S. Gauthier1,2, J. Therriault1,2, P. Rosa-Neto1,2
1. Translational Neuroimaging Laboratory, McGill University Research Centre for Studies in Aging, Alzheimer’s Disease Research Unit, Douglas Research Institute, Le Centre intégré universitaire de santé et de services sociaux (CIUSSS) de l’Ouest-de-l’Île-de-Montréal; Department of Neurology and Neurosurgery, Psychiatry and Pharmacology and Therapeutics, McGill University, Montreal, Canada; 2. Department of Neurology and Neurosurgery, Faculty of Medicine, McGill University, Canada
Corresponding Author: Serge Gauthier, Translational Neuroimaging Laboratory, The McGill University Research Centre for Studies in Aging, 6875 La Salle Blvd – FBC room 3149, Montreal, QC, Canada H4H 1R3, serge.gauthier@mcgill.ca
J Prev Alz Dis 2023;3(10):378-379
Published online May 17, 2023, http://dx.doi.org/10.14283/jpad.2023.58
We are impressed by the scope of the Appropriate Use Recommendations (AUR) by Cummings et al (1) about lecanemab, but we are disappointed by the lack of guidance about the assessment of clinical response in individual patients. We had offered suggestions after the first publication of the aducanumab AUR (2) and we would like to argue in favor of the CDR Global score based on the experience gained over thirty years with cholinesterase inhibitors (ChEIs), the knowledge about the natural history of progression of symptoms in persons with A(+) MCI or mild dementia over 24 months, and the published data from the CLARITY Study (3).
Demonstrating clinical benefit in individual patients with mild dementia has proven to be a challenge with ChEIs since cognitive scales such as the MMSE rarely show an improvement at three and six months, but patients are often considered as responders if stable or having minimal decline at twelve months, justifying a second year of treatment (4). Increase in spontaneity and interest in performance of instrumental activities of daily living (IADL) with ChEIs had been noted as early as three months of treatment, and functional scales have been created in order to capture this change in initiative such as the DAD, the ADCS-ADL-MCI, and the A-IADL-Q in randomized clinical trials (RCTs). Clinical global impression of change detecting short-term improvement in initiation of IADL and overall functional stability over time have been used in RCTs and in clinical practice using semi-structured interviews with patients and family. A list of clinical objectives formulated by the patients at the onset of treatment with a ChEI has been tested using Goal Attainment Scaling (GAS) with modest correlations between GAS scores and psychometric tests (5) but have been used in some jurisdictions as indicators of clinical response in daily practice.
