S. Gauthier1, Z. Ismail2, Z. Goodarzi3, K.P. Ng4, P. Rosa-Neto1
1. Department of Neurology and Neurosurgery, McGill University Research Center for Studies in Aging, Montreal, Canada; 2. Departments of Psychiatry, Clinical Neurosciences, and Community Health Sciences; Hotchkiss Brain Institute and O’Brien Institute for Public Health; University of Calgary, Calgary, Canada;
3. Departments of Medicine and Community Health Sciences, Hotchkiss Brain Institute and O’Brien Institute for Public Health, University of Calgary, Calgary, Canada; 4. Department of Neurology, National Neuroscience Institute, Singapore, Singapore
Corresponding Author: Serge Gauthier, Translational Neuroimaging Laboratory, The McGill University Research Centre for Studies in Aging, 6875 La Salle Blvd – FBC room 3149, Montreal, QC, Canada H4H 1R3, serge.gauthier@mcgill.ca
J Prev Alz Dis 2023;3(10):339-341
Published online June 13, 2023, http://dx.doi.org/10.14283/jpad.2023.72
Abstract
Clinicians specialized in the diagnosis and management of persons living with early-stage Alzheimer’s disease need to enable access, for those meeting criteria, to the new class of disease modifying drugs (DMDs). These drugs act on amyloid β42 and delay progression of symptoms. Thus, there will be interest from patients and families. Over the short term, the use of antibodies administered intravenously with serial MRIs to detect amyloid-related imaging abnormalities (ARIA) may require participation in structured phase 4 studies or in registries with third party funding for support staff and MRI scans. In the mid term, the availability of oral anti-amyloid therapy, likely with lower risk of ARIA, may transform clinical practice to a model of screening suitable patients using plasma biomarkers, with a subsequent rapid referral to a specialized memory clinic. Eventually, the biological profile of patients for amyloid, tau, and inflammation will determine which type of DMD to use. We are optimistic that clinicians will gain confidence with the use DMDs and answer the increasing needs of our aging population.
Key words: Alzheimer disease, disease-modifying treatments, patients selection, health care resources.
Introduction
Alzheimer’s Although human anti-amyloid monoclonal antibodies such as aducanumab and lecanemab are not currently approved by regulatory authorities outside the USA, clinicians specialized in the diagnosis and treatment of persons living with Mild Cognitive Impairment (MCI) or mild dementia due to Alzheimer’s disease (AD) are thinking about how best to use this new class of disease modifying drugs (DMDs) in their practice.
It is still early days to formulate Appropriate Use Recommendations (AURs) that take into account local/regional/national resources, and we thank the ADRD Therapeutics Work Group for publishing the first AUR for use of aducanumab and lecanemab in the USA as a starting point (1, 2). Special thanks to Dr Jeff Cummings who shared slides made from his latest publication (2) that are used in the current document as Tables 1 and 2.
In this short perspective we take the point of view of clinicians in specialty practice, e.g., neurologists, geriatric psychiatrists, and geriatricians, who will be asked by front-line clinicians if a DMD is appropriate for individual patients.
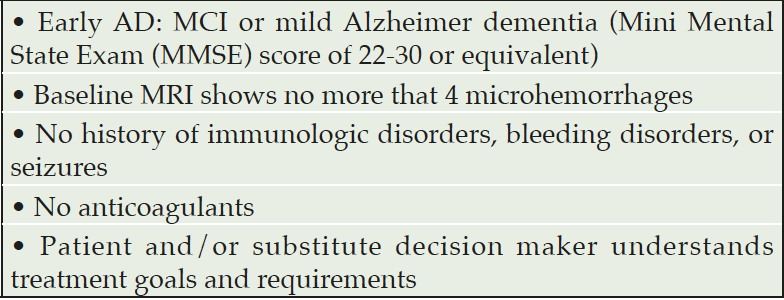
Table 1. Appropriate patient characteristics for use of a DMD such as lecanemab to refer to a specialty clinic (2)
Mise-en-contexte
Drugs acting on amyloid β42 that have completed phase 3 randomized clinical trials (RCTs) include aducanumab (3), lecanemab (4) and donanemab (5). These agents are administered intravenously once or twice a month, for 18 months in these trials. Monitoring for amyloid-related imaging abnormalities (ARIA) requires serial brain MRI. Clinical efficacy in the RCTs was measured through the Clinical Dementia Rating Scale, Sum of Boxes (CDR-SB) as the primary or secondary outcome (6): published results with lecanemab showed a difference of 0.45 on a scale of 18 points (4). Questions that clinicians, persons living with early AD, and their care partners are asking is whether the clinical benefits outweigh the risks of ARIA, what are the logistics and burden of monitoring for the infusions and brain imaging, and the costs of treatment (7). The answers to these questions will also be determined in the context of system readiness for this new model of care.
