S.M. Pruntel1, B.C. van Munster2, J.J. de Vries3, A. Vissink1, A. Visser1,4,5
1. Department of Oral and Maxillofacial Surgery, University Medical Center Groningen and University of Groningen, Groningen The Netherlands; 2. Department of Internal Medicine, University Medical Center Groningen and University of Groningen, Groningen, The Netherlands; 3. Department of Neurology, University Medical Center Groningen and University of Groningen, Groningen, The Netherlands; 4. Department of Gerodontology, Center for Dentistry and Oral Hygiene, University Medical Center Groningen and University of Groningen, Groningen, The Netherlands; 5. Department of Gerodontology, College of Dental Sciences, Radboud University Nijmegen Medical Center, Nijmegen, The Netherlands
Corresponding Author: Anita Visser, Department of Gerodontology, Center for Dentistry and Oral Hygiene, University Medical Center Groningen and University of Groningen, Antonius Deusinglaan 1, Groningen, Groningen, 9713 AV, The Netherlands, Tel: 050 361 3840, E-mail: a.visser@umcg.nl
J Prev Alz Dis 2024;1(11):249-258
Published online June 28, 2023, http://dx.doi.org/10.14283/jpad.2023.82
Abstract
In patients with Alzheimer’s disease pathophysiological changes of the brain that initiate the onset of Alzheimer’s disease include accumulation of amyloid-β plaques and phosphorylation of tau-tangles. A rather recently considered risk factor for the onset of Alzheimer’s disease is poor oral health. The aim of this systematic review of the literature was to assess the potential association(s) of oral health as a risk factor for the onset of Alzheimer’s disease. After a systematic search of Pubmed, Embase and Web of Science. A total of 1962 studies were assessed, of which 17 studies demonstrated possible associations between oral health diseases and Alzheimer’s disease. 4 theories could be distinguished that describe the possible links between oral health and the development or onset of Alzheimer’s disease; 1) role of pathogens, 2) role of inflammatory mediators, 3) role of APOE alleles and 4) role of Aβ peptide. The main common denominator of all the theories is the neuroinflammation due to poor oral health. Yet, there is insufficient evidence to prove a link due to the diversity of the designs used and the quality of the study design of the included studies. Therefore, further research is needed to find causal links between oral health and neuroinflammation that possibly can lead to the onset of Alzheimer’s disease with the future intention to prevent cognitive decline by better dental care.
Key words: Alzheimer’s disease, oral health, cognitive decline, periodontitis.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease and the most common cause of dementia. AD accounts for 50–75% of all causes of dementia (1–4). Dementia is the fastest-growing cause of death in the Netherlands (5) and considered as the disease with the most negative impact on quality of life of patients and their caregivers and high costs. Worldwide the total costs for dementia care are around 10 billion euros annually (5). Multiple clinical manifestations can be seen in patients with AD including severe memory loss, problems with spatial recognition, and impaired reasoning or judgment (6–8). The course of AD is slow and irreversible, and finally leads to death (9, 10). In literature a diversity of physiological changes in the brain has been described and several risk factors for the onset of AD have been discovered, however the cause of AD and the precise pathway to it’s onset are still not fully known (5, 11).
Pathophysiological changes of the brain of AD patients include accumulation of amyloid-β plaques (Aβ) and phosphorylation of tau-tangles (12–14). These changes are considered to be the most important pathological causes for AD as they show a specific spatial and temporal dispersion resulting in degeneration of the affected cerebral cortices (13, 15). Other pathological processes, such as cerebrovascular abnormalities, inflammation and oxidative stress, have also been shown as contributors to AD pathogenesis in imaging and obduction studies (16). It is believed that the buildup and deposition of Aβ in the brain occurs early in the course of the disease and sets off subsequent processes such as tau phosphorylation, inflammation, and oxidative stress that all result in neurodegeneration (17). Several risk factors for AD are known such as an unhealthy life style, malnutrition, brain injury, low education attainment, depression, social isolation, cognitive inactivity and air pollution (18–21).
A rather recently considered risk factor for the onset of AD is poor oral health (22–24). It has been reported that poor oral health is often seen in patients with AD (23, 24). This phenomenon might be the result of cognitive decline which can affect oral hygiene routines and dental visits (25, 26), but it has also been suggested that oral health illness, in particular periodontitis, is a risk factor for a exacerbation of the neuroinflammation that, e.g., could result in a chronically elevated proinflammatory status. This status could lead to neurodegeneration through various pathways (27–29). It already has been shown that a systemic effect of oral illness on general health may be caused by the invasion of oral pathogens into the airway (e.g., due to dysphagia) and or the bloodstream (e.g., via the periodontal tissues) (30). Given the impact of AD on patients, their caretakers and society, it is important to prevent the onset of AD where ever possible. Oral health in particular periodontal disease is preventable and treatable. Therefore, the aim of this systematic review of the literature was to assess the potential association(s) of oral health as a risk factor for the onset of AD.
Methods
This systematic review is reported according to the PRISMA statement to ensure quality and completeness. This study’s protocol was registered in PROSPERO prior to the systematic literature search (registration number CRD42023402291) (31).
