H.N. Yassine1,2, I.C. Arellanes1, A. Mazmanian1, L. De La Cruz1, J. Martinez1, L. Contreras1, N. Kono3, B.S. Liu1, D. Badie1, M.A. Bantugan1, A. Grindon1, T. Urich1, L. D’Orazio2, B.A. Emmanuel2, H.C. Chui2, W.J. Mack3, M.G. Harrington2, M.N. Braskie4, L.S. Schneider5
1. Departments of Medicine, University of Southern California, Los Angeles, CA, USA; 2. Departments of Neurology, University of Southern California, Los Angeles, CA, USA; 3. Department of Population and Public Health Sciences, Keck School of Medicine, University of Southern California, Los Angeles, California, USA; 4. Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, USC, USA; 5. Departments of Psychiatry and Behavioral Medicine, University of Southern California, Los Angeles, CA, USA
Corresponding Author: Hussein Yassine, M.D., Keck School of Medicine, University of Southern California, Los Angeles, CA, 90033, hyassine@usc.edu
J Prev Alz Dis 2023;4(10):810-820
Published online June 19, 2023, http://dx.doi.org/10.14283/jpad.2023.77
Abstract
INTRODUCTION: Lower blood levels of the omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA) are correlated with worse cognitive functions, particularly among APOE ε4 carriers. Whether DHA supplementation in APOE ε4 carriers with limited DHA consumption and dementia risk factors can delay or slow down disease progression when started before the onset of clinical dementia is not known.
METHODS: PreventE4 is a double-blind, single site, randomized, placebo-controlled trial in cognitively unimpaired individuals with limited omega-3 consumption and dementia risk factors (n=368). Its objectives are to determine (1) whether carrying the APOE ε4 allele is associated with lower delivery of DHA to the brain; and (2) whether high dose DHA supplementation affects brain imaging biomarkers of AD and cognitive function.
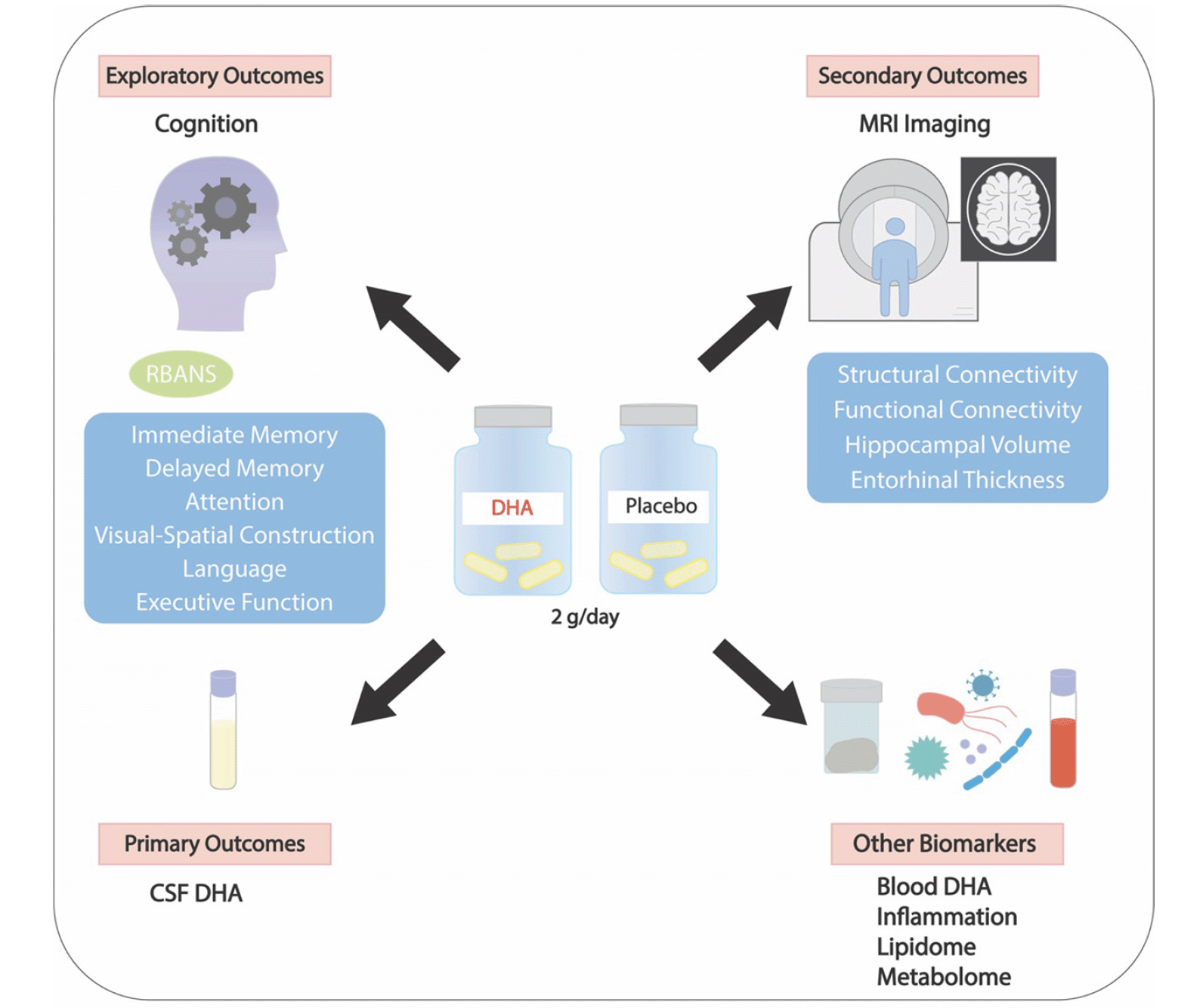
RESULTS: 365 cognitively unimpaired individuals between 55 and 80 (mean age 66) were randomized to 2 grams of DHA per day or identically appearing placebo for a period of 2 years. Half the participants were asked to complete lumbar punctures at baseline and 6-month visits to obtain cerebrospinal fluid (CSF). The primary trial outcome measure is the change in CSF DHA to arachidonic acid ratio after 6 months of the intervention (n=181). Secondary trial outcomes include the change in functional and structural connectivity using resting state functional MRI at 24 months (n=365). Exploratory outcomes include the change in Repeatable Battery of the Assessment of Neuropsychological Status at 24 months (n=365).
CONCLUSIONS: Findings from PreventE4 will clarify the brain delivery of DHA in individuals carrying the APOE ε4 allele with implications for dementia prevention strategies. Trial was registered as NCT03613844.
Key words: ApoE, lipids, brain, Alzheimer’s, DHA.