The use of amyloid-specific DMDs requires an etiologic diagnosis for MCI and mild dementia, confirmed using biomarkers. Until blood biomarkers such as amyloid β40, amyloid β42, pTau-181 and pTau-217 are fully validated to determine amyloid and tau positivity, respectively (8), clinicians must use cerebrospinal fluid (CSF) analysis and/or positron emission tomography (PET) imaging, which are more invasive and costly (9).
Who should be referred for a specialty assessment for suitability of DMD use?
The current DMDs appear to be most effective in the early stages of AD, hence the need for an accelerated referral process to specialty clinics who can initiate a DMD and monitor safety and efficacy for at least the first year. It will be important for clinicians in primary care to perform the initial clinical and laboratory diagnostic work-up for MCI or mild dementia and complete a pre-screen of suitability for the current anti-amyloid drugs, which should include a brain MRI read by a radiologist trained in the detection of microhemorrhages (thus, persons with pacemakers are excluded) (Table 1). In the near future, plasma pTau isoforms specific for AD will be available for biological screening of amyloid and tau positivity, which will simplify and speed up the biomarker confirmation of AD. Again, system readiness subsequent steps will be an important issue.
Clinicians in primary care will remain responsible for the management of comorbidities including deprescription of drugs with a high anticholinergic load, initiation of cholinesterase inhibitors (which should not prevent referral to access DMD), offering education about the disease stages of AD, and referral to community resources (10). The concept of disease modification will require a sustained education effort starting in professional schools and through continuous medical education.
How does the specialist without resources for the safe use of DMDs handle someone who may be suitable for a DMD?
Many resources are needed for optimal care of persons on anti-amyloid drugs. For lecanemab the list can be overwhelming, outside of a well-established clinical trial site (table 2).
Currently, health care systems across Canada are underprepared for these medications. Thus, drug use requirements may necessitate participation in a structured phase 4 study or enrollment in a registry with third party funding for support staff and MRI until oral agents such as ALZ-801 successfully complete phase 3 testing and regulatory review (11).
Thus, for most specialists there will be a need to refer to specific sites with resources to support the treatment when DMDs are initiated and monitored, at least for the near future. This referral should be fast-tracked with pre-agreed requirements for second-level review of inclusion and exclusion criteria. The receiving specialists must also be responsive to the needs of persons with MCI or mild dementia not associated with AD, or those who are excluded from DMD use because of comorbidities.
How can specialists offering DMD treatment cope with the load of persons on DMDs?
Depending on human (nurses and other allied health professionals) and technical (MRI, infusion site) resources, multiple persons can be treated simultaneously. However, clinicians will face the issue of stopping treatment at some point. The current AURs offer guidance about treatment cessation in case of ARIA and related clinical complications but are still vague about what to do once the stage of moderate dementia has been reached. Some commentaries are emerging suggesting the use of CDR global stage as an objective framework for clinical decline (12), and the concept of ‘time saved’ may offer a clearer understanding of the clinical benefit of DMD (13). More information will be forthcoming as third-party payers review the DMD dossiers and as clinicians gain experience with a broader range of patients than were enrolled in the phase 3 trials. National and regions such as Australasia are already looking at resources required to facilitate access to DMD (14).
