investigated the effectiveness of either physical or cognitive training or a combined intervention, while study (32) tested the effect of a nutritional intervention, i.e. controlled consumption of mold-fermented cheese.
Several trials tested interventions against an active control group, e.g. a light exercise condition (26, 28, 29, 31, 33) or similar foods with different nutritional properties than those in the intervention group (32). One trial applied a waitlist control-design (34), while the control group of (27) included regular care and health education. Sample sizes of trials targeted at older individuals with MCI ranged from n = 28 (31) to n = 152 (30); mean: n = 71). Duration of interventions ranged from 8 weeks (31, 34) to one year (30).
Three trials (20, 26, 28) did not report the necessary data to be included in the meta-analysis and did not respond to several attempts to contact the corresponding authors and principal investigators. Results of the respective trials are briefly described below.
Study (26) found beneficial effects of a high-intensity aerobic intervention on cognition in older men and women with MCI when compared to a stretching exercise-control group. Six months of aerobic exercise improved several measures of executive function and verbal fluency in women and revealed favourable effects on executive function (Trail Making Test B) in men. Intervention effects were more pronounced in women than in men across all cognitive tests. Study (28) reported beneficial effects of six months of resistance training in older women with MCI. Resistance training improved measures of selective attention and associative memory, while an aerobic exercise intervention showed beneficial effects on physical, but not cognitive functioning. The FINGER-multi-domain intervention (physical, cognitive, social activity, optimization of nutrition, management of cardiovascular risk factors; study (20) improved global cognitive function, processing speed, memory and executive function, without differences in effectiveness between men and women.
Effect sizes
To increase comparability of results and facilitate interpretation of findings, we decided to focus on global cognitive function, and, additionally, on a selection of cognitive outcomes which are of high relevance for independence in daily functioning and exhibit high sensitivity to age-related decline and neurodegenerative diseases, i.e. executive function, memory and verbal fluency (35–37). This constitutes a clarification of processes outlined in the review protocol. All modifications and clarifications of procedures described in the review protocol are outlined in Additional File 2.
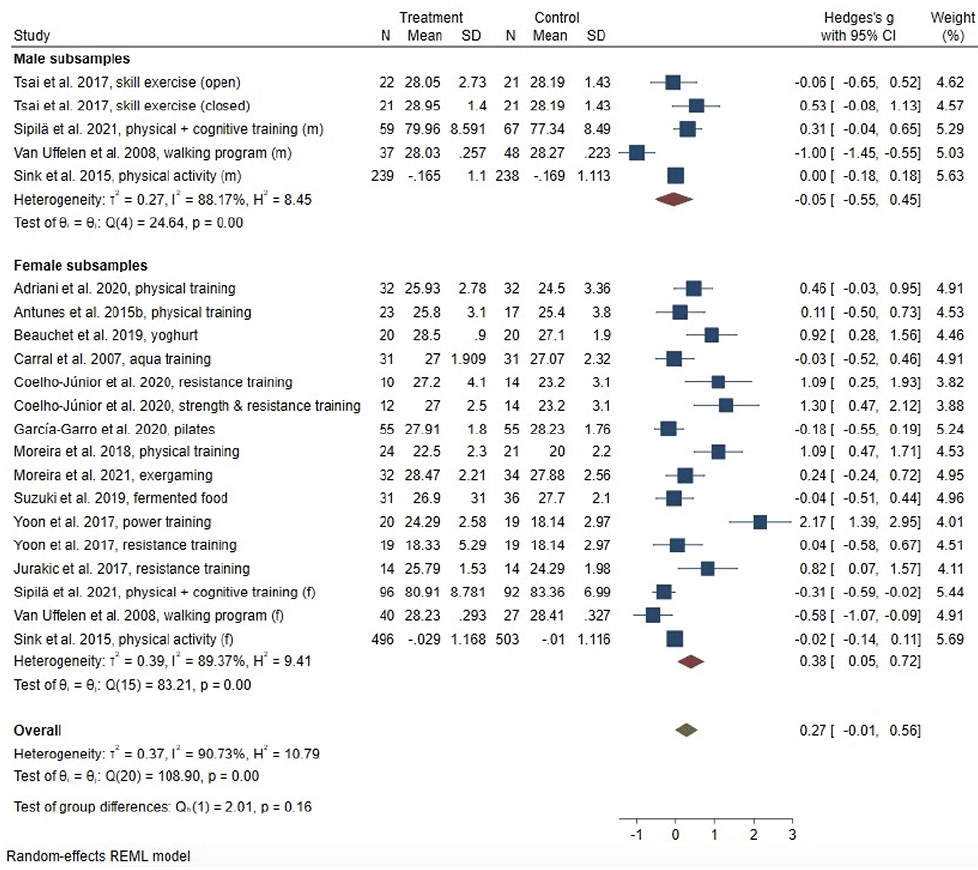
Intervention effects for global cognitive function, memory, executive function and verbal fluency are described using forest plots, providing information on overall-effect sizes and effectiveness by gender. The forest plot of between-group effect sizes for global cognitive function is displayed in Figure 2. Overall, 15 individual studies targeting global cognitive function were analysed meta-analytically, comprising 18 interventions. One study (33) assessed global cognitive function with two tests, respectively, i.e. the MMSE and the MoCA. Due to its higher sensitivity, the MoCA was chosen as outcome for this study. The meta-analysis revealed no effect of lifestyle-interventions on measures of global cognition in the overall study sample (g = .27, 95% CI: -.01; .56), displaying a high level of heterogeneity (I² = 90.73%). Stratified analyses by gender revealed a small intervention effect for women (g = .38, 95% CI: .05; .72) and a high level of heterogeneity (I² = 89.37%). Intervention effects were non-significant in male subsamples (g = -.05; 95% CI: -.55; .45), with high levels of heterogeneity (I² = 88.17%).
