S.B. Hendrix1, H. Soininen2,3, A. Solomon2,4,5, P.J. Visser6,7, A.M.J. van Hees8, D.S. Counotte8, J. Nicodemus-Johnson1, S.P. Dickson1, K. Blennow9,10, M. Kivipelto2,4,5, T. Hartmann11,12, on behalf of the LipiDiDiet clinical study group
1. Pentara Corporation, Millcreek, UT, USA; 2. Department of Neurology, Institute of Clinical Medicine, University of Eastern Finland, Kuopio, Finland; 3. Neurocentre, Department of Neurology, Kuopio University Hospital, Kuopio, Finland; 4. Department of Clinical Geriatrics, Department of Neurobiology, Care Sciences and Society, Karolinska Institute, Huddinge, Sweden; 5. Clinical Trials Unit, Theme Aging, Karolinska University Hospital, Huddinge, Sweden; 6. Department of Psychiatry and Neuropsychology, Alzheimer Centre Limburg, University of Maastricht, Maastricht, the Netherlands; 7. Department of Neurology, Alzheimer Center, Amsterdam Neuroscience, VU University Medical Center, Amsterdam, the Netherlands; 8. Danone Nutricia Research, Utrecht, the Netherlands; 9. Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, The Sahlgrenska Academy at University of Gothenburg, Mölndal, Sweden; 10. Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden; 11. Deutsches Institut für Demenz Prävention (DIDP), Medical Faculty, Saarland University, Homburg, Germany; 12. Department of Experimental Neurology, Saarland University, Homburg, Germany
Corresponding Author: Suzanne B Hendrix, Pentara Corporation, 2180 Claybourne Avenue, Salt Lake City, UT 84109 USA, E-mail: shendrix@pentaracorp.com, Phone: +1 (801) 898-7241
J Prev Alz Dis 2023;3(10):464-470
Published online March 26, 2023, http://dx.doi.org/10.14283/jpad.2023.29
Abstract
The LipiDiDiet randomized clinical trial is evaluating the long term effects of a multinutrient intervention (Fortasyn Connect) compared with control in participants with prodromal AD. In this post-hoc analysis we used the Alzheimer’s Disease Composite Score (ADCOMS) as a measure of cognition and global function, together with a global statistical test (GST) and Bayesian hierarchical modelling (BHM) to evaluate the totality of evidence for an effect of the intervention over 36 months. The analysis includes 67 participants (39 active, 28 control) with change from baseline data after 36 months intervention. All outcome measures showed a statistically significant effect for the intervention: ADCOMS (P =0.045), GST (P <0.001), and BHM (P =0.008 based on 3 outcomes and P <0.001 including all primary and secondary quantitative clinical outcomes). Fortasyn Connect was associated with significantly less clinical decline over 36 months, suggesting the long-lasting beneficial effects of the multinutrient in prodromal AD.
Key words: Cognitive function, nutrients, Souvenaid, Fortasyn Connect.
Introduction
Modifiable risk factors, such as diet and nutrition, can have a positive effect on maintaining cognitive function in older adults at risk of cognitive decline and those with MCI (1). Regular uptake of seafood, fruit, vegetables, nuts and other foods and nutrients are associated with reduced cognitive decline and dementia risk (2-8). This includes complex healthy dietary patterns like the Mediterranean or Nordic diet, or combination diets like the MIND diet. Such findings indicate a large prevention potential for dementia through diet and lifestyle (9-13). Although potentially protective associations for specific nutrients and other biologically active food compounds are believed to form the actual molecular basis for the observed benefits, the WHO and others concluded that addressing several aspects of dietary intake at once, as exemplified by the Mediterranean diet, are more likely to promote better cognition compared to single nutrient supplementation or other less complex interventions. This is possibly due to the cumulative beneficial effect of the individual food components (13-21). Observational studies rather consistently report an association (22, 23), however, evidence from randomized clinical trials (RCT) is more sparse and inconsistent. Indeed, there is no strong RCT evidence to support the use of single-agent supplements. Effects appear to be stronger with more complex multinutrient interventions. Some RCTs observed benefits only in the presence of preexisting nutritional deficiencies or amyloid positivity. Beneficial effects also varied considerably, from reduced brain pathology only, to substantially broader effects including significant cognitive and functional improvements and decelerated brain atrophy. Some of the most pronounced benefits were observed with the multinutrient LipiDiDiet Fortasyn Connect (Fortasyn Connect; [Souvenaid]; Nutricia, Zoetermeer, the Netherlands) trial. Like several other recent nutrition RCTs, the original 24-months LipiDiDiet results raised the question of the need to study nutrient/dietary intervention for longer time-periods, which was supported by the expanded benefits observed in the 36-months LipiDiDiet results (18-21, 24-30). The LipiDiDiet RCT is evaluating the long-term effects of Fortasyn Connect compared with control in participants with mild cognitive impairment (MCI) due to Alzheimer’s Disease (AD) (24, 25). Fortasyn Connect contains docosahexaenoic acid and eicosapentaenoic acid, uridine monophosphate, choline, vitamins B12, B6, C, E, and folic acid, phospholipids, and selenium, and has been shown in preclinical studies to be neuroprotective (31). Analysis of the first 24- and 36-months intervention periods showed significant benefits for clinical dementia rating – sum of boxes (CDR-SB), hippocampal atrophy, cognition and memory and other outcomes. For the primary endpoint, a cognition focused subset of the Neuropsychological Test Battery, statistically significant benefit was shown over 36 months, but not in the primary analysis of the trial over 24 months. LipiDiDiet is a long-term trial allowing participants the option to continue for up to 6 years, which provides an opportunity to determine whether the effects of the specific multinutrient intervention change over time, and to evaluate results post hoc using contemporary research tools that were not available when the trial was first designed.