Introduction
The omega-3 (ω-3) polyunsaturated fatty acid (PUFA) docosahexaenoic acid (DHA) has important roles in synaptic plasticity and membrane fluidity (1). The brain requires DHA for maintenance of neuronal membranes, clearance of beta amyloid (Aβ) proteins and modulation of inflammation. Several studies have reported lower DHA levels with neuroinflammation, oxidative stress, lower synaptic activity, and hippocampal functions (2, 3). DHA brain levels and the ratio of DHA to Arachidonic Acid (AA) have been observed to be diminished in individuals with Alzheimer’s Disease (AD) (4), although these findings have been inconsistent (5, 6). Previous research evaluating the efficacy of DHA supplementation on cognition and dementia have shown mixed results. As a treatment for patients with dementia, DHA supplementation has not been effective (7, 8).
To determine if DHA supplementation is a viable prevention for AD, the target population that would most benefit from supplementation must be identified. The apolipoprotein E ε4 (APOE4) allele is the greatest genetic risk factor for development of late-onset AD (9). APOE4 modifies DHA metabolism in the brain and in the periphery (10), and reduces brain DHA delivery before the onset of dementia (11). There is an association between greater blood omega-3 levels in APOE4 carriers during mid-life with markers of lower AD risk (12) but this relationship is not present in patients with dementia. Therefore, cognitively unimpaired APOE4 carriers might represent a target population that could benefit from this intervention (13). The main objective of the PreventE4 trial is to test the delivery of high dose DHA to cerebrospinal fluid (CSF) after supplementation and whether DHA supplementation improves brain structure and connectivity and measures of cognitive outcomes. The objectives of the paper are to: (1) summarize background literature on DHA in relation to brain health; and (2) summarize the PreventE4 trial protocol (the complete protocol is included as a supplement) (3) and report the baseline findings.
Background
APOE4 and brain DHA metabolism
ApoE proteins have pleotropic functions that include the exchange of cholesterol and lipids between astrocytes and neurons; this function is intricately involved in synaptic plasticity and reinnervation after injury (14-16). In that regard, APOE4 proteins appear to be less efficient in lipid exchange to support injured neurons compared to ApoE3 and ApoE2 (17). In cells, APOE4 impacts the metabolism of PUFAs in the brain. For example, in astrocytes, APOE4 activates ca2+ dependent phospholipase A2 (18) to liberate AA from membrane phospholipids favoring a decrease in the DHA to AA ratio. In postmortem human brains, CSF and plasma, a lower DHA/AA was found in APOE4 carriers with AD pathology (4, 19, 20). In one study in mice, APOE4 was associated with an increased consumption of omega-3 fatty acids via beta-oxidation compared to ApoE2 (21), resulting in lower plasma and adipose tissue omega-3 levels. In humans, APOE4 carriers given a dose of 40 mg of 13C DHA had 31% lower increase in plasma levels compared to non-carriers (22). Combined, these findings support a shift toward lower DHA/AA and greater loss of DHA in APOE4 carriers compared to non-carriers. Using DHA PET scans, we identified greater brain uptake of DHA in cognitively unimpaired APOE4 carriers in their mid-thirties compared to non-carriers (10), suggesting compensation for greater consumption of brain DHA. Since DHA levels are largely determined by the diet, a greater need for DHA brain consumption in APOE4 has negative implications for brain functions in those with limited DHA intake.
Response of APOE4 carriers to DHA supplementation
APOE4 carriers with dementia do not respond to DHA supplementation (7). In postmortem brain tissues of patients with APOE4 dementia, there is evidence of greater eicosanoid lipid profiles than non-carriers suggestive of breakdown of AA and manifesting in a state of chronic sustained inflammation (4, 18). This is not observed in the APOE4 post-mortem brain without dementia raising the prospects that the effects of APOE4 on the brain are modifiable. However, DHA supplementation trials even in patients without dementia report inconsistent results, with some finding improvement in memory outcomes while others report no cognitive benefits (23). Several factors may affect the response of APOE4 carriers to ω-3 supplementation. First, not all APOE4 carriers will progress to dementia. The risk of developing AD dementia increases in individuals with cardiovascular disease (CVD) risk factors, lower education, higher age, and limited seafood consumption (24-27). CVD subgroup analyses across clinical trials have demonstrated that individuals with these risk factors may cognitively benefit most from omega-3 supplementation. Second, individuals that regularly consume fatty fish may not benefit from DHA supplementation because of their adequate intake. Third, dosage and duration of omega-3 supplementation have important roles on its brain delivery. In trials that used low doses (<1gram per day), omega-3 supplementation may have not been sufficient to effectively deliver DHA to the brain (28, 29). Higher DHA doses may be needed to effectively deliver DHA to the brain to reduce neurodegeneration, and this concept is supported by high dose DHA supplementation in APOE4 animal models preventing cognitive deficits (30, 31). The DHA Brain delivery pilot trial tested the effect of high dose (>2grams per day) DHA supplementation on CSF DHA levels. High dose DHA supplementation led to a modest 28% increase in CSF DHA (11), confirming the importance of greater DHA doses for brain delivery.