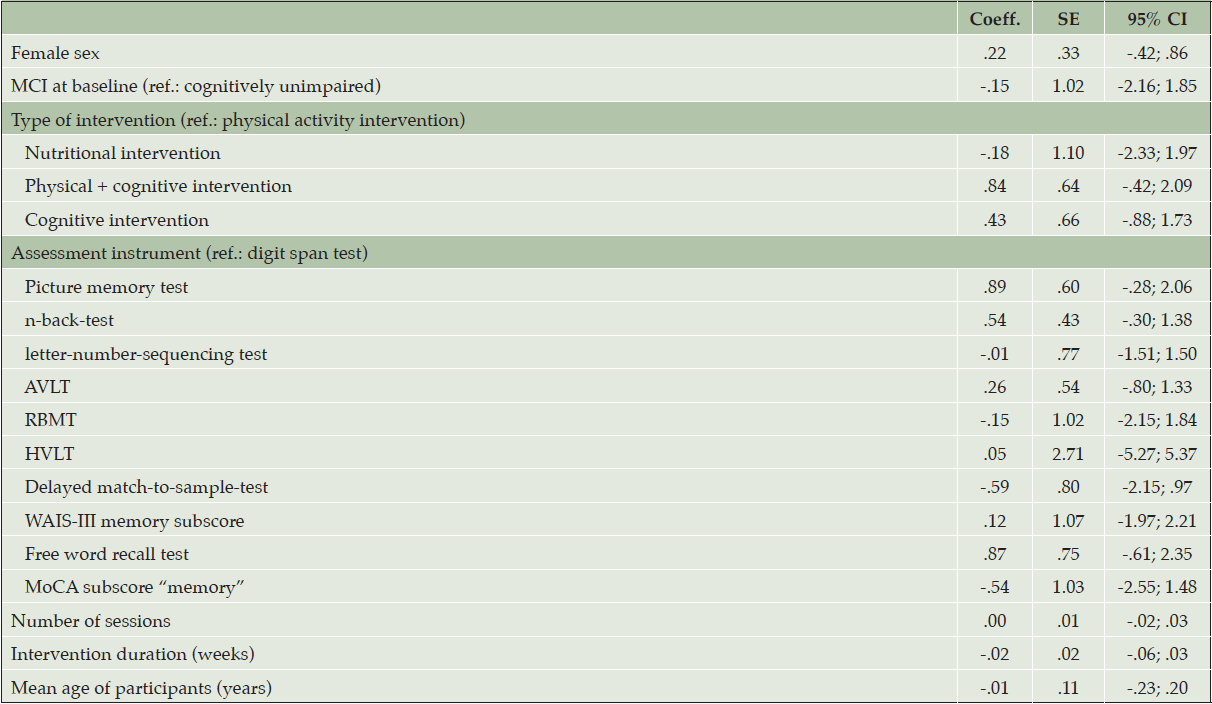
Only one study (30) investigated intervention effects in a sample of older adults with MCI at baseline, therefore, we did not stratify meta-analyses by baseline cognitive function. Random effects meta-regression revealed no effect of the considered determinants on the pooled effect sizes for the overall-sample (Table 2). We found evidence for possible small-study effects, as indicated be Egger’s test (p = .044). The results of the non-parametric trim-and-fill analysis indicated no difference in effect size due to potentially unpublished studies.
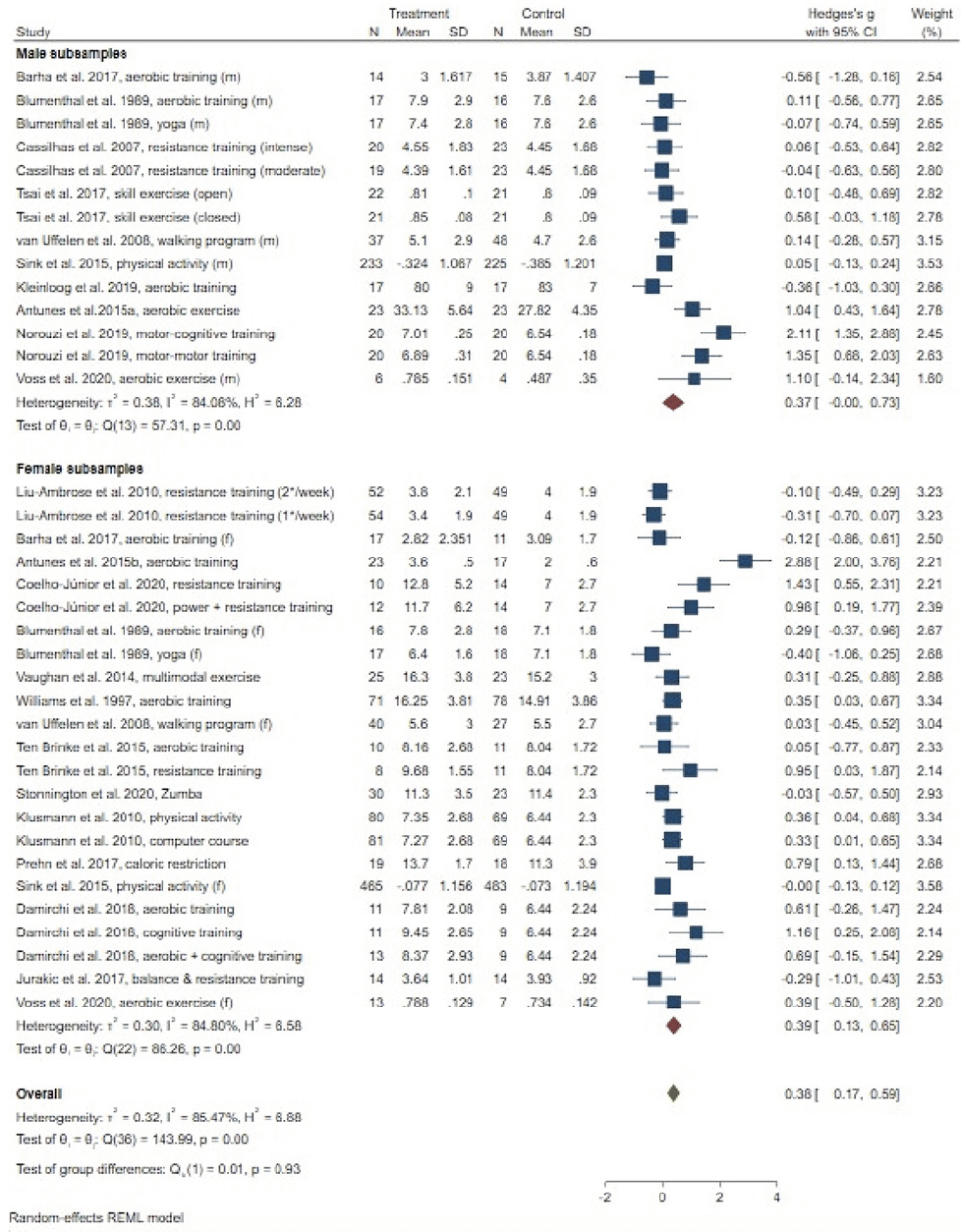
Meta-analysis for memory (19 studies, testing 27 interventions) revealed a small beneficial overall effect (g = .38, 95% CI = .17; .59; Figure 3), with high levels of heterogeneity (I² = 85.47%). Stratifying results by gender, we observed significant small effects only in women (g = .39, 95% CI = .13; .65; men: g = .37, 95% CI = .00; .73), with high levels of heterogeneity in male (I² = 84.08%) and female subsamples (I² = 86.26%), respectively. Random effects meta-regression revealed no influence of considered determinants on the pooled effect sizes for memory (Table 3). Egger’s test indicated possible small-study effects (p = .007). Publication bias was suspected as suggested by trim-and-fill analysis, with an adjusted effect size of g = .09 (95% CI: -.18; .36) in the absence of publication bias (eight studies imputed).