Previously, we reported a post-hoc analysis of 24-month data from the LipiDiDiet trial using the Alzheimer’s Disease Composite Score (ADCOMS), which identified the cognitive and functional benefits of the specific multinutrient intervention and confirmed the value of this composite tool as a potentially more sensitive measure of intervention effects than the NTB used in the primary analysis of the trial (24, 32). We have now updated our post-hoc analysis based on 36-month data from the LipiDiDiet trial. The update is important because multiple outcome measures, including the primary endpoint, showed significant benefits over 36 but not over 24 months, providing evidence for improved results with long-term outcomes. In addition, we used a global statistical test (GST) and Bayesian hierarchical modelling (BHM) to evaluate the totality of evidence for an effect of the intervention over 36 months.
Methods
LipiDiDiet (Netherlands Trial Registry NTR1705, new ID NL1620) is a 6-year, double-blind, parallel-group, multi-center, randomized controlled clinical trial comparing Fortasyn Connect (Souvenaid) with a control product in participants with prodromal AD defined according to the International Working Group (IWG)-1 criteria (33). Detailed methods were published previously (24). Participants provided written consent and the trial was approved by ethics committees of all sites and done in accordance with the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines.
After the 24-month intervention period reported in the primary analysis (24), participants could continue in their initially assigned group for a maximum total of 72 months. Efficacy was evaluated every 12 months and this report is based on data collected after 36 months of intervention. Results are reported for the ‘active’ group receiving the multinutrient intervention and the ‘control’ group. The main aim of this post-hoc analysis was to explore the effects of 36-month multinutrient intervention on cognition and global function, and its subdomains, by using ADCOMS, an outcome optimized for progression in this stage of AD, as described previously (32). ADCOMS consists of 4 subscale items of the Alzheimer’s disease assessment scale–cognitive subscale (ADAS-Cog: delayed word recall, orientation, word recognition, and word finding difficulty), two MMSE items (orientation time and drawing), and all 6 CDR-SB items (personal care, community affairs, home and hobbies, judgement and problem solving, memory, and orientation) (34). ADCOMS is an appropriate outcome measure for participants with prodromal AD because the composite outcomes from these scales are sensitive to changes and intervention effects over time in this population (34).
We calculated ADCOMS scores using the selected items (4-item ADAS-cog, 2-item MMSE, 6-item CDR-SB) and corresponding partial least squares (LS) coefficients in a modified intention-to-treat (mITT) population (24). Composite scores range from 0.0 to a maximum of 1.97, where higher values indicate worse performance. The measured outcome was change from baseline to 36 months. LS-means over 36 months were calculated using a linear mixed model for repeated measures that included baseline, baseline MMSE, continuous time, treatment, and time by treatment as fixed effects (primary model) (25). A random intercept was used within sites (small sites were pooled within country) and a random intercept and slope for time was used within participants. Sensitivity analyses comprised a sensitivity model with planned visit as categorical variable, primary and sensitivity models with baseline in the outcome vector, a 2-sided, independent t-test, and a non-parametric Mann-Whitney U test. Cohen’s d standardized effect sizes were calculated based on the mean treatment difference for the change from baseline over 36 months and pooled standard deviation (SD) with sample size value based on the first follow-up visit in the mixed model.
Two integrated statistical tests were employed to evaluate the totality of data from the 3 main trial outcomes representing neuropsychological performance (5-item NTB), daily function and cognitive effects (CDR-SB), and a brain structure biomarker (MRI hippocampal volume). A global statistical test (GST) (35) based on summary statistics for each item from the mixed model combines probabilities (p-values) using a joint normal distribution that accounts for correlations between these items directly. Bayesian hierarchical modelling (BHM) assesses the overall probability that treatment effects are real by combining probabilities (p-values from the mixed model) as if they are sampled from the same underlying distribution. This allows us to assess the degree to which the underlying disease progression is affected by treatment across multiple outcomes. BHM also provides individual estimates for each outcome that are adjusted to borrow strength from the other assessments. The 2-level BHM analysis and the GST both combine 3 outcomes reflecting neuropsychological performance (5-item NTB), daily function and cognitive effects (CDR-SB), and a biomarker of brain structure (hippocampal volume). A second version of the BHM was also performed and included 3 levels, including all primary and secondary quantitative clinical outcomes in a cognitive composite (5-item NTB, NTB memory, NTB executive function, and NTB total), and combining hippocampal volume, whole brain volume and ventricular volume (reversed) into a volumetric composite also on the first level. The second level includes this cognitive composite and this volumetric composite combined with a global scale (CDR-SB [reversed]). The third level is then the BHA composite.
Results
The 36-month post-hoc analysis includes participants with change from baseline scores available: there were 278 (138 active and 140 control) participants at baseline, 211 (104 active, 107 control) at month 12, 151 (71 active, 80 control) at month 24, and 67 (39 active, 28 control) at month 36.
