S.P. Dickson1, A.M. Wessels2, S.A. Dowsett2, C. Mallinckrodt1, J.D. Sparks2, S. Chatterjee2, S. Hendrix1
1. Pentara Corporation, Millcreek, UT, USA; 2. Eli Lilly and Company, Indianapolis, IN, USA
Corresponding Author: Alette M Wessels, Eli Lilly and Company, Indianapolis, IN, USA, E-mail: wessels_alette_maria@lilly.com, Tel: 317-276-9502
J Prev Alz Dis 2023;3(10):595-599
Published online May 3, 2023, http://dx.doi.org/10.14283/jpad.2023.50
Abstract
In Alzheimer’s disease (AD) clinical trials, disease-modifying therapies are expected to slow the rate of disease progression. Treatment effects are evaluated using a validated clinical scale as the difference between treatment and placebo in mean change from baseline to endpoint. Understanding the clinical relevance of this metric is not necessarily intuitive. Expressing active treatment-placebo difference as a time metric (i.e., months saved with treatment) has potential to provide a metric that is more easily and consistently interpreted. Using data from the TRAILBLAZER-ALZ study, time component tests (TCTs) were employed to determine the time saved with donanemab (an amyloid lowering drug) treatment. At study endpoint (Week 76), disease progression was delayed by 5.3 months and 5.2 months as measured by the Integrated Alzheimer’s Disease Rating Scale (iADRS) and the Clinical Dementia Rating Sum of Boxes (CDR-SB), respectively.
Key words: Clinical trial, Alzheimer’s disease, time component test, iADRS, slowing of disease progression.
Introduction
In the clinical study of treatments for Alzheimer’s disease (AD), there is currently a strong focus on disease-modifying treatments (DMTs) that are expected to slow the rate of clinical decline. This focus poses some analytic challenges. Based on their mechanism of action, it is optimal to commence treatment with DMTs early in disease continuum. At this stage, disease progression is often slow and effect sizes for even highly effective drugs will be small. Therefore, DMT trials typically need to have a large sample size to provide adequate power and a long follow up period to show meaningful separation between treatment groups.
In clinical trials, the treatment effect is evaluated using a validated clinical scale with results summarized as the difference between DMT and placebo groups in mean change from baseline to endpoint – that is, through assessment of the vertical difference between treatment groups (Fig 1, purple line). Translating this vertical separation, whether representing the absolute point difference between placebo and DMT or a percentage slowing in decline with DMT, into the impression of a clinical effect is not necessarily intuitive. Moreover, this assessment also creates challenges in comparing findings across outcomes and between studies where different outcome measures have been employed.
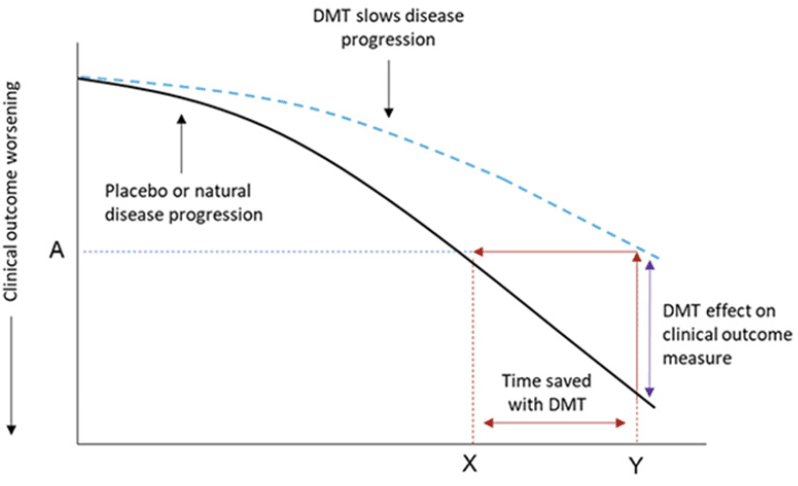
Figure 1. An illustration of a disease-modifying effect whereby treatment delays disease progression
Footnote: At time point Y, the change on the clinical outcome over time is less with a disease modifying therapy (DMT) than placebo (or natural disease progression). At this time point, the decline on the clinical outcome with DMT treatment (A) was reached at time point X with placebo. The difference between X and Y is the time saved with DMT. As an example, a 25% reduction in the progression on an outcome measure with a DMT is equivalent to delaying disease progress by 3 months over 12 months of treatment.