CERAD: Consortium to Establish a Registry for Alzheimer’s Disease; CI: confidence interval; Coeff.: coefficient; MCI: mild cognitive impairment; MMSE: Mini Mental State Examination; MoCA: Montreal Cognitive Assessment; SE: standard error; #: standardized composite score as applied in [38], consisting of Digit Symbol Coding task, revised Hopkins Verbal Learning Test immediate and delayed recall, n-back task, reaction time on task switching and Flanker task.
AVLT: Auditory Verbal Learning Test; CI: confidence interval; Coeff.: coefficient; HVLT: Hopkins Verbal Learning Test; MCI: mild cognitive impairment; MoCA: Montreal Cognitive Assessment; RBMT: Rivermead Behavioural Memory Test; SE: standard error; WAIS-III: Wechsler Adult Intelligence Scale-Third Edition
Results for executive function and verbal fluency are displayed in Additional File 3. A total of 17 studies, comprising 21 interventions, targeted executive function. A non-significant overall-effect size of g = -.16 (95% CI = -.31; .00) was detected, whereas negative values indicated positive intervention effects. Level of heterogeneity was moderate (I² = 70.98%). Stratified analysis by gender revealed a non-significant effect size of g = .01 (95% CI = -.21; .23) for men and a small but significant effect (g = -.24; 95% CI = -.43; -.04) for women, with moderate and considerable levels of heterogeneity for men (I² = 39.17%) and women (I² = 76.34%), respectively. Egger’s test did not suggest influence of small-study effects (p = .275). Publication bias was suspected as indicated by trim-and-fill analysis, with an adjusted effect size of g = -.11 (95% CI: -.29; .07) if one further study was imputed.
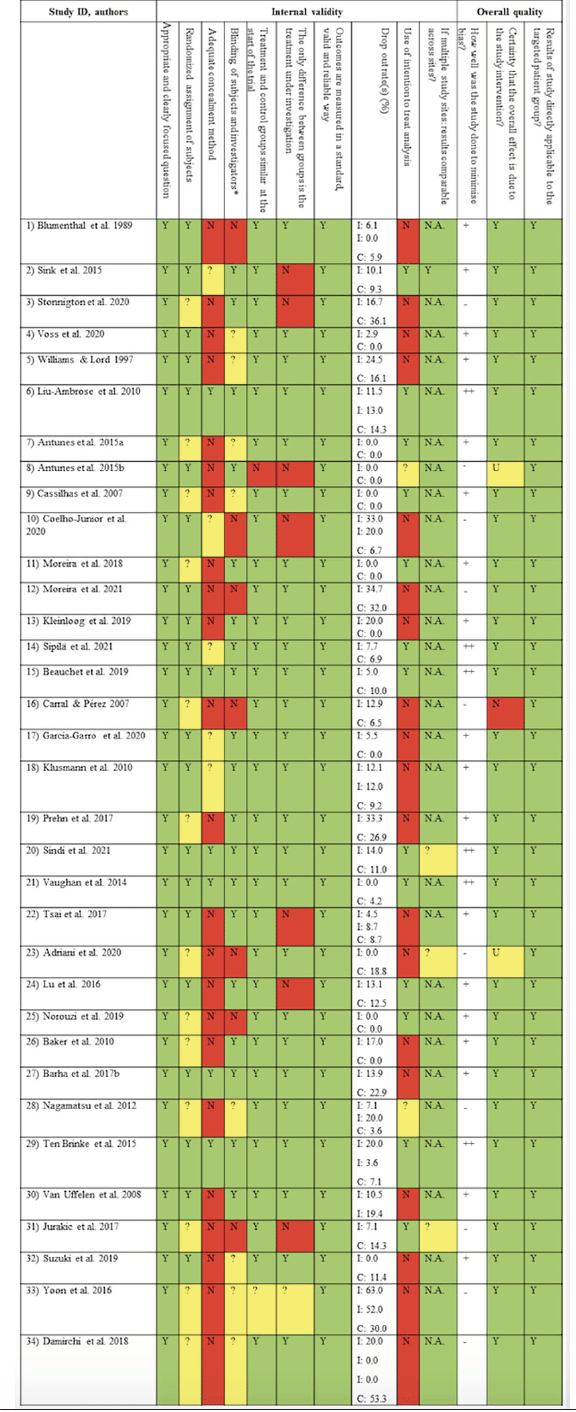
Figure 4. Methodological quality of studies (Scottish Intercollegiate Guidelines Network (SIGN) Methodology Checklist for randomized controlled trials)
* Scored “yes” if statistician or other researchers were blinded; N: no (red); N.A.: not applicable (no color); Y: yes (green); ?: can’t say (yellow); ++: high quality; +: acceptable; -: low quality; CMP: complete sample; I: intervention group; C: control group; U: unsure
Interventions targeted at verbal fluency (n = 8 studies, testing 10 interventions) revealed an overall effect size of g = .12 (95% CI = .01; .24), with low levels of heterogeneity (I² = 0.00%). We observed non-significant effects in male subsamples (g = .01; 95% CI: -.28; .25), with low levels of heterogeneity (I² = 17.40%). Investigating female subsamples revealed an effect size of g = .16 (95% CI: .02; .29), with low levels of heterogeneity (I² = 0.00%). We found no indication of small study effect, as suggested by Egger’s test (P = .735). Trim-and-fill analysis suggested an adjusted effect size of g = .11 (95% CI: .00; .22) if one further study had been available for analysis.
Gender-specific aspects in design and effectiveness of included trials
In addition to gender-specific effectiveness, we assessed whether aspects of gender were discussed in the study design, interpretation of results and respective conclusions of included trials. Study (26), which included both men and women, found evidence for gender-specific effects of physical activity on cognition, with beneficial effects on three measures of executive function in women but only on one measure in men. While no gender differences regarding effectiveness of the FINGER-intervention were detected, study (20) provides evidence for different risk profiles in older men and women at increased risk for dementia. Regarding adherence to the intervention and participant feedback, no gender differences were observed. However, more men than women (49% vs. 39%, p = .008) in the intervention group believed they had been part of the control group, i.e. these participants did not perceive themselves as having taken part in an intensive lifestyle intervention (13). Study (27) reports gender differences in effectiveness of an aerobic training intervention, with greater improvement on the TMT-B in women. However, no differences were observed for the outcomes Stroop and Digit Span Test. The authors report that baseline levels of physical activity were lower in women than in men, leaving greater room for improvement (39). No gender differences regarding intervention effectiveness were reported in studies (1, 2, 14).