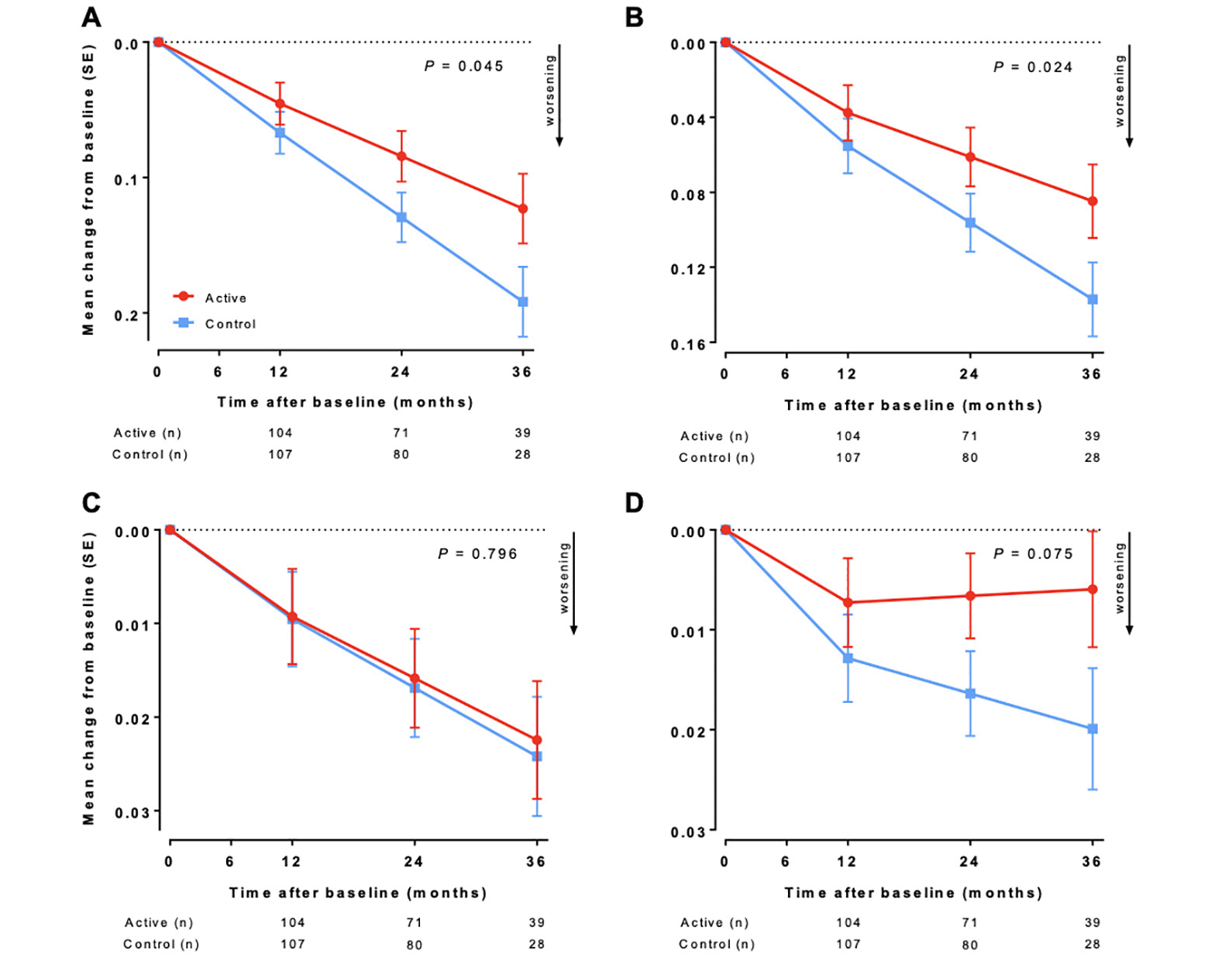
ADCOMS analysis showed a statistically significant (P = 0.045) slowing in change from baseline scores over 36 months in the active group compared with the control group (Figure 1A). The difference between active and control groups was −0.069 (95% confidence interval, −0.136 to −0.002), representing a 36% less worsening of disease in the active group over 36 months. Cohen’s d for effect size was −0.25 (95% confidence interval, −0.519 to 0.011). If ADCOMS subdomains are separately assessed (Figures 1B-D), differences are seen between active and control groups for the 6-item CDR-SB subdomain (38% less worsening; P = 0.024) and the 2-item MMSE subdomain (70% less worsening; P = 0.075) over 36 months, but not for the 4-item ADAS-cog subdomain. Sensitivity analyses supported the main findings on ADCOMS (i.e., model with planned visit as categorical variable: p=0.052, primary model with baseline in outcome vector: p=0.027, t-test: p=0.104, and Mann-Whitney U: p=0.178).
(A) Alzheimer’s Disease Composite Score. (B) Clinical Dementia Rating – Sum of Boxes 6-item subdomain. (C) Alzheimer’s Disease Assessment Scale–cognitive subscale 4-item subdomain. (D) Mini-Mental State Examination 2-item subdomain. Data are mean change from baseline as estimated by the primary linear mixed model; error bars are standard error (SE); n is the number of participants with change from baseline data in the mixed model. P value for linear mixed model (modified intention-to-treat).