Cofactors that affect systemic and brain DHA levels
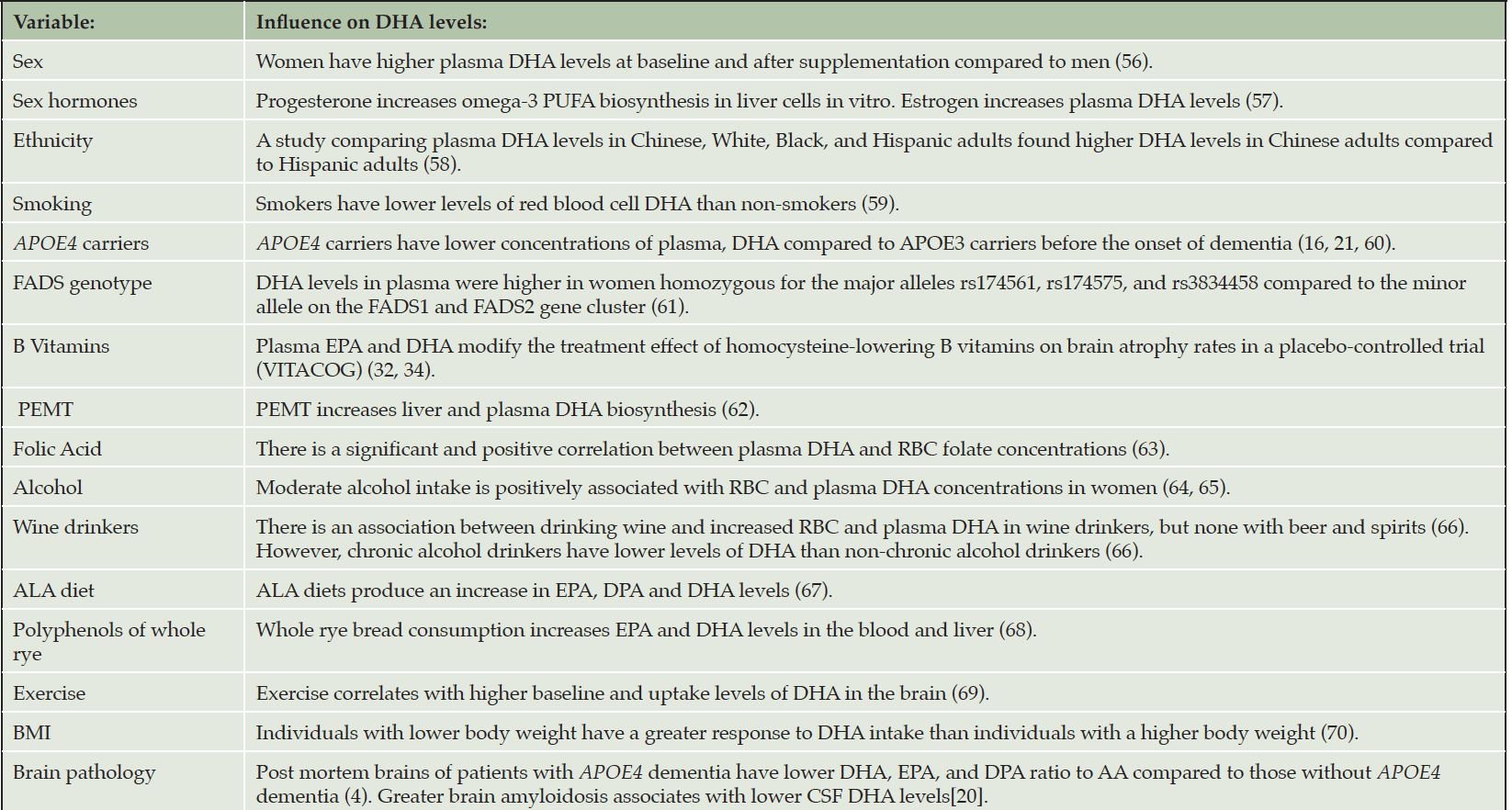
Many factors affect DHA incorporation into brain tissues. These factors are listed in Table 1 and include in addition to DHA dose, age, sex, physical activity, body mass index, smoking, homocysteine levels, B vitamin status and brain AD pathology. B vitamin supplementation, combined with high levels of blood DHA, is associated with a reduced rate of brain atrophy (32). B vitamins were found not to have significant cognitive effects on individuals with low levels of DHA (33). In the VITACOG trial, the brain’s atrophy rate, along with cognitive decline, were slowed by the intake of B vitamins and high levels of ω-3 fatty acids (34). DHA dose also has important implications on levels and outcomes. Clinical trials that have investigated DHA supplementation have used doses of DHA that vary greatly. DHA doses >1 gram per day have been associated with greater drug-placebo differences on cognitive outcomes (35). We have reported that greater brain amyloidosis, as assessed by lower CSF amyloid β-42 (Aβ-42), was associated with lower CSF DHA levels after supplementation (20).
Polyunsaturated fatty acids (PUFA); docosahexaenoic acid (DHA); docosapentaenoic acid, (DPA); alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA); Fatty Acid Desaturases (FADS); Body Mass Index (BMI); Phosphatidylethanola-mine methyltransferase (PEMT).
DHA, brain volume, structural and functional connectivity
The Aging Brain study identified a correlation between lower serum DHA levels and lower hippocampal and entorhinal cortex (ERC) volumes (23). The ERC is one of the sites of early neurofibrillary tangle deposition and is found to be thinner in cognitively healthy adults with APOE4 in comparison to non-carriers (37). A retrospective study examining brain volume and ω-3 found an association between ω-3 and increases in hippocampal volume in APOE4 non-carriers (38). Additionally, a study by Witte et al., found an increase in white matter integrity in several regions of the brain after 26 weeks of high dose supplementation of ω-3s.
DHA and cognitive outcomes
DHA contributes to several neurological pathways, including maintenance of myelination, neuron growth and survival, reduction or resolution of inflammation, synaptic plasticity, membrane receptor function and lipid raft organization (39). DHA also enhances neurite outgrowth and neurogenesis which can improve learning and cognition (40, 41). The role of DHA supplementation on cognitive functions in the aging brain is not fully understood. Although DHA is essential for the development of certain brain regions during gestation, such as the frontal lobes, which are implicated in higher-order cognitive functions (42-44), it is not clear whether it is required to maintain cognitive functions in the aging brain. It is important to recognize that the adult brain consumes only 4 mg of DHA per day (45). How APOE4 affects DHA brain requirements is not fully understood but there is evidence to support that brains of younger APOE4 carriers have a greater demand for plasma derived DHA than non-carriers (10).
DHA intake appears to affect memory, executive functions, and processing speed. In one study, omega-3 consumption was associated with greater hippocampal volume, suggesting that DHA may improve memory consolidation and learning. The ERC of the brain receives input from various regions and is the main provider of information to the hippocampus. The ERC is mainly implicated in spatial processing and spatial memory (46). Studies of cognitively unimpaired APOE4 carriers found greater cortical thickness compared to non-carriers and this was significantly related to DHA levels (47). Increased thickness of the ERC from supplementation can potentially improve orientation and processing speed, two functions affected by AD pathology. APOE4 cognitively healthy adults already exhibit changes in white matter (WM) integrity prior to exhibiting signs of dementia (48). A study examining the effects of omega-3s on brain microstructure found neuroprotective signs that were strongest in cortico-striatal fasciculus, an area particularly vulnerable to degeneration (49). Their data also suggested greater WM integrity, which was associated with higher omega-3 consumption (49). Increases in the integrity of this structural component mediated by omega-3 intake is associated with improvement in memory and executive functioning (50). Changes in WM integrity has also been linked to changes in processing speed (51).