Regarding included trials with gender-homogenous samples, n = 18 did not state reasons for recruiting only one gender (3-5, 7-11, 16, 17, 19, 24, 25, 28, 31-34). Studies (6, 13, 15, 21, 22, 29, 34) stated that a gender-homogenous sample was recruited in order to eliminate a potential influence of gender as an effect modifier. Studies (12) and (23) reported that trial participation was open to both men and women in their respective studies, however, only women were recruited into the trials. The authors provide no further information on possible underlying reasons or how this might have influenced their findings. Study (18), which tested both an intensive physical and cognitive intervention (i.e. computer course), stated that use of advanced technology might still be less common in older women than in older men. Therefore, a guided course for use of computers might be more challenging and provide more pronounced effects on cognition in older women.
Methodological quality
Of all included studies in the systematic review, 21 (61.8%) provided sufficient information on randomization technique; the remaining studies were randomized trials but failed to provide information on randomization method. Six studies (17.6%) applied an adequate concealment measure, while allocation was not concealed in 22 studies (66.7%) and insufficiently described in six (17.6%) studies. Intention-to-treat-analyses were applied in 12 studies (35.3%). Overall, 17.6% (n = 6) of studies were deemed “high quality”, n = 18 (52.9%) were judged as “acceptable” and n = 10 (29.4%) as being of “low quality”. The results of the methodological quality assessment, providing information on internal validity and overall-quality of studies, is displayed in Figure 4. Quality assessments for individual studies are provided in Additional File 4.
Discussion
Our study is the first attempt to systematically assess gender aspects in the design and effectiveness of a broad variety of non-pharmacological RCTs against cognitive decline in older adults free from dementia. Effects of lifestyle interventions on global cognition were non-significant in the overall-sample, whereas a small effect was detected in female subsamples. When investigating intervention effects on memory and verbal fluency, small effect sizes were observed, which were mostly due to effectiveness of interventions in female subsamples. For executive function, small effects sizes were observed only in female samples. The results were unaffected by type of intervention (e.g. physical, cognitive activity or nutrition), as indicated by meta-regression results. Most interventions were tested in female samples and included participants with unimpaired cognitive function at baseline, complicating statements on intervention effectiveness in men and older adults with MCI. However, random effects meta-regression did not reveal effects of baseline cognitive function on the observed estimates, aligning with results from a recent review reporting no interaction effect of baseline cognitive function and intervention in multi-domain lifestyle trials (22). Our findings are in line with previous reviews examining effects of physical activity interventions on cognitive function, reporting more pronounced effects in studies with larger proportions of women (40, 41). Only few included trials applied interventions targeted at nutrition or cognitive activity, while other potentially beneficial domains like e.g. social activity were not applied in identified trials. Likely due to ethical reasons prohibiting a non-active control group, no intervention trials targeted at tobacco or alcohol consumption were identified. Moreover, identified studies aimed at cognitive activity and nutrition applied very heterogeneous approaches (e.g. caloric restriction in study (19) (42) vs. consumption of specific foods in studies (15) and (32) (43, 44) as nutritional interventions). The majority of trials included in our study applied physical activity or exercise interventions, either as single-domain interventions or in combination with cognitive activity.
The meta-analyses focused on specific domains of cognitive function known to be sensitive to age-related decline. Choice of outcomes and availability of data across identified trials might, however, have influenced the findings: Several studies report a general advantage of older women in verbal fluency and memory (45, 46) which is preserved over the lifespan even despite increased pathology for AD (27). This could have lead to the slightly larger intervention effects observed in female samples regarding the respective domains. Men, on the other hand, have been reported to show better performance in tasks of visuospatial abilities (46), a measure for which insufficient amounts of data were available in the current study.
The observed differences between men and women might partly be explained by gender differences in education and occupational history. Older women, especially in less recent trials including earlier birth cohorts, might have been less likely to have been employed generally and, more specifically, in higher-ranking occupations which have been found to contribute to better cognitive function in older age (27, 47). These differences due to interactions of socio-economic status and gender might imply greater room for improvement of cognitive function brought about by lifestyle interventions. However, due to large heterogeneity between included samples and limited numbers of trials investigating men and women simultaneously, this line of thought should be interpreted with caution.
Another line of thought points to baseline differences in health and cardiovascular risk factors between older men and women. Single trials reported differences in body fat and fitness (48), BMI, total and high-density lipoprotein cholesterol (13) and physical activity (39) between male and female participants in favor of men, leaving more room for improvement in cardiovascular risk factors for dementia in women. On the other hand, more favorable dietary habits (regular intake of fruit and vegetables, fish, little or no alcohol consumption) were observed in women in single trials (13). Due to a limited number of studies assessing respective risk profiles by gender, this explanation could not be further assessed in the current review and demands further attention. Numerous trials following the FINGER-model are currently conducted around the globe (25), likely improving knowledge on the impact of baseline dementia risk factors on intervention effectiveness in men and women.
The majority of trials included in our review and meta-analyses tested interventions against an active control group, which might have resulted in a possible underestimation of the true effect sizes observed in included studies. Further, most included studies assessed intervention effects using single measures of global or domain-specific cognitive function, while composite scores and comprehensive neurocognitive assessment batteries have been pointed out to provide higher reliability than single tests (22). Therefore, choice of outcome measures and design of control groups might have impacted our results.
We identified only few studies with mixed samples reporting intervention effects for men and women separately. Trials investigating either men or women commonly explained this recruitment strategy by possible differences in brain structure and hormonal influences in women and men. To date, this has resulted in an underrepresentation of men in prevention trials against cognitive decline which has been observed in earlier reviews (41), highlighting the need for increased efforts in targeting older men. Two studies, however, were by design open to both men and women but failed to include both genders without discussing possible reasons for gender imbalance. This raises the question whether different interventions, e.g. physical activity or optimization of nutrition, might appeal differently to older men and women and whether needs and expectations towards lifestyle interventions differ by gender. Future trials might counteract this by discussing aspects of gender in early stages of study design, e.g. feasibility of intervention components for men and women, and by cooperation with relevant interest groups, e.g. older men and women in the community or health service practitioners with close contact to the targeted groups. The presented results align with previous reviews and guidelines, suggesting a mismatch between evidence from observational studies and intervention trials (22, 28, 49). While epidemiological cohort studies provide evidence for several potentially modifiable risk factors impacting on cognitive function in older age, results from randomized controlled trials are still inconclusive, (50). Existing studies often exhibit low or moderate methodological quality and suffer from small sample sizes and lack of statistical power, a limitation addressed already in previous reviews (50). Substantial differences between trials in study- and intervention design, as observed in the trials included for our review, resulted in high levels of heterogeneity, as observed in earlier reviews (28). Identifying and addressing populations with increased risk for dementia, assessed e.g. using biomarkers, cardiovascular risk factors or validated dementia risk scores has been highlighted as a promising strategy for future trials (28). As insufficient data on baseline dementia risk factors were available, we cannot rule out possible influences of baseline dementia risk on the respective findings. Tailoring interventions to at-risk-populations and reporting on baseline dementia risk factors in future trials might further increase knowledge on intervention effectiveness in older men and women, as first trials have reported different risk profiles between male and female trial participants (13, 39).