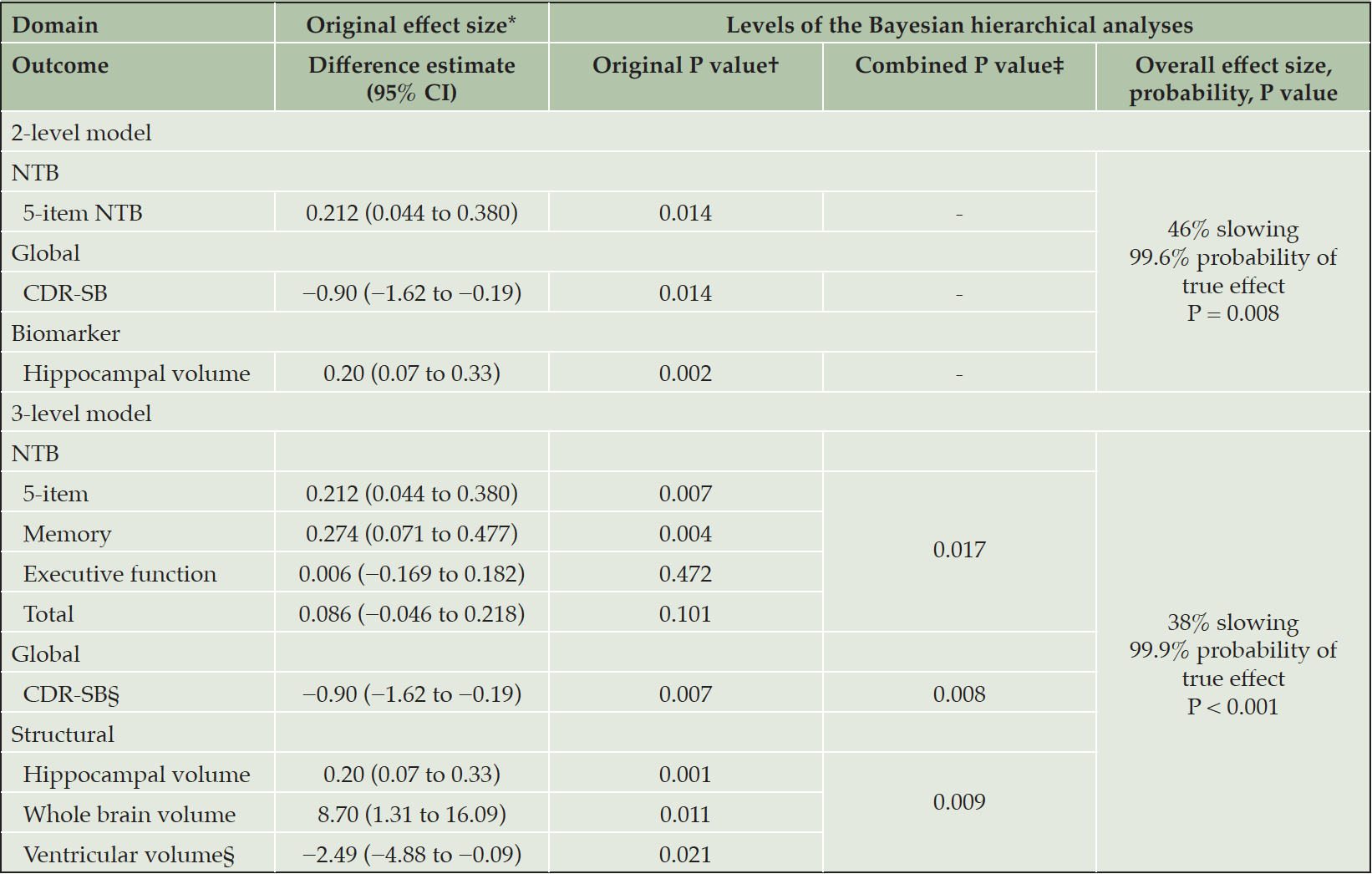
A GST comprising 5-item NTB, CDR-SB, and MRI hippocampal volume showed a statistically significant difference (~46% slowing, P < 0.001) between active and control groups at 36 months. The results from the BHM are summarized in Table 1. The 2-level BHM analysis of 3 outcomes combining the 5-item NTB, the CDR-SB, and hippocampal volume, showed a 46% slowing in the active group and a 99.6% chance of the difference being in favor of the active group (P = 0.008). The 3-level BHM analysis, including all primary and secondary clinical outcomes, showed a 38% slowing and a 99.9% probability of a true effect for active intervention (P < 0.001).
*Original effect size = estimated mean treatment difference (active – control) over 36 months (25); †Original P value for linear mixed model for longitudinal data (25); two-sided for the 2-level model, one-sided for the 3-level model; ‡One-sided; §Reversed.
Discussion
We assessed 3 different ways of analyzing the totality of evidence for an effect of a specific multinutrient intervention (Fortasyn Connect; Souvenaid) over 36 months in participants with prodromal AD adding to the previously published 24-month results. All measures showed a statistically significant effect for the intervention: ADCOMS (P = 0.045), GST (P < 0.001), and BHM (P = 0.008 for 2-level BHM and P < 0.001 for 3-level BHM including all primary and secondary quantitative clinical outcomes). The trio of tests demonstrated a similar effect for the intervention on multiple outcomes and are consistent with an overall effect of the specific multinutrient intervention on AD progression over 36 months (25).