Search strategy
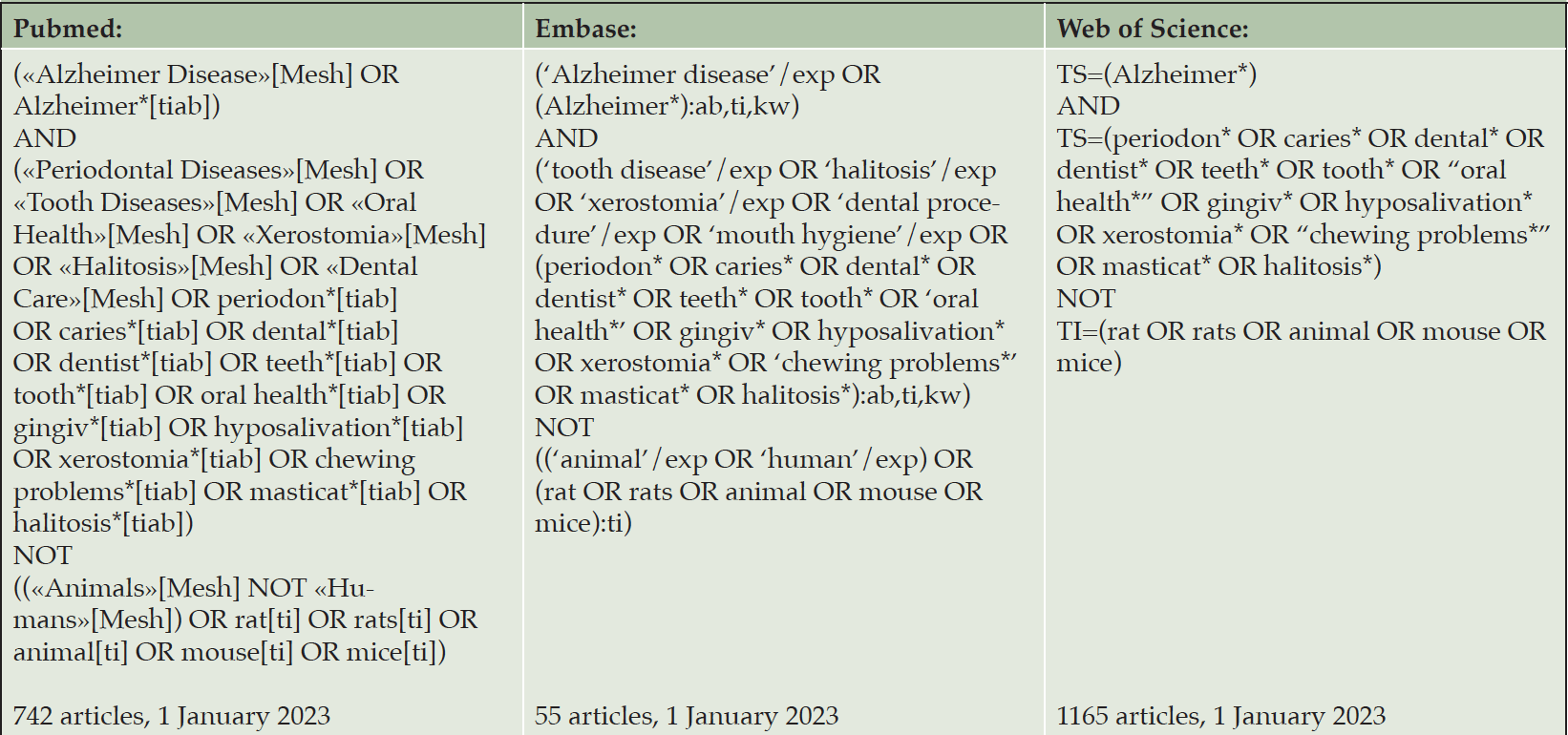
Pubmed, Embase and Web of Science databases were searched. Search terms were: Alzheimer’s disease, periodontal disease, caries, dental infections, mobile teeth, loss of teeth, periodontitis, gingivitis, dental health, oral health, hyposalivation, xerostomia, oral pain, broken teeth, dry mouth, and chewing problems. For the complete search strategy see Table 1. The literature search was searched on 01-01-2023. The sensitive search strategy was developed together with an information specialist from the university library (Karin Sijtsma) and adapted appropriately to each database.
Study selection
Studies were included when they reported on the potential association of oral health as a risk factor for the onset of AD. Excluded were articles reporting on animals, cellular level, case reports, review articles, letters to the editors and trial designs. There were no exclusions based on study design or language.
First, duplicates found in Pubmed, Embase and Web of Science were removed. Titles and abstracts were screened for selection by two observers independently (SP and AnV). Articles with a title that had insufficient information and/or a lacking abstract were screened by full text assessment. If an abstract provided insufficient information or disagreement existed between observers, the full text was also checked. If no full text was available or if parts of the study were unclear, the authors of the study were contacted for additional information. The reference sections of all included and review articles in the full text phase were scrutinized for additional articles. Librarians in the university were able to provide translations when needed.
Quality assessment
The quality of the studies was analyzed by SP and AnV based on the Methodological Index for Non-Randomized Studies (MINORS) (32). 32 contains 12 methodological points, the first eight apply to both non-comparative and comparative studies, while the remaining four relate only to studies with two or more groups. The items were scored 0 if not reported; 1 when reported but inadequate; and 2 when reported and adequate. The ideal score is 16 for non-comparative studies and 24 for comparative studies. MINORS is a valid instrument designed to assess the methodological quality of non-randomized studies, whether comparative or non-comparative.