PreventE4 Protocol
General Trial Design and Objective
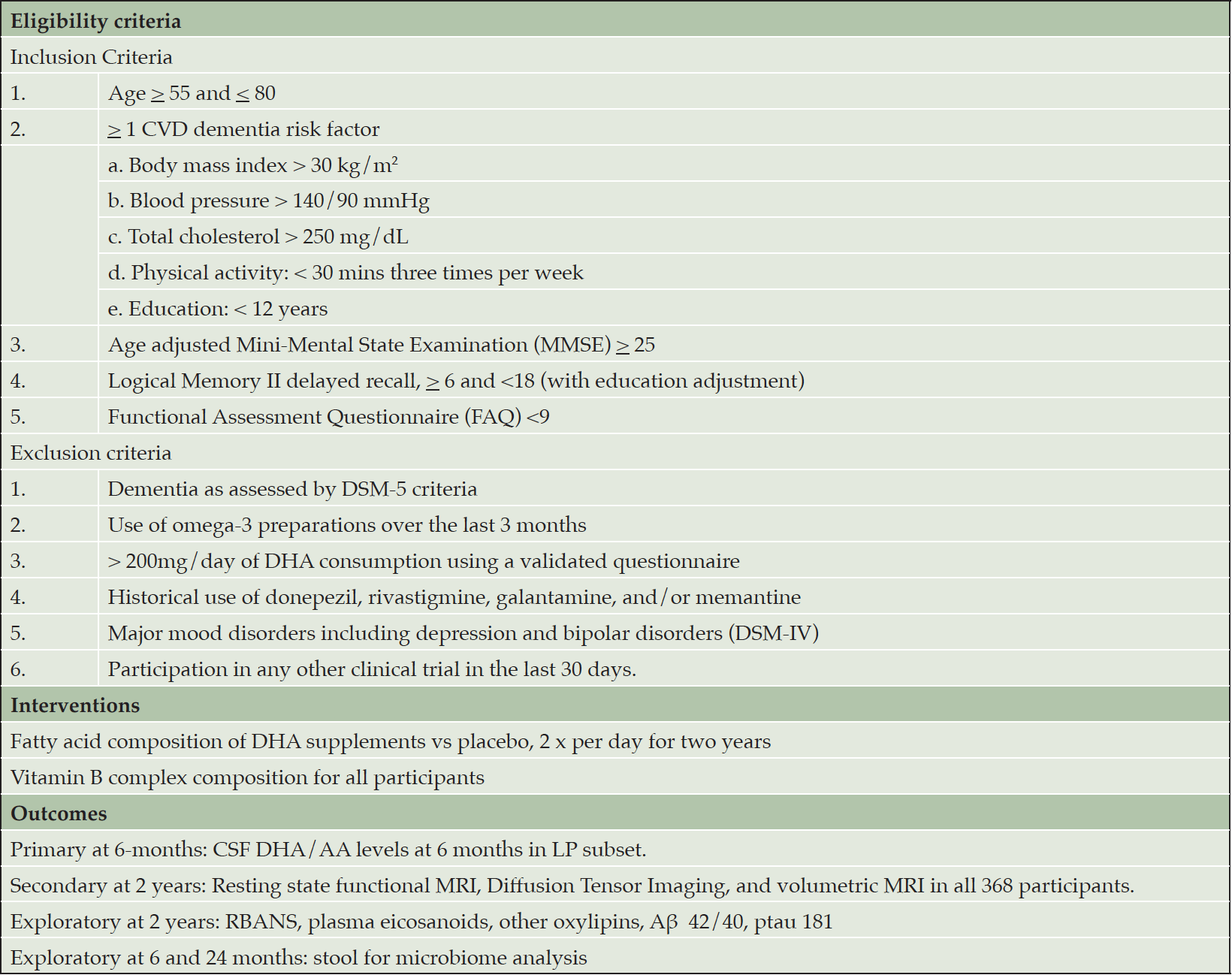
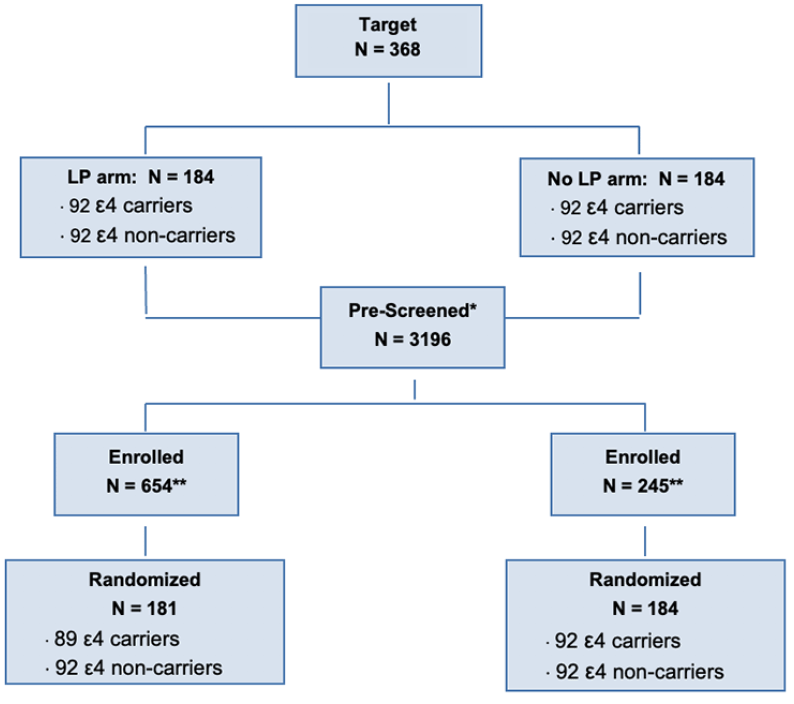
PreventE4 is a double-blind single-site placebo-controlled parallel group randomized trial with 1:1 allocation to identify whether carrying the APOE ε4 allele is associated with reduced delivery of DHA to the brain. The goal of PreventE4 is to recruit 368 cognitively unimpaired individuals who are between the ages of 55-80 with cardiovascular dementia risk factors; 184 of these individuals are APOE4 carriers and 184 are non-APOE4 carriers. The main objective of the trial is to examine whether the delivery of high dose DHA to the brain following supplementation differs by APOE genotype, informing future AD prevention strategies. The primary objective will be tested in 184 eligible participants consenting to lumbar puncture (LP) to assess brain delivery of DHA. Secondary and exploratory objectives examining DHA supplementation effects on neuroimaging and cognitive outcomes will be tested in the entire sample of 368 participants. PreventE4 is approved by the University of Southern California Institutional Review Board and is registered on clinicaltrials.gov (NCT03613844). Trial eligibility criteria, interventions and outcomes are summarized in Table 2.
Participants
The trial includes participants between the ages of 55 and 80 with at least 1 cardiovascular dementia risk factor but with no evidence of clinical dementia. The complete inclusion/exclusion criteria are listed in Table 2.
Recruitment
Participants were recruited from the public residing in the state of California, United States. The USC Keck Hospital EMR query was used to identify potential participants; recruitment letters were sent to these individuals. Other sources of recruitment included flyer postage on campus grounds, community events, and news advertisements in the local Los Angeles area. In addition to the main clinical site at USC Health Sciences Campus, the study has a site in East Los Angeles (Roybal Clinic) to enhance the recruitment of Latinos. The recruitment strategy, especially for underrepresented groups (URGs), was direct community outreach. For this strategy, we utilized both virtual and in-person approaches. During COVID restrictions, we utilized virtual outreach recruitment strategies, which included but were not limited to, social media, internet, e-newsletters, virtual education events such as the annual USC ADRC Memory Caregiver Symposium, and streaming internet radio educational programs. A significant proportion of this outreach was purposely targeted towards Latinos and included Facebook postings and streaming internet ads, which discussed health disparities. In-person community activities recruited prospective participants at health fairs and various community venues. Other examples included educational talks and setting up tables at Latino churches, community organizations, and professional organizations.