Strengths and limitations
To the best of our knowledge, our study is the first to describe gender differences in the effectiveness of a broad range of lifestyle interventions against cognitive decline, therefore contributing to the growing field of research on prevention of dementia. Further, while much of current evidence on lifestyle factors and cognitive function in older age stems from observational studies, our review relies on data from randomized controlled trials, therefore providing a higher quality of evidence regarding effectiveness of lifestyle changes against cognitive decline. Our review solely included studies using standardized objective measures of cognitive function, therefore allowing for differentiated statements on effectiveness of interventions on cognition and avoiding risk of self-report bias by excluding subjective measures of cognitive function. Where possible, we stratified analyses by cognitive function at baseline, as subjects with MCI might likely differ from cognitively unimpaired older adults in ability to participate in ambitious intervention trials. Due to the small number of eligible studies investigating men and women with MCI, however, further studies are warranted to ascertain these findings and provide evidence for possible gender differences. Exclusion of clinical samples and differentiation for various cognitive outcomes should improve comparability of reported results. Lastly, our study assessed methodological quality of included trials using a standardized, objective quality assessment.
This review has several limitations. Due to the focus on gender-specific aspects, many published studies reporting effectiveness of lifestyle trials for cognition had to be excluded from the initial search results if studies did not investigate results stratified by gender. Especially, only a limited number of trials including male (sub)samples or older adults with MCI was identified or provided the necessary data, therefore prohibiting stratified meta-analyses by gender and MCI for several outcomes. Further, only few included studies tested interventions targeting nutrition or cognitive activity or applied the same outcome measures across studies, prohibiting subgroup analyses of intervention effectiveness by type of intervention. Although we took great efforts to maximize the number of studies to include in the meta-analysis, several suitable articles could only be presented narratively due to non-reported data and non-response of corresponding authors to our inquiry. Most trials included in our review covered rather small samples, and possibility of small-study-effects was confirmed in the meta-analyses. This might have led to an underestimation of effect sizes and points towards the need for large-scale lifestyle intervention trials providing gender-specific results. Although we investigated possible determinants of intervention effectiveness by applying a meta-regression analyses, certain potential covariates which were not reported across all studies might have impacted on the pooled effect sizes, e.g. adherence to the intervention protocol, which certain trials reported to be important predictors of intervention effects (51). Lastly, due to high heterogeneity between studies regarding choice and assessment of outcome(s), it was decided to limit the number of cognitive outcomes covered in the meta-analysis. Heterogeneity in methodology and outcome assessment has been recognized as a problematic feature in lifestyle trials targeting cognitive function in older age and attempts at data harmonizing in current trials in order to increase comparability of findings have been launched (26).
Conclusion
Our systematic review found evidence for small differences in the effectiveness of lifestyle interventions on cognition in favour of women. However, we were able to point out several weaknesses and knowledge gaps in the growing field of lifestyle interventions against cognitive decline. Despite growing numbers of RCTs aiming at prevention of cognitive decline around the globe, questions of gender are only seldom addressed in trial design and interpretation of results to date, limiting our knowledge on intervention effectiveness especially in men and older adults with MCI. Future studies investigating mixed-gender samples and reporting stratified results by gender are highly warranted to improve our understanding of the effectiveness of lifestyle interventions in older adults. Applying a greater variety of recruitment strategies and discussing aspects of study design with older men and women from the respective target population might likely contribute to more gender-balanced samples in future trials.
Growing numbers of multi-domain lifestyle trials with large sample sizes are currently being conducted, which will increase the evidence base for effectiveness of lifestyle interventions against cognitive decline in men and women. Many of these trials follow the FINGER-approach of targeting at-risk-individuals based on modifiable risk factors for dementia, allowing for the investigation of the impact of baseline risk factors on intervention effectiveness in men and women, respectively. In addition to cognitive function, future studies are encouraged to assess intervention effects on surrogate outcomes, e.g. dementia risk scores or changes in risk behaviour for men and women, as respective effects might lead to reduced risk for cognitive decline and dementia in the long run.
Lastly, harmonization of outcome assessments and trial conduct constitutes another aim for future research, as highlighted by expert consortia in the field. Beyond that, enabling active participation of older men and women in the design and implementation of future interventions may constitute a promising approach to conduct tailored, individualized interventions in the future. The potential of lifestyle interventions against cognitive decline and dementia has been shown. Addressing gender differences in trial design and effectiveness will enhance precision and personalization of interventions, which might thereby improve effectiveness.
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Availability of data and materials: The data generated for this study are available from the corresponding author upon request.
Competing interests: The authors declare that they have no competing
interests.
Funding: This work was supported by the German Federal Ministry of Health (BMG) with a grant awarded to SR-H and SR (grant number: ZMVI1-2520FSB510). SR is an Atlantic Fellow for Equity in Brain Health and is supported by the Global Brain Health Institute (GBHI). The funders had no role in study design, data collection and analysis, interpretation of the data, decision to publish, or preparation of the manuscript. Open Access funding enabled and organized by Projekt DEAL.
Authors’ contributions: Conceptualization: SR.-H, SR, ML; Funding acquisition: SR-H, SR; Data curation: AZ, FW; Visualization: AZ, FW; Investigation: AZ, FW; Methodology: AZ, ML, AP; Project administration: AZ, SR-H, ML.; Supervision: SR-H, ML; Writing, original draft: AZ; Writing, review and editing: AZ, FW, AP, SR-H, SR, ML.
Acknowledgments: The authors want to thank Sina K. Gerhards and Rosa Siemensmeyer for assistance in conducting the systematic review.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Gauthier S, Rosa-Neto P, Morais JA, Webster C. World Alzheimer Report 2021: Journey through the diagnosis of dementia 2021. London: Alzheimer’s Disease International.
2. Prince MJ, Wimo A, Guerchet MM, Ali GC, Wu Y-T, Prina M. World Alzheimer Report 2015-The Global Impact of Dementia: An analysis of prevalence, incidence, cost and trends. 2015.
3. Yiannopoulou KG, Papageorgiou SG. Current and future treatments in Alzheimer disease: an update. Journal of central nervous system disease. 2020;12:1179573520907397. https://doi.org/10.1177%2F1179573520907397.
4. Lisko I, Kulmala J, Annetorp M, Ngandu T, Mangialasche F, Kivipelto M. How can dementia and disability be prevented in older adults: where are we today and where are we going? J Intern Med. 2021;289:807–30. https://doi.org/10.1111/joim.13227.
5. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396:413–46. https://doi.org/10.1016/S0140-6736(20)30367-6.
6. Pase MP, Satizabal CL, Seshadri S. Role of improved vascular health in the declining incidence of dementia. Stroke. 2017;48:2013–20. https://doi.org/10.1161/STROKEAHA.117.013369.
7. Ding M, Qiu C, Rizzuto D, Grande G, Fratiglioni L. Tracing temporal trends in dementia incidence over 25 years in central Stockholm, Sweden. Alzheimer’s & Dementia. 2020;16:770–8. https://doi.org/10.1002/alz.12073.
8. Tom SE, Hubbard RA, Crane PK, Haneuse SJ, Bowen J, McCormick WC, et al. Characterization of dementia and Alzheimer’s disease in an older population: updated incidence and life expectancy with and without dementia. American journal of public health. 2015;105:408–13. https://doi.org/10.2105/AJPH.2014.301935.
9. Anstey KJ, Peters R, Mortby ME, Kiely KM, Eramudugolla R, Cherbuin N, et al. Association of sex differences in dementia risk factors with sex differences in memory decline in a population-based cohort spanning 20-76 years. Sci Rep. 2021;11:7710. doi:10.1038/s41598-021-86397-7.
10. Pankratz VS, Roberts RO, Mielke MM, Knopman DS, Jack CR, Geda YE, et al. Predicting the risk of mild cognitive impairment in the Mayo Clinic Study of Aging. Neurology. 2015;84:1433–42. https://doi.org/10.1212/WNL.0000000000001437.
11. Blanken AE, Nation DA. Does Gender Influence the Relationship Between High Blood Pressure and Dementia? Highlighting Areas for Further Investigation. J Alzheimers Dis. 2020;78:23–48. doi:10.3233/JAD-200245.
12. Gilsanz P, Mayeda ER, Glymour MM, Quesenberry CP, Mungas DM, DeCarli C, et al. Female sex, early-onset hypertension, and risk of dementia. Neurology. 2017;89:1886–93. doi:10.1212/WNL.0000000000004602.
13. Sindi S, Kåreholt I, Ngandu T, Rosenberg A, Kulmala J, Johansson L, et al. Sex differences in dementia and response to a lifestyle intervention: Evidence from Nordic population-based studies and a prevention trial. Alzheimers Dement. 2021;17:1166–78. doi:10.1002/alz.12279.
14. Sachdev PS, Lipnicki DM, Crawford J, Reppermund S, Kochan NA, Trollor JN, et al. Risk profiles for mild cognitive impairment vary by age and sex: the Sydney Memory and Ageing study. The American journal of geriatric psychiatry. 2012;20:854–65. https://doi.org/10.1097/JGP.0b013e31825461b0
15. Kim S, Kim MJ, Kim S, Kang HS, Lim SW, Myung W, et al. Gender differences in risk factors for transition from mild cognitive impairment to Alzheimer’s disease: A CREDOS study. Comprehensive Psychiatry. 2015;62:114–22. https://doi.org/10.1016/j.comppsych.2015.07.002
16. Chireh B, D’Arcy C. A comparison of the prevalence of and modifiable risk factors for cognitive impairment among community-dwelling Canadian seniors over two decades, 1991-2009. PLoS One. 2020;15:e0242911. doi:10.1371/journal.pone.0242911.
17. Chatterjee S, Peters SAE, Woodward M, Mejia Arango S, Batty GD, Beckett N, et al. Type 2 Diabetes as a Risk Factor for Dementia in Women Compared With Men: A Pooled Analysis of 2.3 Million People Comprising More Than 100,000 Cases of Dementia. Diabetes Care. 2016;39:300–7. doi:10.2337/dc15-1588.
18. Gong J, Harris K, Peters SAE, Woodward M. Sex differences in the association between major cardiovascular risk factors in midlife and dementia: a cohort study using data from the UK Biobank. BMC Med. 2021;19:110. doi:10.1186/s12916-021-01980-z.
19. Reifegerste J, Veríssimo J, Rugg MD, Pullman MY, Babcock L, Glei DA, et al. Early-life education may help bolster declarative memory in old age, especially for women. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2021;28:218–52. doi:10.1080/13825585.2020.1736497.
20. van Zutphen EM, Rijnhart JJM, Rhebergen D, Muller M, Huisman M, Beekman A, et al. Do Cardiovascular Risk Factors and Cardiovascular Disease Explain Sex Differences in Cognitive Functioning in Old Age? J Alzheimers Dis. 2021;80:1643–55. doi:10.3233/JAD-201173.
21. Najar J, Aakre JA, Vassilaki M, Wetterberg H, Rydén L, Zettergren A, et al. Sex Difference in the Relation Between Marital Status and Dementia Risk in Two Population-Based Cohorts. J Alzheimers Dis. 2021;83:1269–79. doi:10.3233/JAD-210246.
22. Hafdi M, Hoevenaar-Blom MP, Richard E. Multi-domain interventions for the prevention of dementia and cognitive decline. Cochrane Database Syst Rev. 2021;11:CD013572. doi:10.1002/14651858.CD013572.pub2.
23. Röhr S, Riedel-Heller SG. Viel Luft nach oben: Verhältnis-und Verhaltensprävention von kognitiven Störungen und Demenz aus Public-Health-Perspektive. Psychiatrische Praxis. 2021;48:391–4. https://doi.org/10.1055/a-1666-8540
24. Meng X, Fang S, Zhang S, Li H, Ma D, Ye Y, et al. Multidomain lifestyle interventions for cognition and the risk of dementia: A systematic review and meta-analysis. Int J Nurs Stud. 2022;130:104236. doi:10.1016/j.ijnurstu.2022.104236.
25. Röhr S, Kivipelto M, Mangialasche F, Ngandu T, Riedel-Heller SG. Multidomain interventions for risk reduction and prevention of cognitive decline and dementia: current developments. Curr Opin Psychiatry. 2022;35:285–92. doi:10.1097/yco.0000000000000792.
26. Kivipelto M, Mangialasche F, Snyder HM, Allegri R, Andrieu S, Arai H, et al. World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16:1078–94. doi:10.1002/alz.12123.
27. Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Schumacher Dimech A, Santuccione Chadha A, et al. Sex differences in Alzheimer disease – the gateway to precision medicine. Nature Reviews Neurology. 2018;14:457–69. doi:10.1038/s41582-018-0032-9.
28. Stephen R, Barbera M, Peters R, Ee N, Zheng L, Lehtisalo J, et al. Development of the First WHO Guidelines for Risk Reduction of Cognitive Decline and Dementia: Lessons Learned and Future Directions. Front Neurol. 2021;12:763573. doi:10.3389/fneur.2021.763573.