Results
Search result
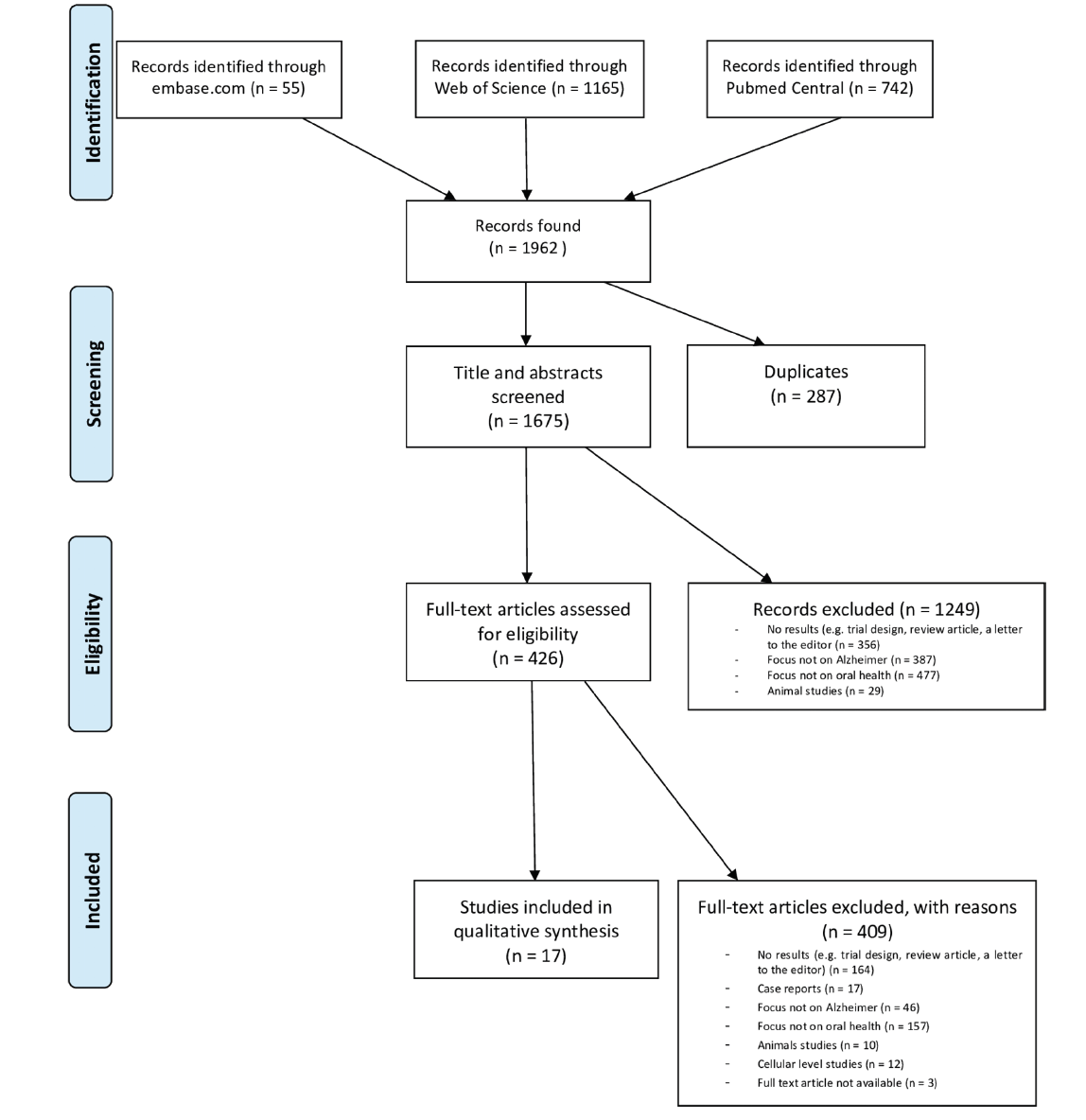
The database search resulted in 1962 hits. After removing duplicates, 1675 unique papers remained (shown in Fig. 1). After title and abstract screening 426 articles remained of which 409 were excluded after full text reading. Hence 17 studies could be included for quality / risk of bias assessment and data extraction.
Study characteristics
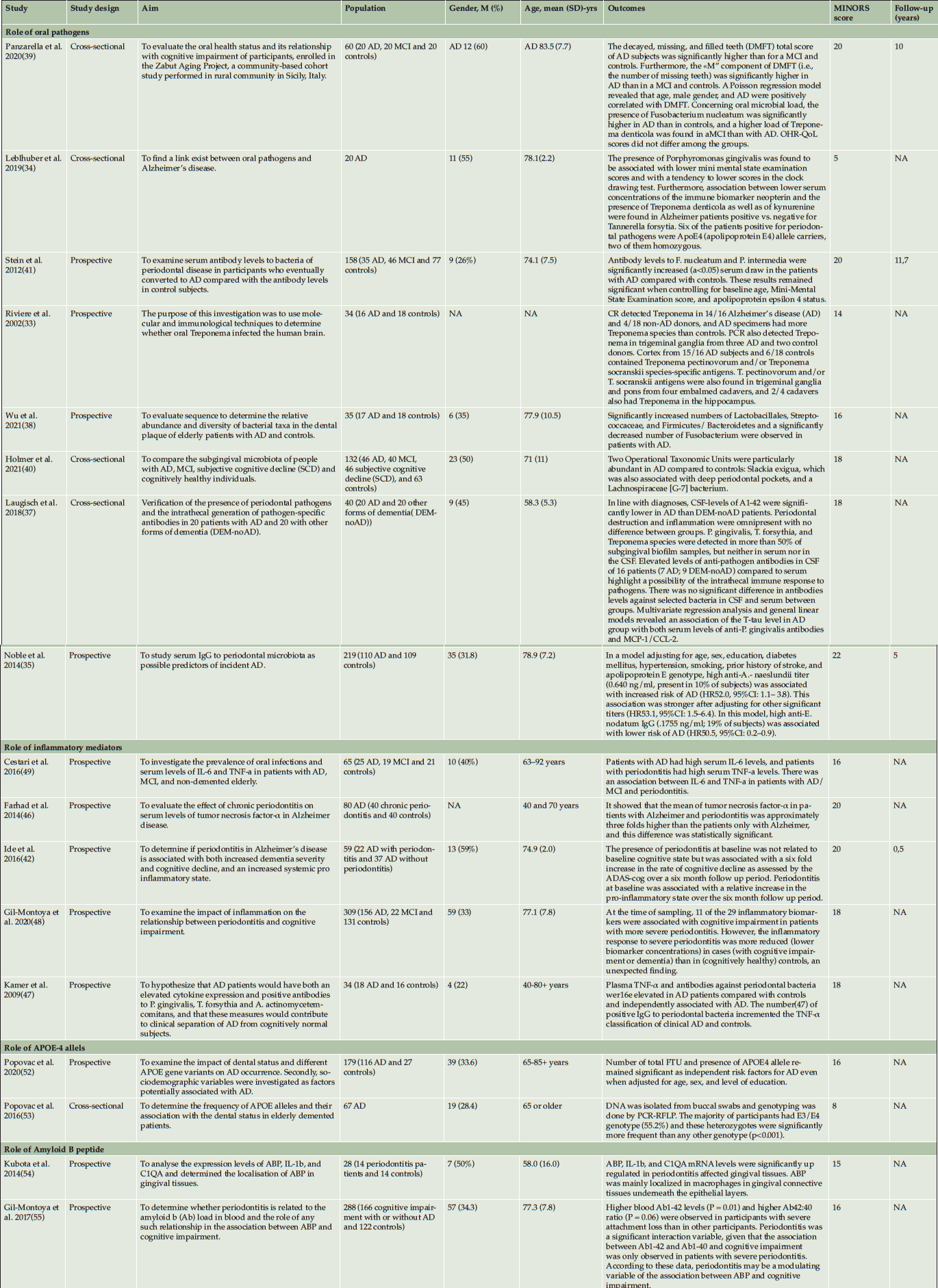
All included papers could be characterized as prospective and or cross sectional studies describing different aspects of oral health as potential factors for the development of AD (table 2). As shown in table 2, there was considerable clinical heterogeneity in sample size, age and design. The number of included patients in the studies varied from 14 to 309 with a total of 1807 patients. Reported age differed between the studies, with a mean age of 72 years (range 40-104). The mean age of the AD groups were 74,5 and control groups were 70,4. In one study, age was not described (33). The mean follow-up was 6.8 years (range: 0.5-10). The included studies could be categorized according to 1) role of pathogens, 2) role of inflammatory mediators, 3) role of APOE alleles and 4) role of Aβ peptide (Table 2). According to MINORS, the quality of most included studies was reasonable to good (Table 3).