Interventions (supplement dose and administration)
A total of 2 grams of DHA or placebo are consumed a day along with 1 supplement of vitamin B for a 2-year intervention period. The DHA intervention capsules contain algal-derived triglyceride DHA-S. The placebo is composed of 50% corn oil and 50% soy oil. The specific fatty acid profile of DHA and the placebo are listed in Supplementary Table 1A. Both DHA and placebo are identical in appearance and size, as well as containers, and are distributed by DSM nutritional products. Supplements are given to participants to take home and consume twice daily. All participants are also provided and given instructions to take one vitamin B complex supplement (composition provided in Supplementary Table 1B) a day and are given written instructions to limit their DHA intake during the trial.
Trial outcomes
The primary trial outcome measure is the 6-month change in cerebrospinal fluid DHA/AA levels in 184 subjects consenting to lumbar puncture (LP). Baseline and 6-month cerebrospinal fluid DHA levels will be assessed by mass spectrometry. Secondary outcome measures include the 2-year change in functional and structural connectivity using resting state functional MRI in all 368 participants. Exploratory trial outcomes include the 2-year change in cognitive measures using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), plasma biomarkers of inflammation (eicosanoids and oxylipins), and amyloidosis (plasma Aβ 42/40, ptau 181) in 368 participants. In a small subset of participants consenting (n=30), a stool sample is obtained at baseline, 6 months, and 24 months to study the microbiome in an exploratory analysis.
Study Procedures and Assessments
Potential participants complete a pre-screening questionnaire to determine their eligibility in the study. The pre-screening includes questions about demographics, diet, exercise, medical history, and omega-3 consumption. If trial inclusion criteria are met, DNA is obtained through saliva collection for APOE genotyping and initial eligibility is reviewed.
Participants who meet pre-screening eligibility complete additional questionnaires including neuropsychology questionnaires, targeted history and physical exam, and dietary and exercise questionnaires. The MMSE test is conducted to assess cognitive health. After all criteria are reviewed and the participant is deemed eligible, they are invited to participate in the randomized study. During this screening, informed consent is obtained along with blood work for biomarkers. Participants are also notified of the dietary restrictions to consume less than one portion of fatty fish per week.
In the 184 participants in the LP arm, a lumbar puncture is completed at baseline and 6 months to obtain cerebrospinal fluid to analyze fatty acid levels of DHA. In all participants, blood is obtained at baseline, 6 months, and 24 months. At the baseline visit and 24 months, MRI is completed in all participants to assess brain volume and connectivity. Cognitive testing is obtained in all participants at baseline and repeated at the 6-, 12-, 18-, and 24-month visits.
Sample size
The original sample size estimation for the DHA trial was based on the primary outcome of 6-month change in CSF DHA/AA in the LP arm. Sample size was estimated to test an interaction hypothesis between DHA intervention and APOE4 genotype (i.e., that the DHA intervention effect on CSF DHA levels will differ by APOE4 genotype) at 80% power. The interaction effect size (standard deviation of interaction mean effects, divided by pooled between subject SD) of 0.25 relates to a 50% difference in the DHA effect in APOE4 positive compared to APOE4 negative individuals. The required sample size of 32 subjects per DHA/APOE4 cell (128 total) was increased to a sample size of 160 to accommodate an anticipated 20% dropout. Since initial measures of the dropout rate increased to 30% during trial conduct due to the COVID pandemic, the original sample size of 160 would provide reduced power of 74.6% to detect this interaction effect size. To obtain 80% power considering the 30% dropout, the sample size was increased to 184 (46 per cell) for the LP arm of the trial.
The secondary and exploratory trial outcomes will be assessed in the combined sample (LP and no-LP arms). The original sample size estimate of 320 participants (160 in LP arm and 160 in no-LP arm, with 80 APOE4 participants in each arm) was based on a detectable effect size of 0.5 SD for the main effect of DHA compared to placebo on cognitive change among APOE4 participants, with a 20% dropout. To achieve 80% power considering a 30% dropout, we obtained NIH, DSMB and IRB approval to increase the sample to 368 (184 for the LP arm and 184 for the no-LP arm).
Randomization
Following informed consent and determination of eligibility into the LP or no-LP arm, participants are randomized in a 1:1 allocation to two grams of DHA or placebo per day in identically appearing capsules and treated over two years. Within each LP and no-LP arm, randomization is stratified by APOE4 carrier status (E4, no E4) and recruitment site (2 levels), with a blocking factor that is not revealed to investigators. Participant selection to LP and no-LP arms is not randomized and is based on consenting to the LP procedure. The randomization sequence was developed and is monitored by trial statisticians. After verification of trial eligibility and randomization strata, randomization is completed by an unblinded trial statistician. All researchers and participants are blind to the specific intervention.
Statistical analysis plan
Baseline demographics and clinical, laboratory, and questionnaire data will be characterized and compared between randomized groups using frequency tabulations for binary/categorical variables and means (SD)/medians (inter-quartile range) for continuous variables. Baseline characteristics of participants in the LP vs no-LP arms and in those who complete and do not complete the study will also be compared. Initial analyses on trial outcomes will follow the intent-to-treat (ITT) principle by which participants are analyzed according to their randomized group, using complete data (participants who complete trial follow-up outcome measures). The primary outcome of CSF DHA/AA will be evaluated in the LP arm using a general linear model that includes main effects of randomized treatment, the APOE4 carrier and clinical site stratification factors, and the interaction between APOE4 stratum and treatment group. Sensitivity analyses will (1) use multiple imputation procedures on the primary outcome that will involve analyses of all randomized subjects, (2) limit analysis to “compliant” subjects (defined as at least 80% capsule count over the trial period). Secondary and exploratory outcomes, including changes in blood and CSF lipid and inflammation biomarkers, cognition, structural and functional connectivity by MRI and will be analyzed in the entire sample using the same general linear model; additional covariates will include an indicator variable for LP vs. no-LP arm and baseline variables that differ between the two LP arms. Analysis of secondary outcomes will not adjust for multiple comparisons (except for voxel-wise analyses). If there is a significant group difference found (p<0.05) in the primary or secondary outcomes (carriers vs. non-carriers), we will conduct analyses to identify potential mediation of the treatment effect.
