D. Blasco1, J.S. Roberts1
1. Department of Health Behavior and Health Education, University of Michigan School of Public Health, Ann Arbor, MI, USA
Corresponding Author: Drew Blasco, Department of Health Behavior and Health Education, School of Public Health, University of Michigan, 1415 Washington Heights, Ann Arbor, MI 48109, Email: blasco@umich.edu
J Prev Alz Dis 2023;3(10):359-361
Published online April 17, 2023, http://dx.doi.org/10.14283/jpad.2023.46
Introduction
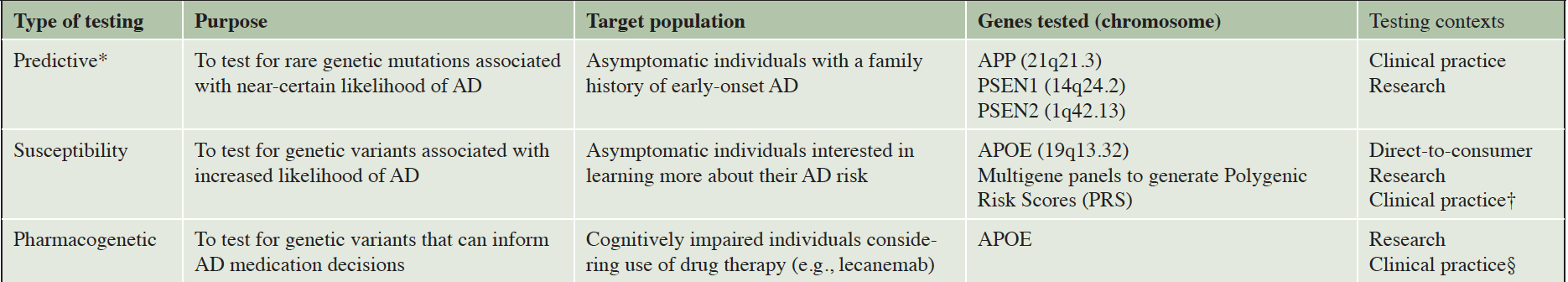
The recently released appropriate use recommendations (AURs) for lecanemab provide welcome guidance for clinicians considering use of this new therapy for the treatment of Alzheimer’s disease (AD) (1). The AUR statement includes a recommendation for APOE genotyping of all patients considering uptake of lecanemab, given that such testing can identify patients at elevated risk for drug side effects and inform treatment monitoring decisions (1). Given that millions of older adults are potential candidates for lecanemab, this would mark a dramatic expansion of genetic testing for Alzheimer’s disease, which has long been available but utilized relatively infrequently in clinical practice (and for different purposes than informing medication use). In this commentary, we briefly survey the broader landscape of genetic testing for AD (see Table 1) and then highlight the ethical, practical, and policy implications of APOE testing to inform use of lecanemab and other emerging treatments for AD.
*Testing for these mutations can also be used for diagnostic purposes in symptomatic individuals; †Discouraged by practice guidelines but in occasional use (4); § Most recent appropriate use recommendations include a recommendation that all individuals considering lecanemab treatment have APOE genotyping prior to uptake (1)
Predictive and Susceptibility Testing
In the 1990s, rare genetic mutations causing early-onset AD were identified on chromosomes 1 (PSEN2), 14 (PSEN1), and 21 (APP) (2). These pathogenic variants account for a very small proportion of AD cases, but allow for predictive genetic testing if there is a known or suspected mutation within a given family (2). Predictive testing for AD is recommended within a multi-session genetic education and counseling protocol originally developed for Huntington’s disease (3). Given limitations in AD treatment and prevention options, and the potential for stigma and distress in response to a positive genetic test result, most at-risk individuals elect not to pursue predictive testing; however, others may find such results useful to make life or advanced planning decisions (3).
Susceptibility testing via APOE genotyping has also been available for decades, given that APOE4 carriers have a higher likelihood of developing AD (2). However, APOE testing has not been widely implemented in clinical practice given the aforementioned limitations in disease treatment and prevention options and because the ε4 allele is neither necessary nor sufficient to cause AD (2–4). Nevertheless, in 2017 the US Food & Drug Administration (FDA) approved APOE testing to be offered via the direct-to-consumer (DTC) genetic testing company 23andMe. Although psychological distress and decisional regret have fortunately been rare for those disclosed their APOE results within a research context, it is less clear how individuals have been impacted when learning such results through other methods (e.g., DTC testing) where results are conveyed without the involvement of a genetic counselor or other medical professional (5).