29. Solomon A, Stephen R, Altomare D, Carrera E, Frisoni GB, Kulmala J, et al. Multidomain interventions: state-of-the-art and future directions for protocols to implement precision dementia risk reduction. A user manual for Brain Health Services-part 4 of 6. Alzheimers Res Ther. 2021;13:171. doi:10.1186/s13195-021-00875-8.
30. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi:10.1371/journal.pmed.1000097.
31. Zuelke AE, Riedel-Heller SG, Wittmann F, Pabst A, Roehr S, Luppa M. Gender-specific design and effectiveness of non-pharmacological interventions against cognitive decline and dementia-protocol for a systematic review and meta-analysis. PLoS One. 2021;16:e0256826. doi:10.1371/journal.pone.0256826.
32. Scottish Intercollegiate Guidelines Network, editor. SIGN 50:: A Guideline Developer’s Handbook; 2001.
33. Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic press; 2013. https://doi.org/10.4324/9780203771587
34. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–34. https://doi.org/10.1136/bmj.315.7109.629
35. Banich MT. Executive function: The search for an integrated account. Current directions in psychological science. 2009;18:89–94. https://doi.org/10.1111%2Fj.1467-8721.2009.01615.x
36. Foster TC, DeFazio RA, Bizon JL. Characterizing cognitive aging of spatial and contextual memory in animal models. Frontiers in aging neuroscience. 2012;4:12. https://doi.org/10.3389/fnagi.2012.00012
37. Saxton J, Lopez OL, Ratcliff G, Dulberg C, Fried LP, Carlson MC, et al. Preclinical Alzheimer disease: neuropsychological test performance 1.5 to 8 years prior to onset. Neurology. 2004;63:2341–7. https://doi.org/10.1212/01.WNL.0000147470.58328.50
38. Sink KM, Espeland MA, Castro CM, Church T, Cohen R, Dodson JA, et al. Effect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the LIFE randomized trial. Jama. 2015;314:781–90. https://doi.org/10.1001/jama.2015.9617
39. Barha CK, Hsiung G-YR, Best JR, Davis JC, Eng JJ, Jacova C, et al. Sex difference in aerobic exercise efficacy to improve cognition in older adults with vascular cognitive impairment: secondary analysis of a randomized controlled trial. Journal of Alzheimer’s Disease. 2017;60:1397–410. https://doi.org/10.3233/JAD-170221
40. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological science. 2003;14:125–30. https://doi.org/10.1111/1467-9280.t01-1-01430
41. Barha CK, Davis JC, Falck RS, Nagamatsu LS, Liu-Ambrose T. Sex differences in exercise efficacy to improve cognition: a systematic review and meta-analysis of randomized controlled trials in older humans. Frontiers in neuroendocrinology. 2017;46:71–85. https://doi.org/10.1016/j.yfrne.2017.04.002
42. Prehn K, Jumpertz von Schwartzenberg R, Mai K, Zeitz U, Witte AV, Hampel D, et al. Caloric restriction in older adults—differential effects of weight loss and reduced weight on brain structure and function. Cerebral cortex. 2017;27:1765–78. https://doi.org/10.1093/cercor/bhw008
43. Suzuki T, Kojima N, Osuka Y, Tokui Y, Takasugi S, Kawashima A, et al. The effects of mold-fermented cheese on brain-derived neurotrophic factor in community-dwelling older Japanese women with mild cognitive impairment: a randomized, controlled, crossover trial. Journal of the American Medical Directors Association. 2019;20:1509-1514. e2. https://doi.org/10.1016/j.jamda.2019.06.023
44. Beauchet O, Launay CP, Galery K, Vilcocq C, Dontot-Payen F, Rousseau B, et al. Effects of vitamin D and calcium fortified yogurts on gait, cognitive performances, and serum 25-hydroxyvitamin D concentrations in older community-dwelling females: results from the gait, memory, dietary and vitamin D (GAME-D2) randomized controlled trial. Nutrients. 2019;11:2880.
45. Golchert J, Roehr S, Luck T, Wagner M, Fuchs A, Wiese B, et al. Women Outperform Men in Verbal Episodic Memory Even in Oldest-Old Age: 13-Year Longitudinal Results of the AgeCoDe/AgeQualiDe Study. J Alzheimers Dis. 2019;69:857–69. doi:10.3233/jad-180949. https://doi.org/10.3390/nu11122880
46. Luck T, Pabst A, Rodriguez FS, Schroeter ML, Witte V, Hinz A, et al. Age-, sex-, and education-specific norms for an extended CERAD Neuropsychological Assessment Battery-Results from the population-based LIFE-Adult-Study. Neuropsychology. 2018;32:461–75. doi:10.1037/neu0000440.
47. Then FS, Luck T, Heser K, Ernst A, Posselt T, Wiese B, et al. Which types of mental work demands may be associated with reduced risk of dementia? Alzheimers Dement. 2017;13:431–40. doi:10.1016/j.jalz.2016.08.008.
48. Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Archives of neurology. 2010;67:71–9. https://doi.org/10.1001/archneurol.2009.307
49. Lee Y. Primary Prevention of Dementia: The Future of Population-Based Multidomain Lifestyle Interventions. The Journal of Prevention of Alzheimer’s Disease. 2018;5:5–7. doi:10.14283/jpad.2017.17.
50. Brasure M, Desai P, Davila H, Nelson VA, Calvert C, Jutkowitz E, et al. Physical Activity Interventions in Preventing Cognitive Decline and Alzheimer-Type Dementia: A Systematic Review. Ann Intern Med. 2018;168:30–8. doi:10.7326/M17-1528.
51. van Uffelen JGZ, Chinapaw MJM, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. British journal of sports medicine. 2008;42:344–51. doi:10.1136/bjsm.2007.044735.