With recent developments in clinical and statistical thinking, there may be benefits in translating a point change difference into a time component (1, 2). With this conversion, focus is shifted from an absolute point difference on a clinical outcome scale at endpoint to the point difference between DMT and control in the time to reach a specified degree of worsening (e.g., change from baseline with placebo at study endpoint or the threshold for clinically meaningful worsening on that outcome measure) or time saved with treatment. This can be measured as the horizontal difference between treatment groups (Fig 1, red double headed arrow line). This time saved approach aligns with the slowing of disease progression with a DMT in a continuously accumulating disease. Of note, for treatments generally thought to have primarily symptomatic benefit, related approaches have been used previously (3, 4) but they have usually been based on time-to-event analysis.
A ‘time saved’ approach has potential for providing a metric that is more easily and consistently interpreted because it is a direct measure of the time by which decline of a patient’s current functional abilities is delayed, thereby potentially extending the time patients remain independent and engaged in daily activities.
Assessment of the time component also facilitates comparison of findings across outcomes (both within and between trials) since converting to a time component places different aspects of disease pathology on a common measurement scale, time.
The aim of the current study was to apply time component tests (TCTs) to the Integrated Alzheimer’s Disease Rating Scale (iADRS) and Clinical Dementia Rating Sum of Boxes (CDR-SB) data from TRAILBLAZER-ALZ, a Phase 2 study of donanemab (a DMT, specifically an amyloid lowering therapy) (5), to ascertain how the 32% slowing of disease progression seen with donanemab treatment over 76 weeks translates to time saved.
Methods
TCT methodology
The TCT can be used to assess treatment effect when the treatment is expected to be disease modifying and is applicable only when the placebo group exhibits decline.
The TCT uses the least squares mean estimates from a primary analysis, such as mixed models with repeated measures (MMRM) over time, rather than individual participant data. Instead of focusing on absolute differences between mean changes in units of the outcome scale, the TCT aligns the least squares means from a primary analysis in the DMT group with the means in the placebo group at earlier time points using linear interpolation to assess the horizontal distance between treatments. For example, if the iADRS change from baseline at 76 weeks is -8 for the DMT group and for the placebo group this iADRS change from baseline of -8 occurs at 52 weeks then the time saving with the DMT is 24 weeks. The resulting summary measure of the treatment effect is in units of time – the amount saved with treatment. The standard error of the estimate is also converted to the time scale. TCTs can be applied to individual outcome measures and also combined to provide integrated evidence of efficacy across outcomes (6). In the latter case, the TCT methodology is used for all outcomes individually, and then estimates are combined accounting for the correlation between each outcome included in the TCT.
TRAILBLAZER-ALZ
TRAILBLAZER-ALZ (5) was a multicenter, randomized, double-blind, placebo-controlled Phase 2 trial designed to assess the safety and efficacy of donanemab in patients with early symptomatic AD. The primary outcome of the study was the iADRS (7, 8), an integrated assessment of cognition and daily function comprised of items from the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog13) (9, 10) (and the Alzheimer’s Disease Cooperative Study- Activities of Daily Living (ADCS-iADL) (11, 12), measuring the AD core domains across its continuum. In the pre-specified primary MMRM analysis, the mean change from baseline to endpoint (76 weeks) in the iADRS score was −10.06 in the placebo group and −6.86 in the donanemab group (p=0.04); the 3.2-point difference (Fig 1, purple line) translates to a 32% slowing of disease progression with donanemab. The first key secondary outcome of TRAILBLAZER-ALZ was the CDR-SB (13); the change from baseline at 76 weeks was 1.58 in the placebo group and 1.22 in the donanemab group; the 0.36 point difference (Fig 1, purple line) translates to a 23% slowing with DMT (difference not statistically significant).
Using the TRAILBLAZER-ALZ dataset, the TCT was applied to both the iADRS and the CDR-SB individually, and also with outcomes combined.
Results
Figures 2a and 2b (Part I) show the least squares means (+/- standard error) by treatment over time for the iADRS, and the CDR-SB, respectively, based on pre-specified MMRM analyses. At study endpoint (Week 76), disease progression was delayed by 5.3 months and 5.2 months as measured by the iADRS and CDR-SB, respectively. Figures 2a and b (Part II) show time saved in the donanemab treatment group relative to placebo at various time points during the study. Based on results from the MMRM analyses of the two endpoints combined, disease progression was delayed by 5.2 months (Figure 2c). The baseline to endpoint findings for the iADRS, CDR-SB individually and combined are summarized in Figure 2d.