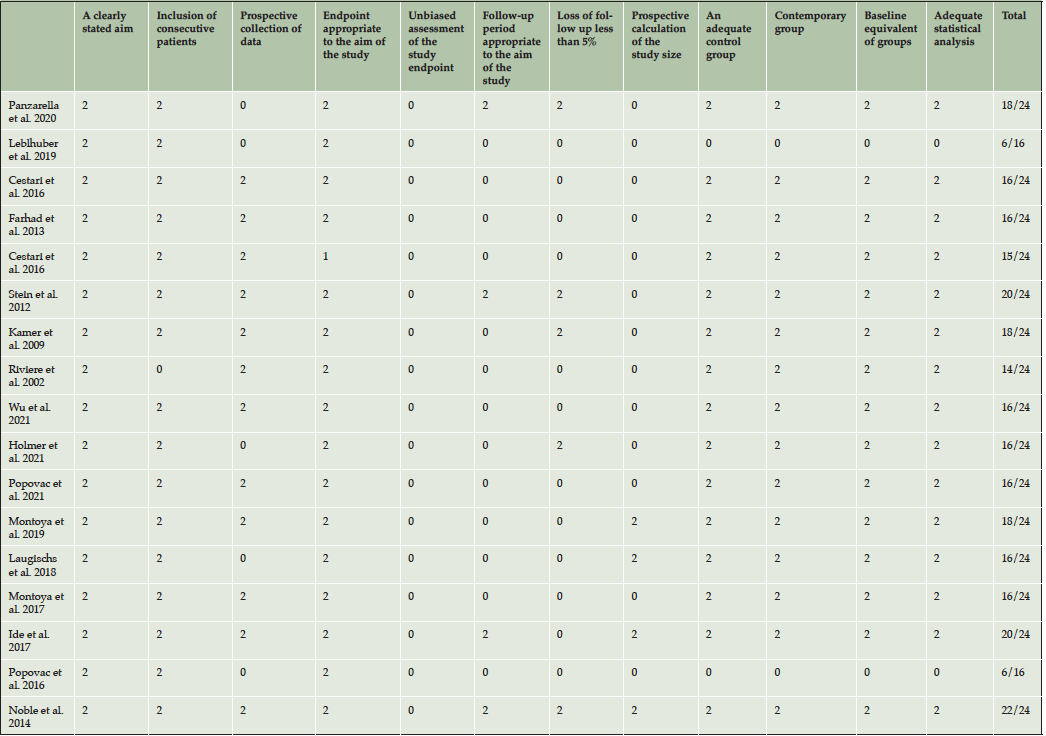
Table 3. MINORS score (0 if not reported; 1 when reported but inadequate; and 2 when reported and adequate). The ideal score is 16 for non-comparative studies and 24 for comparative studies
The role of oral pathogens
Eight out of the 17 studies described associations between oral pathogens and AD (33–41). Multiple studies showed that oral bacterial species (e.g., Fusobacterium nucleatum and Prevotella intermedia), who are known to be involved in the development of periodontitis, were found in higher percentages in AD patients than in non-AD patients (34, 36, 39, 41, 42). In the study of Stein et al. (41) antibody levels towards the periodontal bacteria P. intermedia and F. nucleatum were significantly increased at baseline in serum in patients with AD when compared to controls. In this study the response to periodontal bacteria in AD patients years before cognitive impairment suggests that the bacterial load of periodontal disease could potentially contribute to the risk of the development of AD (41). The median time from baseline assessment to diagnosis for the AD subjects was 9.6 years (41).
Maurer et al. investigated a possible link between bacterial infestation of the mouth, oral health and AD (43). They discovered that resistant oral bacteria (A. actinomycetemcomitans, P. gingivalis, and F. nucleatum) were present on the surface of the maxillary molars in AD patients. These bacteria formed a complex biofilm. The authors hypothesized that bacterial toxins may cause inflammatory processes that trespasses neighboring parts of the central nervous system that are associated with the onset of AD such as the entorhinal cortex and the hippocampus. This might also explain the loss of smell in AD patients (entorhinal cortex) (43). Laugisch et al. concluded from their studies that periodontal pathogens could enter the brain and stimulate a local immune response, however, their data does not support a specific association of periodontal infection with an onset of AD in an age up to 70-year-old patients and early stages of the disease (37). Similar levels of periodontal infection were found in patients with AD and other forms of dementia (37).
Two post-mortem studies from Riviere et al. and Poole et al. compared the microbiome of the brain tissue of AD patients to that of cognitively normal patients (33, 44). Rivière et al. found in the frontal lobe cortex that treponemes (T. amylovorum, T. denticola, T. maltophilum, T. medium, T. pectinovorum, T. socranskii, T. vincentii) which are associated with periodontitis colonized the brain more frequently in AD patients than in controls (33). Next, Poole et al. found P. gingivalis (pg)- lipopolysaccharides (LPS) in early post-mortem and proposed that pg-LPS have a role in brain inflammation associated with AD (45). In non-AD control tissues, no evidence of pg-LPS was seen (45). This study confirms that in AD patients, LPS from periodontal bacteria can access the brain during life (45).