Conclusions
Despite the high technical burden to administer the first anti-amyloid drugs, we should be optimistic that these agents represent the first step towards a personalized treatment for persons with early-stage AD. Using a patient’s biological profile of amyloid, tau, and inflammation, clinicians will be able to determine the type of DMD to use. Education of patients and of healthcare professionals is a sine qua non condition for the therapeutic paradigm shift from symptomatic treatment to disease modification. There are questions yet to be answered about the use of DMDs, especially on how to best monitor for ARIA, when to stop treatment, and the health care system implications of these medications. Nonetheless, clinicians of all disciplines who provide care for persons living with MCI and dementia will gain experience with, and confidence in the use of DMDs, and will be able to answer the increasing needs of the aging population.
Conflicts of interest: All potential conflicts of interest have been submitted to the editor and none are considered as interfering with the opinions expressed in this document.
References
1. Cummings J, Aisen P, Apostolova LG, Atri A, Salloway S, Weiner M. Aducanemab; Appropriate Use Recommendations. J Prev Alz Dis 2021;4(8):398-410. doi: 10.14283/jpad.2021.41.
2. Cummings J, Apostolova L, Rabinovici GD, Atri A, Aisen P, Greenberg S et al. Lecanemab: Appropriate Use Recommendations. J Prev Alz Dis 2023;3(10):362-377. Doi:10.14283/jpad.2023.30.
3. Budd Haeberlein SB, Aisen PS, Barkhof F, Chalkias S, Chen T, Cohen S et al. Two randomized Phase 3 studies of aducanumab in early Alzheimer’s disease. J Prev Alz Dis 2022;2(9):197-210. Doi: 10.14283/jpad.2022.30.
4. Van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M et al. Lecanemab in early Alzheimer’s disease. N Eng J Med 2022. Doi: 10.1056/NUJMoa2212948.
5. Mintun MA, Lo AC, Evans CD, Wessels AM, Ardayfio PA, Andersen SW et al. Donanemab in early Alzheimer’s disease. N Eng J Med 2021: 384:1691-704. Doi:10.1056/NEJMoa2100708.
6. Morris JC. The Clinical Dementia Rating Scale (CDR): current version and scoring rules. Neurology 1993;43:2412-4.
7. Walsh S, Merrick R, Richard E, Nurock S, Brayne C. Lecanemab for Alzheimer’s disease. BMJ 2022;379:o3010. Doi: 10.1136/bmj.o3010.
8. Therriault J, Vermeiren M, Servaes S, Tissot C, Ashton NJ, Benedet AL et al. Association of phosphorylated tau biomarkers with amyloid Positron Emission Tomography vs tau Positron Emission Tomography. JAMA Neurology 2023;80:188-199. Doi:10.1001/jamaneurology.2022.4485.
9. Frölich L, Jessen F. Lecanemab: Appropriate Use Recommendations – a commentary from a European perspective. J Prev Alz Dis 2023;3(10):357-358. Doi: 10.14283/jpas.2023.44.
10. Gauthier S, Webster C, Servaes S, Morais JA, Rosa-Neto P. World Alzheimer Report 2022. Life after diagnosis: navigating treatment, care and support. London, England. Alzheimer’s Disease International. Alzint.org.
11. Tolar M, Abushakra S, Sabbagh M. The paty forward in Alzheimer’s disease therapeutics: reevaluating the amyloid cascade hypothesis. Alzheimer’s Dement 2020;16:1553-1560. Doi: 10.1016/j.jalz.2019.09.075
12. Gauthier S, Therriault J, Rosa-Neto P. Clinical efficacy in individual patients treated with Lecanemab. J Prev Alz Dis 2023;3(10):378-379. Doi: 10.14283/jpad.2023.58.
13. Dickson SP, Wessels AM, Dowsett SA, Mallinkrodt C, Sparks JD, Chatterjee S, Hendrix S. ‘Time saved’ as a demonstration of clinical meaningfulness and illustrated using the donanemab TRAILBLAZER-ALZ study findings. J Prev Alz Dis 2023;3(10):595-599. Doi: 10.14283/jpad.2023.50.
14. Brodtmann A, Darby D, Oboudiyat C, Mahoney CJ, Panegyres PK, Brew B. Assessing preparedness for Alzheimer disease-modifying therapies in Australasian health care systems. MJA 2023; 218(6) 3 April 2023. Doi:10.5694/mja2.51880.
© Serdi 2023