Study approval
The study was approved by University of Southern California IRB# HS-18-00291 and HS-18-00984. All participants signed an informed consent.
Baseline Findings
The onset of the COVID-19 pandemic briefly interrupted participant recruitment and study operations as new protocols and safety measures were put in place. Prior to the pandemic, recruitment of participants included hosting community events which were no longer continued due to safety measures. However, recruitment continued through other means such as mailing letters using the I2B2 database. The majority of pre-screenings and screening visits were conducted over the phone and via zoom video call to limit the number of in-person meetings. Visits that were required to be in-person were conducted with safety protocols and measures, such as wearing a mask and always remaining 6 feet apart.
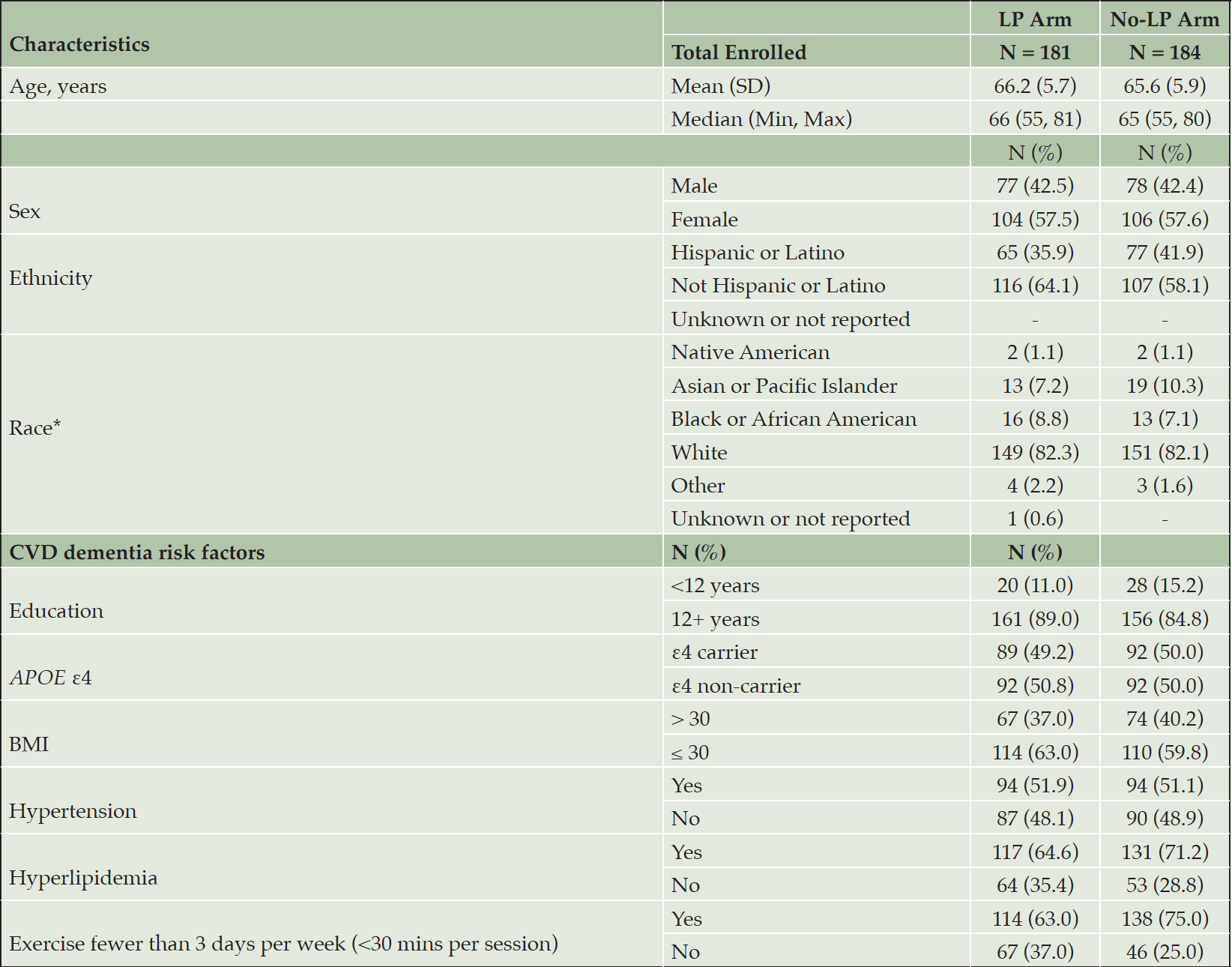
From 2019-2022, 365 individuals were randomized into DHA vs placebo, stratified by LP/no-LP arm, APOE4 status, and clinical site and enriched with CVD risk factors (Figure 1, Table 3). Forty-two % of randomized participants were males, 39% identified as Latino reflecting success in reaching this URG. The cohort was enriched in dementia risk factors. Thirteen% of the cohort had <12 years of education; 39% had a BMI >30 kg/m2, 52% had hypertension, 69% hyperlipidemia, and 69% exercised < 3 days/week. By design, 50% of the randomized individuals were APOE ε4 carriers. Baseline mean MMSE score was 29 (SD 1.6, range 23-30), logical memory II delayed recall score was 11 (SD 3.1, range 3-18), with mean education years at 14 (SD 4, range 1-24).