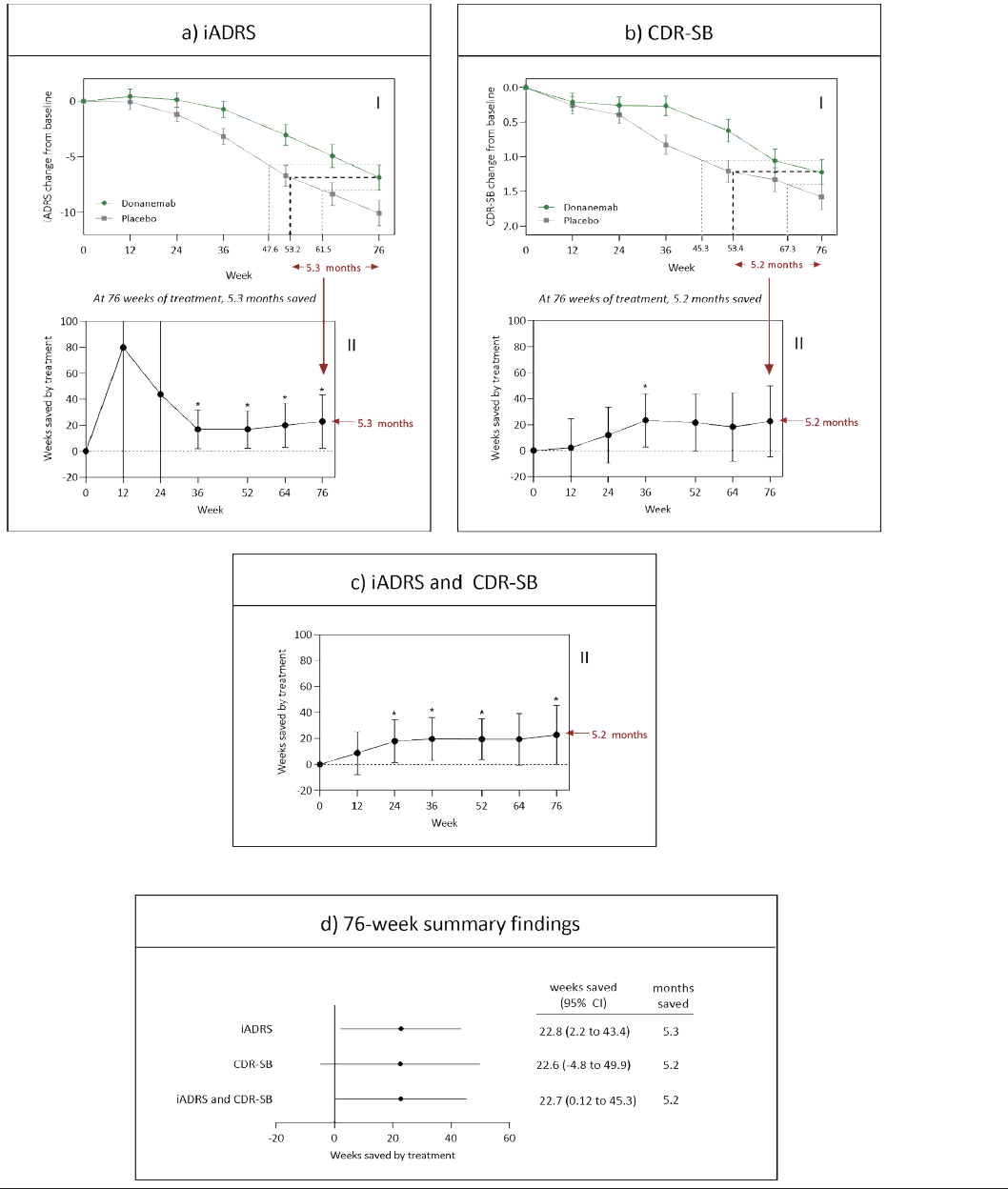
Figure 2. Findings from time component tests (TCTs) applied to a) the iADRS, b) the CDR-SB and c) the iADRS and CDR-SB combined data from TRAILBLAZER-ALZ. Part I shows the absolute point change from baseline on the scale and the weeks saved at 76 weeks of treatment; Part II shows the weeks saved over time. d) summarizes the 76-week findings for the iADRS, CDR-SB, and iADRS and CDR-SB combined
Footnote: For a) to c), Part I shows mean +/- standard error; Part II shows mean and 95% confidence interval; Abbreviations: CDR-SB, Clinical Dementia Rating Sum of Boxes; CI, confidence interval; iADRS, Integrated Alzheimer’s Disease Rating Scale; mo, month; SE, standard error
Discussion
Starting a DMT early in the disease process and assessing a meaningful effect during the relatively brief (e.g., 18-month) clinical trial period is essential in understanding the value of a treatment (2). The standard analysis approach focuses on the difference between treatments in mean change from baseline to endpoint on a validated scale, but the findings expressed this way are open to misinterpretation. For example, a small difference in absolute point change between DMT and placebo groups on a scale with a large range may be viewed as a minimal or modest effect of the DMT. Yet, with a deeper understanding of the scale, it will be evident that this is not necessarily the case. The difference in point change between placebo and DMT should be considered in the context of the dynamic range (i.e., expected change over time for a specific population), which is generally not the full score range (14, 15). In addition, it is often incorrectly assumed that the point difference between DMT and placebo groups at a specified time point (between group difference) should be assessed against the minimal clinically meaningful change (also termed minimal clinically important meaningful difference [MCID]). The MCID is the within-person point change associated with notable impact on the individual/care partner well-being (i.e., not a between-group difference) (14).