The LipiDiDiet trial design includes preplanned analyses up to 72 months, allowing observation of long-term trends and differences between groups over time. This analysis showed that the difference in ADCOMS observed over 24 months was sustained over 36 months (36% less worsening at both time points). The difference was largely driven by a sustainable benefit in CDR-SB, which is a sensitive and meaningful measure of clinical and functional changes in individuals with early AD (36). The 2-level BHM analysis comprising the 5-item NTB, the CDR-SB, and hippocampal volume, also showed statistically significant slowing of disease progression in participants taking the multinutrient.
No other randomized controlled trials of long-term multinutrient intervention in people with prodromal AD or mild cognitive impairment (MCI) have been published. This is the first study to use a global statistical test to integrate multiple outcomes in participants with prodromal AD receiving a long-term multinutrient intervention. In addition, the 3-year data from the LipiDiDiet trial have been used as an example in a recent publication on the application of a multivariate competing risk joint model to model the underlying biological processes in Alzheimer’s disease (37). Other composite endpoints of cognitive decline have been developed for trials in people with prodromal AD (38); for example, the Alzheimer’s Prevention Initiative Composite Cognitive Test (APCC) appears to be sensitive to preclinical AD decline up to 11 years before clinical AD is diagnosed (39).
This analysis is limited by post-hoc use of composite measures and integrated statistical tests using data transcribed from the original study outcomes measures. Use of composite scores has been criticized because for various methodological issues they may not be superior to individual tests or domain-based factor scores (38). ADCOMS was used for this post hoc analysis because it offers improved sensitivity to detect the effect of an intervention in early-stage AD (34), however, another clinical study found that it provided only a marginal improvement in sensitivity to decline compared with CDR-SB or ADAS-Cog (40). The additional integrated statistical tests used in this analysis give confidence in the validity of the ADCOMS analysis to detect differences between intervention and control groups over 36 months in the LipiDiDiet trial. These results therefore provide further support for use of ADCOMS as an outcome measure for long-term intervention studies in early AD. The GST and BHM statistical analyses provide an estimate of slowing of clinical progression which can be interpreted for clinical meaningfulness. The CDR-SB also provides an indication of clinical meaningfulness, because treatment favorably affects the functional deficits affecting people with prodromal AD (41). In this context, the GST and the 2 separate BHM approaches including not only a selection of outcomes (2-level BHM), but also all primary and secondary quantitative clinical outcomes from the trial (3-level BHM) provide a high degree of confidence of a true effect and evidence of clinical meaningfulness in the slowing of clinical progression. In addition, the Cohen’s d score is supportive of a meaningful clinical effect for long-term intervention. As with other long-term studies, there is potential for bias to be introduced by participant dropout disproportionately affecting balance between arms. Previously, we found no evidence of bias due to missing data over 36 months using a joint model combining longitudinal and survival data as additional sensitivity analyses (25).
Results of 3 analyses (ADCOMS, GST, and BHM) coalesce to support an overall effect of 36-month multinutrient intervention on cognitive, functional, and structural endpoints. From a clinical perspective, these findings indicate 38% to 46% slower progression of disease related decline with long-term multinutrient intervention. The progression trajectories for intervention and control groups diverged early, driven predominantly by CDR-SB scores. In a previous analysis of CDR-SB trajectories over 36 months, there was a 45% slowing of disease progression for multinutrient intervention compared with control (25), which we estimate translates for this group of patients with mild cognitive impairment due to AD into a 16-month gain over the control group. Interestingly, BHM showed a 46% slowing of disease progression in this analysis.
When considered with other studies of Souvenaid across the AD spectrum (42-45), these new analyses are in line with evidence showing that this specific multinutrient intervention has its greatest potential for benefit when used earlier rather than later in the disease, when it appears to still be possible to positively affect the dynamic processes involved in synaptic function, neurodegeneration and neuroprotection (46, 47). Studies in aging adults with memory complaints have shown the potential benefits of encouraging healthier lifestyle and diet to reduce dementia progression (48, 49), while a study in individuals with prodromal AD or dementia due to AD found a significant association between nutritional index and risk of disease progression (50), suggesting a beneficial role for specific nutritional interventions in the earliest stages of AD.
Conclusion
In this post-hoc analysis of data from the LipiDiDiet trial, participants taking Fortasyn Connect (Souvenaid) showed significantly less clinical decline over 36 months as measured by ADCOMS, suggesting long-lasting beneficial effects of the multinutrient in a prodromal AD/pre-dementia population with MCI. Multinutrient intervention can be considered as part of an overall strategy to encourage healthy lifestyle and improve diet in people at risk of progression to AD dementia.
Funding: The research leading to these results was mainly funded by the European Commission under the 7th framework program of the European Union (grant agreement number 211696). Additional funding was provided by the EU Joint Programs – Neurodegenerative Disease Research (EU-FINGERS and MIND-AD grants; BMBF 01ED1509 and 01ED2003); Kuopio University Hospital, Finland (EVO/VTR grant); and Academy of Finland (grant 287490). These funders had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript. The LipiDiDiet consortium received funding by Danone Nutricia Research for the intervention period from 25 to 96 months and the consortium distributed the funding to their members to conduct the trial and analysis. This post-hoc analysis was funded by Danone Nutricia Research and performed by Pentara Corporation and Danone Nutricia Research. The corresponding author had final responsibility for the decision to submit for publication.
Acknowledgments: The authors thank all participants and their families, all members of the LipiDiDiet clinical study group, and all investigators and on-site study staff for their efforts in the conduct of the field work. We thank Tim Kelly (Medi-Kelsey Limited), funded by Danone Nutricia Research, for editorial support and language correction.