*LP Arm: 3 participants reported multiple races: white/black, white/other, white/Asian, black/Native American; No-LP Arm: 5 participants reported multiple races: white/black, white/other, white/Asian, white
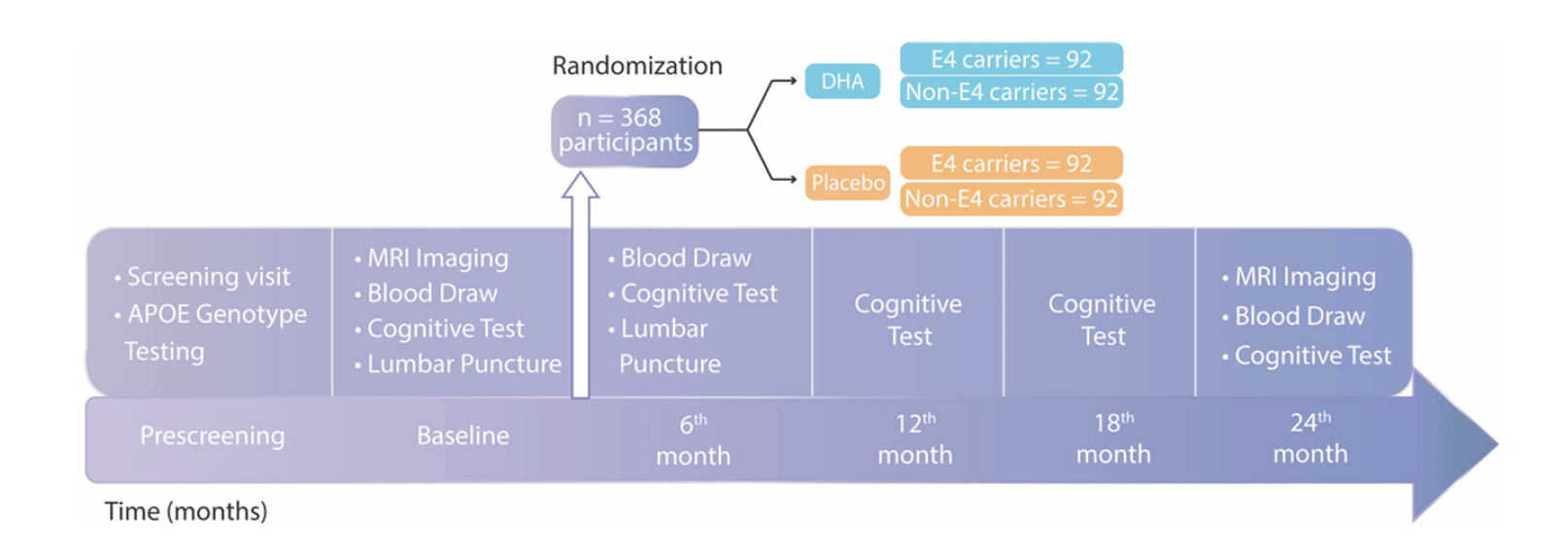
The study timeline and visits are illustrated.
The study target with the number of participants prescreened, enrolled, and randomized is shown.
The figure illustrates the primary, secondary and exploratory outcomes.
Discussion
This study investigates the effects of high dose DHA supplementation (2 grams per day) on DHA brain delivery following 6 months of supplementation in cognitively healthy participants with limited omega-3 consumption and CVD risk factors. It also measures the subsequent effects of DHA supplementation on brain structure and function and cognition over a period of 24 months. The study investigates interaction effects of DHA brain delivery and brain structure and function by APOE4 carrier status. We hypothesize that high-dose DHA supplementation will increase DHA and EPA and their ratio to AA in CSF. We also hypothesize that a lower increase in DHA and EPA levels and their ratio to AA will be observed in APOE4 carriers compared to non-carriers: signifying either lower brain DHA transport across the blood-brain barrier (BBB) or greater brain DHA consumption with APOE4. We expect that a lower increase in CSF DHA/AA will correlate with greater measures of inflammation (such as plasma and CSF eicosanoids and other oxylipins), loss of BBB function (indicated by elevated CSF/plasma albumin or platelet-derived growth factor β (PDGF-β)) or amyloidosis (CSF Aβ42, or plasma Aβ 42/40). This in turn can have implications for understanding the effect of DHA supplementation on brain structure and function to guide future prevention strategies using ω-3 supplements.
In PreventE4, changes in cognition outcomes are exploratory, and include performance on the RBANS immediate and delayed memory measurements. In non-demented APOE4 carriers over the age of 55, we expect that memory function will have the greatest likelihood of decline over two years, and this likelihood increases with aging and other dementia risk factors (52). A meta-analysis of studies involving DHA supplementation and its relation to AD and dementia used omega-3 indexes for analysis as opposed to supplementation. Their analysis revealed that increased omega-3 indexes lead to increased executive function among individuals (53). We hypothesize that DHA will improve memory, executive functions, or both within the context of a 2-year supplementation trial. Several trials investigating whether omega-3 supplementation in cognitively healthy younger adults affects cognitive outcomes had inconclusive findings likely resulting from a relatively short follow up duration (28, 54). A short duration is a potential limitation given the long half-life of DHA in the brain. A study using [11C]-DHA PET scanning revealed that the estimated turnover of DHA is approximately 2.5 years, pointing to a longer duration requirement to remodel neuronal membranes with supplemented DHA (10). Additionally, a longitudinal study found that long term users of ω-3s, over 10 years, had lower amyloid burden and higher memory scores amongst APOE4 carriers, compared to medium duration (1-9 years) and never (<1 year) users. These data further strengthen the need to increase the duration of DHA supplementation, but longer clinical trials are complicated to design and implement (55). Another limitation for this trial is the timing of supplementation. Although participants in this trial are cognitively healthy, at a mean age of 66, APOE4 carriers would have a greater degree of brain amyloidosis, some loss of BBB integrity and markers of brain inflammation more so than non-carriers that can potentially limit benefit from DHA supplementation. We selected this age group to observe a potential decline in cognitive performance over 2 years but whether this is too late for a prevention trial using DHA is not known. The findings generated from this trial are important, nevertheless, and may guide future interventions to younger individuals with longer supplementation duration and more sensitive brain function biomarkers.
The primary, secondary and exploratory results of this study will expand knowledge on how APOE4 affects brain omega-3 metabolism with insights towards AD prevention. Specifically, the trial will provide insights into mechanisms of brain DHA delivery before the onset of dementia, and how DHA supplementation affects brain functions in predementia stages. We hope that upon completion of the trial, changes in inflammation biomarkers, brain imaging and cognitive outcomes in relation to changes in DHA and EPA will help select a population that could benefit from such intervention and guide future steps. Further research using high dose DHA supplementation in a more personalized and larger phase III DHA supplementation trial has the potential of being a feasible prevention approach for individuals at increased risk of dementia.
Author contributions: HNY, WJM and LSS designed the trial. MNB is responsible for brain imaging. MGH and BE led the lumbar punctures. All other authors contributed significantly to the study design, study visits and writing the manuscript.
Competing interests: None.
Acknowledgments: We thank Eileen Bailey from DSM for donating the DHA and placebo supplements and Alemtsehay Wotango from Solgar for donating the Vitamin B complex (homocysteine modulator). We thank Daniella Garofalo and Hira Sherazi from the USC CTSI for help supporting our recruitment through I2B2.
Funding: HNY holds the Kenneth and Bette Volk Endowed Chair of Neurology. HNY is supported by RF1AG076124, RF1AG078362, R01AG067063, R01AG054434, R01AG055770, R21AG056518, and P30AG066530 from the National Institute on Aging, GC-201711-2014197 from the Alzheimer’s Drug Discovery Foundation (ADDF), and generous donations from the Vranos and Tiny Foundations and from Ms. Lynne Nauss. USC CTSI UL1TR001855 from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health supported recruitment for this study.