Role of inflammatory mediators
Five studies assessed the role of oral inflammatory mediators originating from the mouth in patients with AD(46–49). Pro-inflammatory cytokines (interleukin 1(IL-1), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α)) might be a connecting link between poor oral health and AD as these cytokines can penetrate the blood-brain barrier and activate the resident microglial cells (49). Human gingival fibroblasts (HGFs) are the most abundant cells in gingival connective tissues (50). Various HGF responses to periodontal pathogens or inflammatory cytokines contribute to the development of periodontitis. There they can trigger the production of Amyloid Beta Protein (ABP) and Tau phosphorylation, resulting in neuronal damage and cognitive impairment, and thus be involved in AD development (49). IL-6 is an important cytokine involved in the regulation of host response to bacterial infection (50). IL-6 levels in AD patients were substantially higher than in controls, and periodontitis patients had noticeably higher TNF-a levels than AD patients with healthy periodontium (49). In the AD and mild cognitive impairment (MCI) groups, IL-6 and TNF-a were likewise positively associated (49). Thus, patients with AD and poor periodontal health had greater TNF-α levels in their blood compared with healthy controls (49).
Multiple studies found that the levels of TNF-α were significantly increased in AD subjects compared to the controls, while IL-1β and IL-6 levels did not differ between AD subjects and controls (47, 49). These findings suggest that antibodies against periodontal bacteria are linked to AD and may aid in the clinical identification of the disease (47).
The diagnostic signature pointed towards a central role for TNF-α and significant roles for several immune-associated plasma proteins. As antibody response reflects host immune function, we hypothesize that antibody titers to periodontal bacteria may complement plasma TNF-α levels in improving the clinical diagnosis of AD patients and differentiate them from cognitively normal subjects (47, 49).
Ide et al. showed that periodontitis was not associated with baseline cognitive status like onset AD, but periodontitis was linked to a six-fold increase in the rate of cognitive decline throughout a six-month follow-up period (42). A follow up study of Noble et al. studied if IgG to periodontal microbiota as possible predictors of AD (35). They showed that not only IgG levels are associated with risk for developing AD, but also an increase of IgG levels in the time without an obvious cause (35). Both were not found in the control group.
Role of APOE-4 allels
Bergdahl et al. found that the edentate patients (age mean 72 years) had a higher ApoE4 in the AD patients than dentate AD patients (age mean 54 years) (51). Also the results of the studies of Popovac et al. showed that presence of ApoE4 allele and low number of functional tooth units may independently both raise the risk of AD (52, 53). The few studies that looked at dental and genetic factors as predictors of AD showed the association that having at least one ApoE4 allele and fewer than eight teeth increased the likelihood of mild memory impairment, although only the risk factor ApoE4 allele did significantly increase the risk of AD (51). Fewer teeth can be caused by inflammation of the gums, which can be caused by oral pathogens.
Role of Amyloid Beta Protein
ABP may play a role in the development of AD (52–54). Patients with severe periodontal disease have higher plasma ABP concentrations and the presence of periodontitis may modulate the observed association between ABP and cognitive impairment (55). Kubota et al demonstrated an association between periodontitis and AD using a combination of microarray and computer-aided data mining analyses (54). qRT-PCR successfully identified differential expression of specific genes related to periodontitis and AD pathogenesis (54). Immunolocalisation of APP in gingival tissue was also investigated suggesting a potential mechanism by which chronic periodontitis may be biologically linked to the clinical onset or progression of AD (54).
Discussion
This systematic review of the literature aimed to assess the potential association of oral health as a risk factor for the onset of AD. Only a few studies demonstrated possible associations between oral health diseases and AD (34). When summarizing these studies, four theories could be distinguished that describe the possible links between oral health and the development or onset of AD as can be read in the results section. These four theories are discussed below. The main common denominator of all the theories is the ‘inflammatory burden that occurs. The theory of the role of Aβ is the least likely and the theory of oral pathogens is the most likely.
Role of oral pathogens
Some studies suggest that bacteria and their toxins can enter the brain via the central nervous system, and provoke a chain reaction that might lead to AD (42, 56, 57). Several pathways are optional for this theory. In literature the gut–brain axis is a well-known bidirectional link between the gut microbiome and the brain/ central nervous system as has been shown for e.g. Parkinson disease (PD) (42, 56, 57). For PD accumulating evidence suggests that the onset of non-motor symptoms, such as gastrointestinal manifestations, can precedes the onset of motor symptoms and disease diagnosis, supporting a potential role for the microbiome-gut-brain axis in the underlying pathological mechanisms (58–60). This bidirectional relationship is also thought to exist between the oral microbiota and AD (42, 56, 57). Thus, the dysbiotic signature in AD patients particularly with regard to periodontal bacteria, indicates that the mouth bacterial flora and it’s toxins may affect the central nervous system (61). For AD counts that some oral microorganisms and their toxins have already be found to transfer into the brain via cranial nerves, raising the chance of developing AD (57, 61).
F. nucleatum and T. denticola bacterial load in the mouth was significantly higher in AD than controls (39). F. nucleatum is one of the most common species in the human gingival, and may be isolated both from healthy and compromised periodontal teeth, thus the cause between the bacterial load and AD is not yet clear (39, 62). Which pathogens and associated pathways are involved in the development and progression of AD remains to be elucidated, but understanding the specific mechanisms involved in the interaction between these pathogens and the nervous system is vital for the early intervention in AD.
Role of inflammatory mediators
Neuroinflammation is an important process in AD neurodegeneration by the vicious circle of inflammation and cell destruction (63). The presence of lipopolysaccharides from T. denticola, T. forsythia and P. gingivalis in the brain, which can be associated with increased expression of major proinflammatory cytokines (IL-1β, IL-6 and TNFα) and decreased expression of anti-inflammatory cytokines (IL-10), suggest a role for the interaction of inflammatory markers in the development and progression of AD (63, 64). In addition, these periodontal pathogens appear to modulate the immune response in AD patients. This modulation may be related to an alteration in microglial activity, which is associated with an increased risk of AD (64). However, it appears that the oral pathogens and inflammatory mediators amplify a chain reaction, but there is no clear evidence of an association between the mouth inflammatory mediators and AD. More research is needed to understand the relationship with important inflammatory pathways and to assess the detailed interactions between periodontitis and AD.