Ethical standards: The study was approved by ethics committees of all sites and done in accordance with the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines.
Conflict of interest: SBH is founder, owner and an employee of Pentara Corporation, and consults with dozens of companies in the Alzheimer’s space. She has received consulting fees through Pentara from Nutricia related to this work. HS reports grants from Kuopio University Hospital, Finland (EVO/VTR grant), and Academy of Finland grant 287490, during the conduct of the study, and personal fees from the Steering committee of the Evoke study conducted by Novo Nordisk, outside the submitted work. HS is the Lead medical PI of LipiDiDiet. AS reports a grant from the Academy of Finland (grant 287490), during the conduct of the study. PJV reports grants from the European Commission under the 7th framework program of the European Union (grant agreement number 211696), during the conduct of the study. AMJH and DSC are employees of Danone Nutricia Research. JNJ is a full time employee of Pentara Corporation, a company that consults with dozens of companies in the Alzheimer’s space, including Nutricia. SPD is a full time employee of Pentara Corporation, a company that consults with dozens of companies in the Alzheimer’s space, including Nutricia. KB served as a consultant at advisory boards for Acumen, ALZPath, BioArctic, Biogen, Eisai, Julius Clinical, Lilly, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programs for Biogen, Eisai and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. MK reports grants from the EU Joint Program BMBF 01ED1509 MIND-AD and the EU Joint Program BMBF 01ED2003 EU-FINGERS, during the conduct of the study; advisory boards for Combinostics, Biogen, and BioArtic; speaker for Biogen, Roche, Nestle, and Nutricia, and reports other grant support from CIMED, Stiftelse Stockholms Sjukhem, ALF. TH reports grants from the European Commission grant agreement number 211696, EU Joint Program BMBF 01ED1509 (MIND-AD), EU Joint Program BMBF 01ED2003 (EU-FINGERS), and Danone Nutricia Research to LipiDiDiet Consortium (month 25-96), during the conduct of the study. TH is the coördinator of LipiDiDiet.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255-2263. 10.1016/S0140-6736(15)60461-5
2. Bakre AT, Chen R, Khutan R, Wei L, Smith T, Qin G, et al. Association between fish consumption and risk of dementia: a new study from China and a systematic literature review and meta-analysis. Public Health Nutr. 2018;21:1921-1932. 10.1017/S136898001800037X
3. Zhang Y, Chen J, Qiu J, Li Y, Wang J, Jiao J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: a dose-response meta-analysis of 21 cohort studies. Am J Clin Nutr. 2016;103:330-340. 10.3945/ajcn.115.124081
4. Samieri C, Morris MC, Bennett DA, Berr C, Amouyel P, Dartigues JF, et al. Fish Intake, Genetic Predisposition to Alzheimer Disease, and Decline in Global Cognition and Memory in 5 Cohorts of Older Persons. Am J Epidemiol. 2018;187:933-940. 10.1093/aje/kwx330
5. Jiang X, Huang J, Song D, Deng R, Wei J, Zhang Z. Increased Consumption of Fruit and Vegetables Is Related to a Reduced Risk of Cognitive Impairment and Dementia: Meta-Analysis. Front Aging Neurosci. 2017;9:18. 10.3389/fnagi.2017.00018
6. Wu L, Sun D, Tan Y. Intake of Fruit and Vegetables and the Incident Risk of Cognitive Disorders: A Systematic Review and Meta-Analysis of Cohort Studies. J Nutr Health Aging. 2017;21:1284-1290. 10.1007/s12603-017-0875-6
7. Solfrizzi V, Custodero C, Lozupone M, Imbimbo BP, Valiani V, Agosti P, et al. Relationships of Dietary Patterns, Foods, and Micro- and Macronutrients with Alzheimer’s Disease and Late-Life Cognitive Disorders: A Systematic Review. Journal of Alzheimer’s disease : JAD. 2017;59:815-849. 10.3233/JAD-170248
8. Morris MC, Brockman J, Schneider JA, Wang Y, Bennett DA, Tangney CC, et al. Association of Seafood Consumption, Brain Mercury Level, and APOE epsilon4 Status With Brain Neuropathology in Older Adults. JAMA. 2016;315:489-497. 10.1001/jama.2015.19451
9. Singh B, Parsaik AK, Mielke MM, Erwin PJ, Knopman DS, Petersen RC, et al. Association of mediterranean diet with mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Journal of Alzheimer’s disease : JAD. 2014;39:271-282. 10.3233/JAD-130830
10. Wu L, Sun D. Adherence to Mediterranean diet and risk of developing cognitive disorders: An updated systematic review and meta-analysis of prospective cohort studies. Sci Rep. 2017;7:41317. 10.1038/srep41317
11. Morris MC, Tangney CC, Wang Y, Sacks FM, Barnes LL, Bennett DA, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11:1015-1022. 10.1016/j.jalz.2015.04.011
12. Thomas A, Lefevre-Arbogast S, Feart C, Foubert-Samier A, Helmer C, Catheline G, et al. Association of a MIND Diet with Brain Structure and Dementia in a French Population. The journal of prevention of Alzheimer’s disease. 2022;9:655-664. 10.14283/jpad.2022.67
13. Hershey MS, Sotos-Prieto M, Andrieu S, Hofman A, Magiatis P, Martinez-Gonzalez MA, et al. Prevention of Alzheimer’s Disease and Cognitive Decline with Diet and Lifestyle: Proceedings of the A. G. Leventis Foundation Conference. The journal of prevention of Alzheimer’s disease. 2023;10:137-143. 10.14283/jpad.2022.99
14. Radd-Vagenas S, Duffy SL, Naismith SL, Brew BJ, Flood VM, Fiatarone Singh MA. Effect of the Mediterranean diet on cognition and brain morphology and function: a systematic review of randomized controlled trials. Am J Clin Nutr. 2018;107:389-404. 10.1093/ajcn/nqx070
15. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. WHO Guidelines Approved by the Guidelines Review Committee. Geneva2019.
16. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413-446. 10.1016/S0140-6736(20)30367-6
17. Samieri C, Sonawane AR, Lefevre-Arbogast S, Helmer C, Grodstein F, Glass K. Using network science tools to identify novel diet patterns in prodromal dementia. Neurology. 2020;94:e2014-e2025. 10.1212/WNL.0000000000009399
18. D’Cunha NM, Georgousopoulou EN, Dadigamuwage L, Kellett J, Panagiotakos DB, Thomas J, et al. Effect of long-term nutraceutical and dietary supplement use on cognition in the elderly: a 10-year systematic review of randomised controlled trials. The British journal of nutrition. 2018;119:280-298. 10.1017/S0007114517003452
19. Fitzpatrick-Lewis D, Warren R, Ali MU, Sherifali D, Raina P. Treatment for mild cognitive impairment: a systematic review and meta-analysis. CMAJ Open. 2015;3:E419-427. 10.9778/cmajo.20150057
20. Forbes SC, Holroyd-Leduc JM, Poulin MJ, Hogan DB. Effect of Nutrients, Dietary Supplements and Vitamins on Cognition: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. Can Geriatr J. 2015;18:231-245. 10.5770/cgj.18.189
21. Solfrizzi V, Agosti P, Lozupone M, Custodero C, Schilardi A, Valiani V, et al. Nutritional Intervention as a Preventive Approach for Cognitive-Related Outcomes in Cognitively Healthy Older Adults: A Systematic Review. Journal of Alzheimer’s disease : JAD. 2018;64:S229-S254. 10.3233/JAD-179940
22. Berendsen AAM, Kang JH, van de Rest O, Feskens EJM, de Groot L, Grodstein F. The Dietary Approaches to Stop Hypertension Diet, Cognitive Function, and Cognitive Decline in American Older Women. J Am Med Dir Assoc. 2017;18:427-432. 10.1016/j.jamda.2016.11.026
23. Frith E, Shivappa N, Mann JR, Hebert JR, Wirth MD, Loprinzi PD. Dietary inflammatory index and memory function: population-based national sample of elderly Americans. The British journal of nutrition. 2018;119:552-558. 10.1017/S0007114517003804
24. Soininen H, Solomon A, Visser PJ, Hendrix SB, Blennow K, Kivipelto M, et al. 24-month intervention with a specific multinutrient in people with prodromal Alzheimer’s disease (LipiDiDiet): a randomised, double-blind, controlled trial. Lancet Neurol. 2017;16:965-975. 10.1016/S1474-4422(17)30332-0
25. Soininen H, Solomon A, Visser PJ, Hendrix SB, Blennow K, Kivipelto M, et al. 36-month LipiDiDiet multinutrient clinical trial in prodromal Alzheimer’s disease. Alzheimers Dement. 2021;17:29-40. 10.1002/alz.12172
26. Baumel BS, Doraiswamy PM, Sabbagh M, Wurtman R. Potential Neuroregenerative and Neuroprotective Effects of Uridine/Choline-Enriched Multinutrient Dietary Intervention for Mild Cognitive Impairment: A Narrative Review. Neurology and therapy. 2021;10:43-60. 10.1007/s40120-020-00227-y
27. Giudici KV. Nutrition-Based Approaches in Clinical Trials Targeting Cognitive Function: Highlights of the CTAD 2020. The journal of prevention of Alzheimer’s disease. 2021;8:118-122. 10.14283/jpad.2021.6
28. Jerneren F, Elshorbagy AK, Oulhaj A, Smith SM, Refsum H, Smith AD. Brain atrophy in cognitively impaired elderly: the importance of long-chain omega-3 fatty acids and B vitamin status in a randomized controlled trial. Am J Clin Nutr. 2015;102:215-221. 10.3945/ajcn.114.103283
29. Bowman GL, Dodge HH, Guyonnet S, Zhou N, Donohue J, Bichsel A, et al. A blood-based nutritional risk index explains cognitive enhancement and decline in the multidomain Alzheimer prevention trial. Alzheimer’s & dementia (New York, N Y). 2019;5:953-963. 10.1016/j.trci.2019.11.004
30. Delrieu J, Payoux P, Carrie I, Cantet C, Weiner M, Vellas B, et al. Multidomain intervention and/or omega-3 in nondemented elderly subjects according to amyloid status. Alzheimers Dement. 2019;15:1392-1401. 10.1016/j.jalz.2019.07.008
31. van Wijk N, Broersen LM, de Wilde MC, Hageman RJ, Groenendijk M, Sijben JW, et al. Targeting synaptic dysfunction in Alzheimer’s disease by administering a specific nutrient combination. Journal of Alzheimer’s disease : JAD. 2014;38:459-479. 10.3233/JAD-130998
32. Hendrix SB, Soininen H, van Hees AMJ, Ellison N, Visser PJ, Solomon A, et al. Alzheimer’s Disease Composite Score: A Post-Hoc Analysis Using Data from the LipiDiDiet Trial in Prodromal Alzheimer’s Disease. The journal of prevention of Alzheimer’s disease. 2019;6:232-236. 10.14283/jpad.2019.33
33. Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734-746. 10.1016/S1474-4422(07)70178-3
34. Wang J, Logovinsky V, Hendrix SB, Stanworth SH, Perdomo C, Xu L, et al. ADCOMS: a composite clinical outcome for prodromal Alzheimer’s disease trials. J Neurol Neurosurg Psychiatry. 2016;87:993-999. 10.1136/jnnp-2015-312383
35. O’Brien PC. Procedures for comparing samples with multiple endpoints. Biometrics. 1984;40:1079-1087
36. Vellas B, Bateman R, Blennow K, Frisoni G, Johnson K, Katz R, et al. Endpoints for Pre-Dementia AD Trials: A Report from the EU/US/CTAD Task Force. The journal of prevention of Alzheimer’s disease. 2015;2:128-135. 10.14283/jpad.2015.55
37. van Oudenhoven FM, Swinkels SHN, Hartmann T, Rizopoulos D. Modeling the underlying biological processes in Alzheimer’s disease using a multivariate competing risk joint model. Stat Med. 2022. 10.1002/sim.9425
38. Schneider LS, Goldberg TE. Composite cognitive and functional measures for early stage Alzheimer’s disease trials. Alzheimer’s & dementia (Amsterdam, Netherlands). 2020;12:e12017. 10.1002/dad2.12017
39. Langbaum JB, Ellison NN, Caputo A, Thomas RG, Langlois C, Riviere ME, et al. The Alzheimer’s Prevention Initiative Composite Cognitive Test: a practical measure for tracking cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s research & therapy. 2020;12:66. 10.1186/s13195-020-00633-2
40. Satlin A, Wang J, Logovinsky V, Berry S, Swanson C, Dhadda S, et al. Design of a Bayesian adaptive phase 2 proof-of-concept trial for BAN2401, a putative disease-modifying monoclonal antibody for the treatment of Alzheimer’s disease. Alzheimer’s & dementia (New York, N Y). 2016;2:1-12. 10.1016/j.trci.2016.01.001
41. Edgar CJ, Vradenburg G, Hassenstab J. The 2018 Revised FDA Guidance for Early Alzheimer’s Disease: Establishing the Meaningfulness of Treatment Effects. The journal of prevention of Alzheimer’s disease. 2019;6:223-227. 10.14283/jpad.2019.30
42. Olde Rikkert MG, Verhey FR, Blesa R, von Arnim CA, Bongers A, Harrison J, et al. Tolerability and safety of Souvenaid in patients with mild Alzheimer’s disease: results of multi-center, 24-week, open-label extension study. Journal of Alzheimer’s disease : JAD. 2015;44:471-480. 10.3233/JAD-141305
43. Scheltens P, Kamphuis PJ, Verhey FR, Olde Rikkert MG, Wurtman RJ, Wilkinson D, et al. Efficacy of a medical food in mild Alzheimer’s disease: A randomized, controlled trial. Alzheimers Dement. 2010;6:1-10 e11. 10.1016/j.jalz.2009.10.003
44. Scheltens P, Twisk JW, Blesa R, Scarpini E, von Arnim CA, Bongers A, et al. Efficacy of Souvenaid in mild Alzheimer’s disease: results from a randomized, controlled trial. Journal of Alzheimer’s disease : JAD. 2012;31:225-236. 10.3233/JAD-2012-121189
45. Shah RC, Kamphuis PJ, Leurgans S, Swinkels SH, Sadowsky CH, Bongers A, et al. The S-Connect study: results from a randomized, controlled trial of Souvenaid in mild-to-moderate Alzheimer’s disease. Alzheimer’s research & therapy. 2013;5:59. 10.1186/alzrt224
46. Rasmussen J. The LipiDiDiet trial: what does it add to the current evidence for Fortasyn Connect in early Alzheimer’s disease? Clinical interventions in aging. 2019;14:1481-1492. 10.2147/CIA.S211739
47. Baumel BS, Doraiswamy PM, Sabbagh M, Wurtman R. Potential Neuroregenerative and Neuroprotective Effects of Uridine/Choline-Enriched Multinutrient Dietary Intervention for Mild Cognitive Impairment: A Narrative Review. Neurology and therapy. 2020. 10.1007/s40120-020-00227-y
48. Lehtisalo J, Ngandu T, Valve P, Antikainen R, Laatikainen T, Strandberg T, et al. Nutrient intake and dietary changes during a 2-year multi-domain lifestyle intervention among older adults: secondary analysis of the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) randomised controlled trial. The British journal of nutrition. 2017;118:291-302. 10.1017/S0007114517001982
49. Andrieu S, Guyonnet S, Coley N, Cantet C, Bonnefoy M, Bordes S, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16:377-389. 10.1016/S1474-4422(17)30040-6
50. Izquierdo Delgado E, Gutierrez Rios R, Andres Calvo M, Repiso Gento I, Castrillo Sanz A, Rodriguez Herrero R, et al. Nutritional status assessment in Alzheimer disease and its influence on disease progression. Neurologia (Barcelona, Spain). 2021. 10.1016/j.nrleng.2019.11.006
© The Authors 2023