Some of the misunderstanding around difference in absolute point change between treatment groups can be addressed by expressing the study finding as a percent slowing (i.e., mean change from baseline to endpoint on a clinical scale with DMT as a percent of that seen with placebo) (14), although interpretation may still not be straightforward. Moreover, these clinical scale findings expressed at study endpoint fail to account for any nonlinearity in disease progression and treatment response.
We can facilitate a broader understanding of clinical trial findings by converting scale findings to the well-understood metric of time and expressing treatment effects as time saved compared to placebo. This metric may be more closely associated with what patients and their care partners want to know about AD – that is, how long treatment will maintain their current lifestyle and delay transition to a more advanced stage of disease with less independence and increased burden on the care partner (16). An additional benefit of the TCT is that it facilitates comparisons across studies where different outcome measures have been employed, with the caveat that one need to be cognizant of other trial differences (e.g., patient population, time of treatment). Finally, unlike percent slowing measured as a difference in change from baseline on an outcome scale, time component testing allows for non-linearity in disease progression and treatment.
In the current study, we applied straightforward TCT methodology to the TRAILBLAZER-ALZ data, specifically the iADRS and CDR-SB data, to determine the time saved was similar across the 2 measures (5.3 months [p<0.05] and 5.2 [not statistically significant] months as measured by the iADRS and CDR-SB, respectively). This time saving aligns with the difference in percent slowing with the iADRS (32%, p=0.04) versus the CDR-SB ((23%, not statistically significant) (5). The difference in statistical significance of the findings between CDR-SB (not significant) and iADRS (statistically significant) is likely because iADRS is a more robust outcome than CDR-SB in that the score is not as vulnerable to random noise (14).
In addition to the TCT that is based on group level results, TCTs can be formulated for individual participant data and then aggregated into group-level summaries. Using TRAILBLAZER-ALZ data, with the individual participant data TCTs, 4-5 months were saved with donanemab treatment (findings presented at the 15th Clinical Trials on Alzheimer’s Disease conference, 2022 (17)).
While the individual outcomes provide valuable information on treatment effect, combining TCTs across outcomes can create a more robust and accurate summary of time saved; as shown in Figure 2, less variability was seen in combining TCTs for the iADRS and CDR-SB. Of note, this is not combining iADRS and CDR-SB as a composite score but instead the estimates measuring the time components of each outcome measure are combined.
Given the novelty of the TCT when used to assess DMT effect, it is not surprising that for TCTs based on individual participant data several analytic approaches are being considered. For example, Raket recently used progression models for repeated measures (PMRM) to similarly estimate treatment effects as slowing of progression (i.e., time metric) (1). Using time-based PMRM methodology with the TRAILBLAZER-ALZ dataset resulted in similar findings to those presented in this paper (5.4-5.8 months saved) (data on file). A further TCT based on individual participant data was presented recently (18). With the individual participant approach, it is also possible to categorize each participant’s response based on whether a clinically meaningful threshold has been achieved. This approach is an area of ongoing research.
In conclusion, providing information on drug effect as a time metric (i.e., months saved with treatment) is of value to the clinician, patient and care partner. It is easier to understand and more clinically applicable than difference in absolute or percent point change on a clinical scale. It also allows for more valid comparisons across studies.
Funding: The TRAILBLAZER-ALZ study was funded by Eli Lilly and Company. Several authors (AMW, SA, JDS, SC) are full time employees at Eli Lilly and Company.
Ethical standards: TRAILBLAZER ALZ was conducted in accordance with the consensus ethics principles derived from international ethics guidelines, including the Declaration of Helsinki and the Council for International Organizations of Medical Sciences International Ethical Guidelines; see original publication for details (5).