52. Blumenthal JA, Emery CF, Madden DJ, George LK, Coleman RE, Riddle MW, et al. Cardiovascular and behavioral effects of aerobic exercise training in healthy older men and women. Journal of gerontology. 1989;44:M147-M157. https://doi.org/10.1093/geronj/44.5.M147
53. Stonnington CM, Krell-Roesch J, Locke DEC, Hentz JG, Dueck AC, Geda YE, et al. Impact of Zumba on Cognition and Quality of Life is Independent of APOE4 Carrier Status in Cognitively Unimpaired Older Women: A 6-Month Randomized Controlled Pilot Study. American Journal of Alzheimer’s Disease & Other Dementias®. 2020;35:1533317519868370. https://doi.org/10.1177%2F1533317519868370
54. Voss MW, Weng TB, Narayana-Kumanan K, Cole RC, Wharff C, Reist L, et al. Acute exercise effects predict training change in cognition and connectivity. Medicine and science in sports and exercise. 2020;52:131. https://doi.org/10.1249/2FMSS.0000000000002115
55. Williams P, Lord SR. Effects of group exercise on cognitive functioning and mood in older women. Australian and New Zealand journal of public health. 1997;21:45–52. https://doi.org/10.1111/j.1467-842X.1997.tb01653.x
56. Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Archives of internal medicine. 2010;170:170–8. https://doi.org/10.1001/archinternmed.2009.494
57. Antunes HK, Mello MT de, Santos-Galduróz RF, Galduróz JCF, Lemos VA, Tufik S, Bueno OFA. Effects of a physical fitness program on memory and blood viscosity in sedentary elderly men. Brazilian Journal of Medical and Biological Research. 2015;48:805–12. https://doi.org/10.1590/1414-431X20154529
58. Antunes HKM, Santos-Galduroz RF, Lemos VDA, Bueno OFA, Rzezak P, Santana MG de, Mello MT de. The influence of physical exercise and leisure activity on neuropsychological functioning in older adults. Age. 2015;37:1–10. https://doi.org/10.1007/s11357-015-9815-8
59. Cassilhas RC, Viana VAR, Grassmann V, Santos RT, Santos RF, Tufik S, Mello MT. The impact of resistance exercise on the cognitive function of the elderly. Medicine and science in sports and exercise. 2007;39:1401. https://doi.org/10.1249/mss.0b013e318060111f
60. Coelho-Júnior HJ, Oliveira Gonçalves Id, Sampaio RAC, Sampaio PYS, Lusa Cadore E, Calvani R, et al. Effects of combined resistance and power training on cognitive function in older women: A randomized controlled trial. Int J Environ Res Public Health. 2020;17:3435. https://doi.org/10.3390/ijerph17103435
61. Moreira NB, Gonçalves G, da Silva T, Zanardini FEH, Bento PCB. Multisensory exercise programme improves cognition and functionality in institutionalized older adults: a randomized control trial. Physiotherapy Research International. 2018;23:e1708. https://doi.org/10.1002/pri.1708
62. Moreira NB, Rodacki ALF, Costa SN, Pitta A, Bento PCB. Perceptive–cognitive and physical function in prefrail older adults: Exergaming versus traditional multicomponent training. Rejuvenation Research. 2021;24:28–36. https://doi.org/10.1089/rej.2020.2302
63. Kleinloog JPD, Mensink RP, Ivanov D, Adam JJ, Uludağ K, Joris PJ. Aerobic exercise training improves cerebral blood flow and executive function: a randomized, controlled cross-over trial in sedentary older men. Frontiers in aging neuroscience. 2019;11:333. https://doi.org/10.3389/fnagi.2019.00333
64. Sipilä S, Tirkkonen A, Savikangas T, Hänninen T, Laukkanen P, Alen M, et al. Effects of physical and cognitive training on gait speed and cognition in older adults: A randomized controlled trial. Scandinavian Journal of Medicine & Science in Sports. 2021. https://doi.org/10.1111/sms.13960
65. Carral JC, Pérez CA. Effects of high-intensity combined training on women over 65. Gerontology. 2007;53:340–6. https://doi.org/10.1159/000104098
66. García-Garro PA, Hita-Contreras F, Martínez-Amat A, Achalandabaso-Ochoa A, Jiménez-García JD, Cruz-Díaz D, Aibar-Almazán A. Effectiveness of a pilates training program on cognitive and functional abilities in postmenopausal women. Int J Environ Res Public Health. 2020;17:3580. https://doi.org/10.3390/ijerph17103580
67. Klusmann V, Evers A, Schwarzer R, Schlattmann P, Reischies FM, Heuser I, Dimeo FC. Complex mental and physical activity in older women and cognitive performance: a 6-month randomized controlled trial. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2010;65:680–8. https://doi.org/10.1093/gerona/glq053
68. Vaughan S, Wallis M, Polit D, Steele M, Shum D, Morris N. The effects of multimodal exercise on cognitive and physical functioning and brain-derived neurotrophic factor in older women: a randomised controlled trial. Age and ageing. 2014;43:623–9. https://doi.org/10.1093/ageing/afu010
69. Tsai C-L, Pan C-Y, Chen F-C, Tseng Y-T. Open-and closed-skill exercise interventions produce different neurocognitive effects on executive functions in the elderly: a 6-month randomized, controlled trial. Frontiers in aging neuroscience. 2017;9:294. https://doi.org/10.3389/fnagi.2017.00294
70. Adriani D, Imran Y, Mawi M, Amani P, Ilyas E, II. Effect of Brain Gym® exercises on cognitive function and brain-derived neurotrophic factor plasma level in elderly: a randomized controlled trial. Universa Medicina. 2020;39:34–41. https://doi.org/10.18051/UnivMed.2020.v39.34-41
71. Lu X, Siu KC, Fu SN, Hui-Chan CWY, Tsang WWN. Effects of Tai Chi training on postural control and cognitive performance while dual tasking–a randomized clinical trial. Journal of complementary and integrative medicine. 2016;13:181–7. https://doi.org/10.1515/jcim-2015-0084
72. Norouzi E, Vaezmosavi M, Gerber M, Pühse U, Brand S. Dual-task training on cognition and resistance training improved both balance and working memory in older people. The Physician and sportsmedicine. 2019;47:471–8. https://doi.org/10.1080/00913847.2019.1623996
73. Nagamatsu LS, Handy TC, Hsu CL, Voss M, Liu-Ambrose T. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Archives of internal medicine. 2012;172:666–8. https://doi.org/10.1001/archinternmed.2012.379
74. Ten Brinke LF ten, Bolandzadeh N, Nagamatsu LS, Hsu CL, Davis JC, Miran-Khan K, Liu-Ambrose T. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. British journal of sports medicine. 2015;49:248–54. http://dx.doi.org/10.1136/bjsports-2013-093184
75. Jurakic ZG, Krizanic V, Sarabon N, Markovic G. Effects of feedback-based balance and core resistance training vs. Pilates training on cognitive functions in older women with mild cognitive impairment: a pilot randomized controlled trial. Aging clinical and experimental research. 2017;29:1295–8. https://doi.org/10.1007/s40520-017-0740-9
76. Yoon DH, Kang D, Kim H-j, Kim J-S, Song HS, Song W. Effect of elastic band-based high-speed power training on cognitive function, physical performance and muscle strength in older women with mild cognitive impairment. Geriatrics & gerontology international. 2017;17:765–72. https://doi.org/10.1111/ggi.12784
77. Damirchi A, Hosseini F, Babaei P. Mental training enhances cognitive function and BDNF more than either physical or combined training in elderly women with MCI: a small-scale study. American Journal of Alzheimer’s Disease & Other Dementias®. 2018;33:20–9. https://doi.org/10.1177/1533317517727068
© The Authors 2022