Emerging Applications of APOE Genetic Testing
Recent years have seen the emergence of anti-amyloid therapies for AD where genetic testing might help inform treatment decision making. The FDA approved aducanumab (marketed as AduhelmTM) in 2021 and lecanemab (marketed as LeqembiTM) in January 2023 (6). For both of these treatments, clinical trials data suggest that APOE4 carriers, and especially ε4 homozygotes, are at higher risk of amyloid-related imaging abnormalities (ARIA) (7, 8). Two types of ARIA may occur: ARIA-E (edema or effusions) which involves brain swelling, and ARIA-H (hemosiderin deposits), which involves brain bleeds (7, 8). While often benign, ARIA can result in symptoms such as headaches, dizziness, or confusion, with more severe symptoms of seizures possible (6, 8). The effects of APOE status on ARIA risk, among those on lecanemab, are illustrated by results from a Phase 3 (CLARITY AD) lecanemab clinical trial (8). In this trial, the overall proportions of participants with ARIA-E and ARIA-H (alone or concurrent) on lecanemab were 12.6% and 17.3%, respectively; yet among APOE4 homozygotes, the corresponding rates of ARIA were 32.6% and 39% (8). Such differences in ARIA risk led to the inclusion of the recommendation for APOE genotyping in the aforementioned AUR statement for lecanemab: knowing a patient’s APOE status might influence the decision whether to initiate this treatment in the first place, and how aggressively to monitor for side effects once the drug is administered (1). For example, the guidelines stipulate that for APOE4 homozygotes, an additional MRI scan is recommended during the course of lecanemab treatment to assess potential presence of ARIA (1).
Implications of Expanded APOE Testing for AD Treatment Decision Making
The anticipated rapidly expanded clinical use of APOE genotyping in a cognitively impaired population has numerous ethical, legal, social, and policy implications. APOE4 carriers are relatively common among those with an AD diagnosis (40-65%) (2); this group would be at elevated risk of ARIA and therefore faced with complex decisions about if and how to proceed with new AD treatments. Below we build on points made in the AUR statement for lecanemab to highlight opportunities and challenges involved in this new and emerging use of APOE testing.
Psychosocial Impact of Testing and Risk Communication Challenges
Although research on the impacts of receiving APOE risk information has generally shown participants to have less psychological distress in response to their results than many had feared, these studies have mostly involved well-educated, cognitively intact participants of high socioeconomic status, and a limited number of racial/ethnic groups (5, 9). Further, in such studies participants have typically received genetic testing under controlled research conditions where expert genetic counselors provide robust pre- and post-test education and counseling (5, 9). Potential routine uses of APOE testing in the clinical setting with cognitively impaired patients pose a different set of challenges. For example, some patients may lack the decisional capacity and numeracy skills required to effectively weigh probabilistic risk information in already complex treatment decisions (4). Even for patients with minimal cognitive impairment, use of visual aids (e.g., pictographs) to convey differential ARIA risks by APOE status may be warranted, and the use of formal patient decision aids may be a helpful supplement to patient and family education (an existing online DA for APOE testing in asymptomatic adults provides an instructive example) (10).
There is also the issue of “ripple effects” of genetic information within families. Many patients who will be recommended APOE testing to inform treatment decisions have biological family members involved in their care who might discover new information about their own genetic risk for AD (1, 4). In the case of a patient found to be an APOE4 homozygote, any biological children would be obligate APOE4 carriers. Adult children may therefore not only have to face the challenges posed by their parent’s AD diagnosis and care, but also those raised by learning about their own elevated risk for a severe and often feared disease (1, 4). This is not to mention the potential implications of their APOE status for insurance decisions, given that long-term care, life, and disability insurers are not covered under federal legal protections against genetic discrimination. Future offerings of APOE testing to inform patients about risks of AD treatments need to account for how biological family members may be affected by new genetic information, and how to support the coping of both patients and family members.