Conflict of interest: Dickson is a full-time employee of Pentara Corporation (Pentara consults with and receives payment from clients working in the neurodegenerative space, particularly in Alzheimer’s disease [including Eli Lilly and Company]; Pentara was contracted to perform the statistical analyses used in this manuscript); Hendrix is the sole owner of Pentara Corporation; Mallinckrodt is a full-time employee of Pentara Corporation, and a minor stockholder in Eli Lilly and other pharma companies through index mutual funds; Wessels, Dowsett, Sparks, Chatterjee are full time employees and minor stockholders at Eli Lilly and Company.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Raket LL. Progression models for repeated measures: Estimating novel treatment effects in progressive diseases. Statistics in Medicine 2022;41:5537-5557.
2. Petersen RC, Aisen PS, Andrews JS, et al. Expectations and clinical meaningfulness of randomized controlled trials. Alzheimer’s & Dementia 2023.
3. Mohs RC, Doody R, Morris J, et al. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology 2001;57:481-488.
4. Stern Y, Tang M-X, Albert MS, et al. Predicting time to nursing home care and death in individuals with Alzheimer disease. Jama 1997;277:806-812.
5. Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer’s disease. N Engl J Med 2021;384:1691-1704. DOI: doi: 10.1056/NEJMoa2100708.
6. Hendrix S, Knowlton N, Hogan N, Nicodemus-Johnson J, Dickson SP. Identifying Disease-Modifying Treatments in Progressive Diseases Through Delayed Failure Time Models and Delayed Longitudinal Progression Models. Presented at The Joint Statistical Meeting 2022. Availabel at: https://ww2.amstat.org/meetings/jsm/2022/onlineprogram/AbstractDetails.cfm?abstractid=320684. Last accessed April 2023.
7. Wessels A, Siemers E, Yu P, et al. A combined measure of cognition and function for clinical trials: the integrated Alzheimer’s disease rating scale (iADRS). J Prevent Alzheimers Dis 2015;2:227-241. DOI: 10.14283/jpad.2015.82.
8. Liu-Seifert H, Andersen S, Case M, et al. Statistical properties of continuous composite scales and implications for drug development. J Biopharm Stat 2017;27:1104-1114. DOI: 10.1080/10543406.2017.1315819.
9. Mohs RC, Knopman D, Petersen RC, et al. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. Alzheimer Disease & Associated Disorders 1997;11:S13-21.
10. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry 1984;141:1356-1364. DOI: 10.1176/ajp.141.11.1356.
11. Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. Alzheimer Dis Assoc Disord 1997:S33-39.
12. Galasko D, Kershaw PR, Schneider L, Zhu Y, Tariot PN. Galantamine maintains ability to perform activities of daily living in patients with Alzheimer’s disease. J Am Geriatr Soc 2004;52:1070-1076. DOI: 10.1111/j.1532-5415.2004.52303.x.
13. Hughes CP, Berg L, Danziger WL, Coben LA, Martin R. A new clinical scale for the staging of dementia. The British journal of psychiatry 1982;140:566-572.
14. Wessels AM, Dennehy EB, Dowsett SA, Dickson SP, Hendrix SB. Meaningful Clinical Changes in Alzheimer Disease Measured With the iADRS and Illustrated Using the Donanemab TRAILBLAZER-ALZ Study Findings. Neurology: Clinical Practice 2023;13.
15. Mintun M, Wessels A, Sims J. Donanemab in early Alzheimer’s disease: Author reply to letter (author, Espay AJ). The New England journal of medicine 2021;385:666-667.
16. DiBenedetti DB, Slota C, Wronski SL, et al. Assessing what matters most to patients with or at risk for Alzheimer’s and care partners: a qualitative study evaluating symptoms, impacts, and outcomes. Alzheimer’s research & therapy 2020;12:1-15.
17. Dickson S, Hendrix S. The time component test is inherently meaningful because it combines evidence across outcomes to measure the impact of treatment on progression rate in degenerative diseases. J Prev Alzheimer’s Dis 2022;9:P-23.
18. Hogan N, Johnson J, Knowlton N, et al. The Inherent Meaningfulness of a Novel Time Component Test (TCT): Combining Evidence Across Outcomes to Measure the Impact of Treatment on Progression Rate in Degenerative Diseases. Presented at The Joint Statistical Meeting 2022. Availabel at: https://ww2.amstat.org/meetings/jsm/2022/onlineprogram/AbstractDetails.cfm?abstractid=323630. Last accessed April 2023
© The Authors 2023