Role of APOE-4 allels
The APOE-4 allels appears to play a critical role in neuroinflammation and may contribute to promoting P. gingivalis colonisation of the brain (44, 65, 66). Poole et al. observed the presence of P. gingivalis DNA in the brain of APOE mice which were infected at the gingival level with this gram-positive pathogen (44). Interestingly, as shown by Singhrao et al, gingival infection with P. gingivalis also resulted in the early appearance of age-related granules in these mice (66). These data, in line with the results of another study by Hafezi-Moghadam et al., suggest that the lack of APOE allel and the increased systemic inflammation observed in periodontitis induce an impairment of the blood-brain barrier (65). A larger population is needed to confirm these findings (65). It is impossible to conclude that the APOE-4 allele plays a pivotal role in this complex interaction of oral inflammation and AD based on the findings of the preceding investigations.
Role of Amyloid Beta Protein
The passage of ABP from the bloodstream to the brain may explain the previous finding of an association between periodontitis and AD and the present observation that increased plasma ABP concentrations are associated with AD and modulated by periodontal disease (67). Periodontitis has been linked to Aβ accumulation in distant brain areas thought to be sensitive to AD (55). According to Montoya et al., patients with severe periodontal disease and AD had higher plasma ABP concentrations, and the presence of periodontitis may affect the ABP-AD relationship (55). The passage of ABP from the bloodstream to the brain may explain a previous finding of an association between periodontitis and AD (55). The involvement of ABP was validated in a study by Kamer et al., who discovered that cognitively healthy persons with periodontal disease had more Aβ accumulation in their brain than those without poor oral health (47). They found that periodontal disease was linked to amyloid buildup in the brain in areas prone to amyloid accumulation in AD patients (47, 55).
All ABP findings imply that poor oral health may enhance the risk of amyloid accumulation in the brain (47). Plasma ABP levels are higher in individuals who have severe periodontal disease (55). Thus, the presence of periodontitis may modify the association between ABP and cognitive impairment (55). Faulty inflammatory mechanisms may play a role in the onset and progression of age-related chronic diseases such as AD, cardiovascular disease, cancer, diabetes and periodontitis (54, 55). Finally, it remains unclear whether increased concentrations of ABP in the brain are responsible for the neurodegeneration and/or the cause of oral health disease. In the above studies, all patients already had AD (54, 55). Therefore, studies are eagerly awaited in patients who do not yet have AD, but develop it later.
Strengths and limitations
Most of the included studies met the criteria as described in the MINORS qualification (32). The quality of most studies was reasonable to good. Limitations of this systematic review are that the studies differed in study design (cross-sectional and prospective), study protocol, sample sizes, and whether the oral problem was actually present first before the cognitive problem. Furthermore, the definition for oral health in the various studies hampered comparison (39, 68, 69). A proper definition is eagerly awaited. Finally, not all studies compared AD with healthy controls, the mean age in the AD groups was often much higher than the control groups, and in most studies the control group had fewer individuals.
Conclusion
From this review it can be concluded that four main theories can be distinguished that might give an explanation for all associations found between AD and oral health. Yet, there is insufficient evidence to prove a link between AD and oral health due to the diversity of study designs of the included studies. Therefore, further research is needed to search for causal links between oral health (in particular periodontitis) and neuroinflammation that can lead to the onset of AD with the future intention to prevent cognitive decline by better dental care.
Funding: There were no external funding sources for this work.
Conflict of Interests: The authors have no conflicts of interest to declare.
Statement of Ethics: An ethics statement is not applicable because this study is based exclusively on published literature.
Author Contributions: All listed authors (Sanne M. Pruntel, Barbara C. van Munster, Jeroen J. de Vries, Arjan Vissink and Anita Visser) have made substantial contributions to (1) the conception or design of the work or the acquisition, analysis, or interpretation of data for the work; (2) drafting the work or revising it critically for important intellectual content; (3) have given final approval of the version to be published; and (4) have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability Statement: All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Kumar A, Sidhu J, Goyal A, Tsao JW. Alzheimer Disease. In Treasure Island (FL); 2022.
2. Y.Y. Szeto J, J.G. Lewis S. Current Treatment Options for Alzheimer’s Disease and Parkinson’s Disease Dementia. Curr Neuropharmacol. 2016;14(4):326–38.
3. Cicciù M. Neurodegenerative disorders and periodontal disease: Is there a logical connection? Vol. 47, Neuroepidemiology. S. Karger AG; 2016. p. 94–5.
4. Dugger BN, Dickson DW. Pathology of neurodegenerative diseases. Vol. 9, Cold Spring Harbor Perspectives in Biology. Cold Spring Harbor Laboratory Press; 2017.
5. 2020 Alzheimer’s disease facts and figures. Alzheimer’s and Dementia. 2020 Mar 1;16(3):391–460.
6. Xie A, Gao J, Xu L, Meng D. Shared mechanisms of neurodegeneration in alzheimer’s disease and parkinson’s disease. Biomed Res Int. 2014;2014.
7. Qiu C, Kivipelto M, Von Strauss E. Epidemiology of Alzheimer’s disease: Occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11(2):111–28.
8. Tarawneh R, Holtzman DM. The clinical problem of symptomatic Alzheimer disease and mild cognitive impairment. Cold Spring Harb Perspect Med. 2012;2(5):1–16.
9. McKhann G, Drachman D, Folstein M, Katzman R. views & reviews Clinical diagnosis of Alzheimer ’ s disease : Neurology. 1984;34(7):939.
10. Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25(1):59–70.
11. Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016 Jun;8(6):595–608.
12. Rajmohan R, Reddy PH. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. Vol. 57, Journal of Alzheimer’s Disease. IOS Press; 2017. p. 975–99.
13. Deture MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Vol. 14, Molecular Neurodegeneration. BioMed Central Ltd.; 2019.