Implementation in Clinical Practice
Current practice guidelines for all types of genetic testing for Alzheimer’s disease, including APOE genotyping, recommend the use of genetic counseling (4). With the potential for increased APOE testing among symptomatic patients eligible for emerging treatments, these guidelines may need to be revisited. There are already a limited number of genetic counselors working in direct patient care, with most of these in pediatric and oncology settings; therefore, increases in referrals to skilled genetic counselors may overwhelm an already overburdened field (11). Additional training and support should be considered for non-genetics providers to facilitate access to quality education and support for patients and families considering genetic testing. In the case of APOE testing to inform AD treatment decisions, providers from neurology, geriatrics, geriatric psychiatry, and nursing could be appropriate target audiences for such training. At a policy level, the implications of APOE testing for AD treatment regimens should also be considered. As noted earlier, APOE4 carriers may warrant more frequent monitoring for ARIA via MRI (1). Given that new anti-amyloid therapies are already highly expensive, with their coverage by Medicare uncertain, access to appropriate follow-up care indicated by genetic test findings may be challenging. Finally, significant efforts will be needed on multiple levels to avoid the potential exacerbation of racial and other health disparities. Minoritized populations face a wide range of challenges in terms of access to specialty dementia care and culturally component providers, and the evidence base regarding the efficacy of new AD treatments (and associated genetic risks) comes from a disproportionately white population of clinical trial participants.
Conclusion
Genetic testing for AD has long been available for asymptomatic populations but utilized relatively infrequently in clinical practice. Emerging uses, such as APOE genotyping to inform treatment decisions regarding lecanemab and related anti-amyloid AD therapies, require further examination to better understand their risks, benefits, and limitations. Their implications for patients, family members, and providers could be profound and may require proactive efforts in terms of patient education, workforce training, and health policy.
Disclosures and Funding: Dr. Roberts is supported by US National Institutes of Health grant P30 AG072931 and Dr. Blasco is supported by US National Institutes of Health grant T32 HG010030. The sponsors had no role in the preparation, review, or approval of the manuscript.
Conflict of interest: The authors declare there are no conflicts of interest to report.
References
1. Cummings J, Apostolova L, Rabinovici GD, et al. Lecanemab: appropriate use recommendations. J Prev Alz Dis 2023;3(10):362-377; https://doi.org/10.14283/jpad.2023.30
2. Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet Med. 2016;18:421–430. doi: https://doi.org/10.1038/gim.2015.117.
3. Roberts JS, Patterson AK, Uhlmann WR. Genetic testing for neurodegenerative diseases: ethical and health communication challenges. Neurobiol Dis. 2020;141:104871. doi: 10.1016/j.nbd.2020.104871.
4. Goldman JS, Hahn SE, Catania JW, et al. Genetic counseling and testing for Alzheimer disease: joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet Med. 2011;13:597–605. doi: 10.1097/GIM.0b013e31821d69b8.
5. Roberts JS. Assessing the psychological impact of genetic susceptibility testing. Hastings Cent Rep. 2019;49:S38–S43. doi: https://doi.org/10.1002/hast.1015.
6. U.S. Food & Drug Administration (FDA). FDA Grants Accelerated Approval for Alzheimer’s Disease Treatment [Internet]. January 6 2023. Available from: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-disease-treatment.
7. Barakos J, Purcell D, Suhy J, et al. Detection and management of amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with anti-amyloid beta therapy. J Prev Alz Dis. 2022;9:211–220. doi: https://doi.org/10.14283/jpad.2022.21.
8. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9–21. https://www.nejm.org/doi/full/10.1056/NEJMoa2212948
9. Green RC, Roberts JS, Cupples LA, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. N Engl J Med. 2009;361(3):245–254.
doi: https://doi.org/10.1056/NEJMoa0809578.
10. Ekstract M, Holtzman GI, Kim KY, et al. Evaluation of a web-based decision aid for people considering the APOE genetic test for Alzheimer risk. Genet Med. 2017;19:676–682. doi: https://doi.org/10.1038/gim.2016.170.
11. Stoll K, Kubendran S, Cohen SA. The past, present and future of service delivery in genetic counseling: keeping up in the era of precision medicine. Am J Med Genet C Semin Med Genet. 2018;178:24–37.
doi: https://doi.org/10.1002/ajmg.c.31602.
© Serdi 2023