The natural history of A(+) patients at the MCI stage is of small clinical decline over 24 months but variation at the individual level, using the CDR-SB (Figure 1a, derived from the ADNI database, accessed April 5th, 2023). There is more decline and variation for persons at the mild dementia stage (Figure 1b). The changes measured in patients treated with lecanemab in the CLARITY Study showed that the standard deviation of CDR-SB was substantially larger than the mean effect (3). How can deal with this variability?
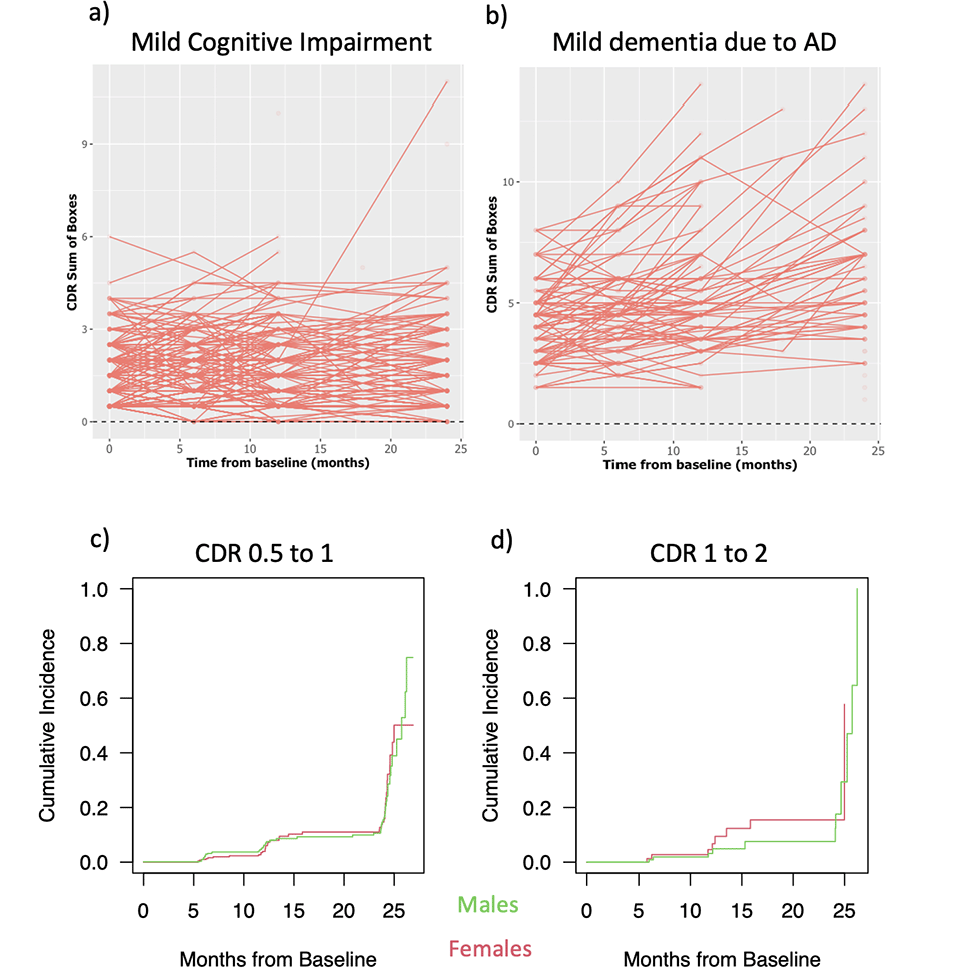
Figure 1. 1a Spaghetti plot of A(+) MCI subjects from ADNI database over 24 months; 1b Spaghetti plot of A(+) mild dementia subjects from ADNI database over 24 months; 1c Rate of progression from CDR 0.5 to 1 in A(+) MCI from ADNI database over 24 months; 1d Rate of progression from CDR 1 to 2 in A(+) mild dementia from ADNI database over 24 months
The CDR was developed originally for the diagnosis of MCI and early dementia, and is using a publicly available algorithm to calculate the global score of 0, 0.5, 1, 2, 3, correlating with clinical stages of normality (0), MCI (0.5) and mild dementia (1). There is more stability in the progression between CDR Global scores at the A(+) MCI and mild dementia stages than the CDR-SB as showed in Figure 1c and 1d, and the CLARITY study did demonstrate a slowing down of the rate of progression using the CDR Global score with a Hazard ratio on lecanemab of 0.69 (3; Supplementary Material, Figure S6). Furthermore, as is done in oncology, discussing a slowing down of transitioning from one stage of disease to the next will have meaning to many eligible patients.
We thus recommend that a global CDR be estimated prior to initiating treatment with disease-modifying drugs at the MCI and mild dementia stages of AD and yearly thereafter, as a way to give structure to the clinical follow-up, particularly if there is requirement in some jurisdictions to stop such treatments when reaching moderate stages of dementia.
Conflict of interest: There are no conflicts of interests relevant to this short text.
References
1. Cummings j, Apostolova L, Rabinovici GD, Atri A, Aisen P, Greenberg S et al. Lecanemab: Appropriate Use Recommendations. J Prev Alz Dis 2023;3(10):362-377, http://dx.doi.org/10.14283/jpad.2023.30.
2. Gauthier S, Rosa-Neto P. The US expert panel on the appropriate use recommendations of aducanumab in clinical practice. J Prev Alz Dis 2021;4(8):411; https://doi.org/10.14283/jpad.2021.44
3. van Dyck CH, Swanson CJ, Aisen P. Bateman RJ, Chen C, Gee M et al. Lecanemab in early Alzheimer’s disease. N Engl J Med 2023:388:9-21. DOI:10.1056/NEJMoa2212948.
4. Winblad Winblad B, Brodaty H, Gauthier S, Morris JC, Orgogozo JM, Rockwood K, Schneider L, Takeda M, Tariot P, Wilkinson D. Pharmacotherapy of Alzheimer’s disease: is there a need to redefine treatment success? Int J Geriatric Psychiatry, 2001: 16: 653-666; DOI: 10.1002/gps.496
5. Rockwood K, Graham J, Fay S. Goal setting and attainment in Alzheimer’s disease patients treated with donepezil. J Neurol Neurosurg Psychiatry 2022; 73(5): 500-507; DOI: 10.1136/jnnp.73.5.500
© Serdi 2023