14. Robbins M, Clayton E, Kaminski Schierle GS. Synaptic tau: A pathological or physiological phenomenon? Vol. 9, Acta Neuropathologica Communications. BioMed Central Ltd; 2021.
15. Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1(1):1–23.
16. Humpel C. Chronic mild cerebrovascular dysfunction as a cause for Alzheimer’s disease? Exp Gerontol. 2011;46(4):225–32.
17. Verdile G, Keane KN, Cruzat VF, Medic S, Sabale M, Rowles J, et al. Inflammation and Oxidative Stress: The Molecular Connectivity between Insulin Resistance, Obesity, and Alzheimer’s Disease. Vol. 2015, Mediators of Inflammation. Hindawi Publishing Corporation; 2015.
18. Ramanathan K, Antognini D, Combes A, Paden M, Zakhary B, Ogino M, et al. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- research that is available on the COVID-19 resource centre – including this for unrestricted research re-use a. 2020;(January):19–21.
19. Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, et al. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7(9):812–26.
20. Hu N, Yu JT, Tan L, Wang YL, Sun L, Tan L. Nutrition and the risk of alzheimer’s disease. Biomed Res Int. 2013;2013.
21. Kiuchi S, Cooray U, Kusama T, Yamamoto T, Abbas H, Nakazawa N, et al. Oral Status and Dementia Onset: Mediation of Nutritional and Social Factors. J Dent Res. 2022 Apr 1;101(4):420–7.
22. Kim JK, Baker LA, Davarian S, Crimmins E. Oral health problems and mortality. J Dent Sci. 2013;8(2):115–20.
23. Gao SS, Chu CH, Young FYF. Oral health and care for elderly people with alzheimer’s disease. Int J Environ Res Public Health. 2020 Aug 2;17(16):1–8.
24. Noble JM, Scarmeas N, Papapanou PN. Poor oral health as a chronic, potentially modifiable dementia risk factor: Review of the literature topical collection on dementia. Vol. 13, Current Neurology and Neuroscience Reports. Current Medicine Group LLC 1; 2013.
25. Gaur S, Agnihotri R. Alzheimer’s disease and chronic periodontitis: Is there an association? Geriatr Gerontol Int. 2015 Apr;15(4):391–404.
26. Kamer AR, Craig RG, Dasanayake AP, Brys M, Glodzik-Sobanska L, de Leon MJ. Inflammation and Alzheimer’s disease: Possible role of periodontal diseases. Alzheimer’s and Dementia. 2008 Jul;4(4):242–50.
27. Wu H, Qiu W, Zhu X, Li X, Xie Z, Carreras I, et al. The Periodontal Pathogen Fusobacterium nucleatum Exacerbates Alzheimer’s Pathogenesis via Specific Pathways. Front Aging Neurosci. 2022 Jun 23;14.
28. Kumar J, Teoh SL, Das S, Mahakknaukrauh P. Oxidative stress in oral diseases: Understanding its relation with other systemic diseases. Vol. 8, Frontiers in Physiology. Frontiers Media S.A.; 2017.
29. Teixeira FB, Saito MT, Matheus FC, Prediger RD, Yamada ES, Maia CSF, et al. Periodontitis and alzheimer’s disease: A possible comorbidity between oral chronic inflammatory condition and neuroinflammation. Front Aging Neurosci. 2017 Oct 10;9(OCT).
30. Scannapieco FA, Cantos A. Oral inflammation and infection, and chronic medical diseases: implications for the elderly. Periodontol 2000. 2016 Oct;72(1):153–75.
31. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339.
32. Arem Lim KS, Mile Ini EN, Amien Orestier DF, Abrice Wiatkowski FK, Ves Anis YP, Acques Hipponi JC, et al. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. Vol. 73, ANZ J. Surg. 2003.
33. Riviere G, Riviere KH, Smith KS. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol Immunol. 2002;17(2):113–8.
34. Leblhuber F, Huemer J, Steiner K, Gostner JM, Fuchs D. Correction to: Knock-on effect of periodontitis to the pathogenesis of Alzheimer’s disease? Vol. 132, Wiener klinische Wochenschrift. Austria; 2020. p. 549–50.
35. Noble JM, Scarmeas N, Celenti RS, Elkind MS V, Wright CB, Schupf N, et al. Serum IgG antibody levels to periodontal microbiota are associated with incident Alzheimer disease. PLoS One. 2014;9(12):e114959.
36. Beydoun MA, Beydoun HA, Hossain S, El-Hajj ZW, Weiss J, Zonderman AB. Clinical and Bacterial Markers of Periodontitis and Their Association with Incident All-Cause and Alzheimer’s Disease Dementia in a Large National Survey. J Alzheimers Dis. 2020 Apr;
37. Laugisch O, Johnen A, Maldonado A, Ehmke B, Burgin W, Olsen I, et al. Periodontal Pathogens and Associated Intrathecal Antibodies in Early Stages of Alzheimer’s Disease. J Alzheimers Dis. 2018;66(1):105–14.
38. Wu YF, Lee WF, Salamanca E, Yao WL, Su JN, Wang SY, et al. Oral microbiota changes in elderly patients, an indicator of alzheimer’s disease. Int J Environ Res Public Health. 2021;18(8).
39. Panzarella V, Mauceri R, Baschi R, Maniscalco L, Campisi G, Monastero R. Oral Health Status in Subjects with Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Data from the Zabút Aging Project. Journal of Alzheimer’s Disease. 2020;1–11.
40. Holmer J, Aho V, Eriksdotter M, Paulin L, Pietiäinen M, Auvinen P, et al. Subgingival microbiota in a population with and without cognitive dysfunction. J Oral Microbiol. 2021;13(1).
41. Sparks Stein P, Steffen MJ, Smith C, Jicha G, Ebersole JL, Abner E, et al. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimer’s and Dementia. 2012;8(3):196–203.
42. Ide M, Harris M, Stevens A, Sussams R, Hopkins V, Culliford D, et al. Periodontitis and cognitive decline in Alzheimer’s disease. PLoS One. 2016;11(3):e0151081.
43. Maurer K, Rahming S, Prvulovic D. Dental health in advanced age and Alzheimer’s Disease: A possible link with bacterial toxins entering the brain? Psychiatry Res Neuroimaging. 2018;282(June):132–3.
44. Poole S, Singhrao SK, Chukkapalli S, Rivera M, Velsko I, Kesavalu L, et al. Active invasion of Porphyromonas gingivalis and infection-induced complement activation in ApoE-/- mice brains. Journal of Alzheimer’s Disease. 2014;43(1):67–80.
45. Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean S. Determining the Presence of Periodontopathic Virulence Factors in Short-Term Postmortem Alzheimer’s Disease Brain Tissue. Journal of Alzheimer’s Disease. 2013;5(4):665–77.
46. Farhad SZ, Amini S, Khalilian A, Barekatain M, Mafi M, Barekatain M, et al. The effect of chronic periodontitis on serum levels of tumor necrosis factor-alpha in Alzheimer disease. Dent Res J (Isfahan). 2014 Sep;11(5):549–52.
47. Kamer AR, Craig RG, Pirraglia E, Dasanayake AP, Norman RG, Boylan RJ, et al. TNF-α and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J Neuroimmunol. 2009;216(1–2):92–7.
48. Gil-Montoya JA, Barrios R, Sanchez-Lara I, Ramos P, Carnero C, Fornieles F, et al. Systemic inflammatory impact of periodontitis on cognitive impairment. Gerodontology. 2020 Mar;37(1):11–8.
49. Cestari JAF, Fabri GMC, Kalil J, Nitrini R, Jacob-Filho W, De Siqueira JTT, et al. Oral Infections and Cytokine Levels in Patients with Alzheimer’s Disease and Mild Cognitive Impairment Compared with Controls. Journal of Alzheimer’s Disease. 2016;52(4):1479–85.
50. Koji Naruishi TN. Biological effects of interleukin-6 on Gingival Fibroblasts: Cytokine regulation in periodontitis. J Cell Physiol. 2018 Sep;233(9):6393–400.
51. Bergdahl M, Bergdahl J, Nyberg L, Nilsson LG. Difference in apolipoprotein E type 4 allele (APOE epsilon 4) among dentate and edentulous subjects. Gerodontology. 2008 Sep;25(3):179–86.
52. Popovac A, Mladenović I, Krunić J, Trifković B, Todorović A, Milašin J, et al. Apolipoprotein ε4 Allele and Dental Occlusion Deficiency as Risk Factors for Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2020;74(3):797–802.
53. Popovac A, Stančić I, Despotović N, Nikolić N, Stefanova E, Milašin J. Difference in apolipoprotein E genotype distribution between dentate and edentulous elderly patients with Alzheimer disease. Genetika. 2016;48(2):699–706.
54. Kubota T, Maruyama S, Abe D, Tomita T, Morozumi T, Nakasone N, et al. Amyloid beta (A4) precursor protein expression in human periodontitis-affected gingival tissues. Arch Oral Biol. 2014;59(6):586–94.
55. Gil-Montoya JA, Barrios R, Santana S, Sanchez-Lara I, Pardo CC, Fornieles-Rubio F, et al. Association Between Periodontitis and Amyloid β Peptide in Elderly People With and Without Cognitive Impairment. J Periodontol. 2017;88(10):1051–8.
56. Foley NC, Affoo RH, Siqueira WL, Martin RE. A Systematic Review Examining the Oral Health Status of Persons with Dementia. JDR Clin Trans Res. 2017 Oct;2(4):330–42.
57. Maitre Y, Mahalli R, Micheneau P, Delpierre A, Amador G, Denis F. Evidence and Therapeutic Perspectives in the Relationship between the Oral Microbiome and Alzheimer’s Disease: A Systematic Review. Int J Environ Res Public Health. 2021 Oct;18(21).
58. Bohnen NI, Postuma RB. Body-first versus brain-first biological subtyping of Parkinson’s disease. Vol. 143, Brain. Oxford University Press; 2020. p. 2871–3.
59. Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015 Oct 1;78(4):522–9.
60. Klann EM, Dissanayake U, Gurrala A, Farrer M, Shukla AW, Ramirez-Zamora A, et al. The Gut–Brain Axis and Its Relation to Parkinson’s Disease: A Review. Vol. 13, Frontiers in Aging Neuroscience. Frontiers Media S.A.; 2022.
61. Narengaowa, Kong W, Lan F, Awan UF, Qing H, Ni J. The Oral-Gut-Brain AXIS: The Influence of Microbes in Alzheimer’s Disease. Front Cell Neurosci. 2021;15(April):1–10.
62. Han Y. Fusobacterium nucleatum: A commensal-turned pathogen. Curr Opin Microbiol. 2015 Feb;23:141–7.
63. Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Vol. 12, Alzheimer’s and Dementia. Elsevier Inc.; 2016. p. 719–32.
64. Hansen D V., Hanson JE, Sheng M. Microglia in Alzheimer’s disease. Vol. 217, Journal of Cell Biology. Rockefeller University Press; 2018. p. 459–72.
65. Hafezi-Moghadam A, Thomas KL, Wagner DD. ApoE deficiency leads to a progressive age-dependent blood-brain barrier leakage. Am J Physiol Cell Physiol. 2007;292:1256–62.
66. Singhrao SK, Harding A, Chukkapalli S, Olsen I, Kesavalu L, Crean S. Apolipoprotein E Related Co-Morbidities and Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2016;51:935–48.
67. Abbayya K, Puthanakar NY, Naduwinmani S, Chidambar YS. Association between periodontitis and alzheimer’s disease. N Am J Med Sci. 2015 Jun 1;7(6):241–6.
68. Hescot P. The New Definition of Oral Health and Relationship between Oral Health and Quality of Life. Vol. 20, The Chinese journal of dental research : the official journal of the Scientific Section of the Chinese Stomatological Association (CSA). 2017. p. 189–92.
69. Li M, Wu Z, Zhang R, Lei L, Ye S, Cheng R, et al. Comparison of oral health behaviour between dental and non-dental undergraduates in a university in southwestern China – – exploring the future priority for oral health education. BMC Oral Health. 2020 Sep 7;20(1).
© The Authors 2023