Ethical standards: The study was approved by University of Southern California IRB# HS-18-00291 and HS-18-00984. All participants signed an informed consent.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Cunnane, S.C., et al., Fish, docosahexaenoic acid and Alzheimer’s disease. Prog Lipid Res, 2009. 48(5): p. 239-56. 10.1016/j.plipres.2009.04.001
2. Cutuli, D., et al., n-3 polyunsaturated fatty acids supplementation enhances hippocampal functionality in aged mice. Front Aging Neurosci, 2014. 6: p. 220. https://doi.org/10.3389/fnagi.2014.00220
3. Okuda, S., H. Saito, and H. Katsuki, Arachidonic acid: toxic and trophic effects on cultured hippocampal neurons. Neuroscience, 1994. 63(3): p. 691-9. 10.1016/0306-4522(94)90515-0
4. Ebright, B., et al., Eicosanoid lipidome activation in post-mortem brain tissues of individuals with APOE4 and Alzheimer’s dementia. Alzheimer’s Research & Therapy, 2022. 14(1): p. 152. https://doi.org/10.1186/s13195-022-01084-7
5. Thomas, J., et al., Omega-3 Fatty Acids in Early Prevention of Inflammatory Neurodegenerative Disease: A Focus on Alzheimer’s Disease. Biomed Res Int, 2015. 2015: p. 172801. 10.1155/2015/172801
6. Safieh, M., A.D. Korczyn, and D.M. Michaelson, ApoE4: an emerging therapeutic target for Alzheimer’s disease. BMC Medicine, 2019. 17(1): p. 64. https://doi.org/10.1186/s12916-019-1299-4
7. Quinn, J.F., et al., Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA, 2010. 304(17): p. 1903-11. 10.1001/jama.2010.1510
8. Freund-Levi, Y., et al., Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol, 2006. 63(10): p. 1402-8. 10.1001/archneur.63.10.1402
9. Lambert, J.-C., et al., Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nature Genetics, 2013. 45(12): p. 1452-1458. https://doi.org/10.1038/ng.2802
10. Yassine, H.N., et al., DHA brain uptake and APOE4 status: a PET study with [1-11 C]-DHA. Alzheimer’s Research & Therapy, 2017. 9(1): p. 23. 10.1186/s13195-017-0250-1
11. Arellanes, I.C., et al., Brain delivery of supplemental docosahexaenoic acid (DHA): A randomized placebo-controlled clinical trial. EBioMedicine, 2020. 59. 10.1016/j.ebiom.2020.102883
12. Satizabal, C.L., et al., Association of Red Blood Cell Omega-3 Fatty Acids With MRI Markers and Cognitive Function in Midlife: The Framingham Heart Study. Neurology, 2022: p. 10.1212/WNL.0000000000201296.
13. Yassine, H.N., et al., Association of Docosahexaenoic Acid Supplementation With Alzheimer Disease Stage in Apolipoprotein E ε4 Carriers: A Review. JAMA Neurol, 2017. 74(3): p. 339-347. 10.1001/jamaneurol.2016.4899
14. Gong, J.-S., et al., Novel action of apolipoprotein E (ApoE): ApoE isoform specifically inhibits lipid-particle-mediated cholesterol release from neurons. Molecular Neurodegeneration, 2007. 2(1): p. 9. 10.1186/1750-1326-2-9
15. Vance, J.E. and H. Hayashi, Formation and function of apolipoprotein E-containing lipoproteins in the nervous system. Biochim Biophys Acta, 2010. 1801(8): p. 806-18. 10.1016/j.bbalip.2010.02.007
16. Yassine, H.N. and C.E. Finch, APOE Alleles and Diet in Brain Aging and Alzheimer’s Disease. Front Aging Neurosci, 2020. 12: p. 150. 10.3389/fnagi.2020.00150
17. Chang, T.Y., et al., Cellular cholesterol homeostasis and Alzheimer’s disease. J Lipid Res, 2017. 58(12): p. 2239-2254. 10.1194/jlr.R075630
18. Wang, S., et al., Calcium-dependent cytosolic phospholipase A2 activation is implicated in neuroinflammation and oxidative stress associated with ApoE4. Molecular Neurodegeneration, 2022. 17(1): p. 42. 10.1186/s13024-022-00549-5
19. Tomaszewski, N., et al., Effect of APOE Genotype on Plasma Docosahexaenoic Acid (DHA), Eicosapentaenoic Acid, Arachidonic Acid, and Hippocampal Volume in the Alzheimer’s Disease Cooperative Study-Sponsored DHA Clinical Trial. J Alzheimers Dis, 2020. 74(3): p. 975-990. 10.3233/JAD-191017
20. Yassine, H.N., et al., The effect of APOE genotype on the delivery of DHA to cerebrospinal fluid in Alzheimer’s disease. Alzheimers Res Ther, 2016. 8(1): p. 25. 10.1186/s13195-016-0194-x
21. Conway, V., et al., Apolipoprotein E isoforms disrupt long-chain fatty acid distribution in the plasma, the liver and the adipose tissue of mice. Prostaglandins Leukot Essent Fatty Acids, 2014. 91(6): p. 261-7. 10.1016/j.plefa.2014.09.007
22. Hauser, P.S., V. Narayanaswami, and R.O. Ryan, Apolipoprotein E: From lipid transport to neurobiology. Progress in Lipid Research, 2011. 50(1): p. 62-74. 10.1016/j.plipres.2010.09.001
23. Yassine, H.N., et al., Association of Serum Docosahexaenoic Acid With Cerebral Amyloidosis. JAMA Neurol, 2016. 73(10): p. 1208-1216. 10.1001/jamaneurol.2016.1924
24. Ferrari, C., et al., How can elderly apolipoprotein E ε4 carriers remain free from dementia? Neurobiol Aging, 2013. 34(1): p. 13-21. 10.1016/j.neurobiolaging.2012.03.003
25. Kivipelto, M., et al., The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimers Dement, 2013. 9(6): p. 657-65. 10.1016/j.jalz.2012.09.012
26. Morris, M.C., et al., Association of Seafood Consumption, Brain Mercury Level, and APOE ε4 Status With Brain Neuropathology in Older Adults. Jama, 2016. 315(5): p. 489-97. 10.1001/jama.2015.19451
27. Jansen, W.J., et al., Prevalence of Cerebral Amyloid Pathology in Persons Without Dementia: A Meta-analysis. JAMA, 2015. 313(19): p. 1924-1938. 10.1001/jama.2015.4668
28. Geleijnse, J.M., E.J. Giltay, and D. Kromhout, Effects of n-3 fatty acids on cognitive decline: a randomized, double-blind, placebo-controlled trial in stable myocardial infarction patients. Alzheimers Dement, 2012. 8(4): p. 278-87. 10.1016/j.jalz.2011.06.002
29. Benton, D., et al., Supplementation with DHA and the psychological functioning of young adults. Br J Nutr, 2013. 109(1): p. 155-61. 10.1017/S0007114512000566
30. Kariv-Inbal, Z., et al., The isoform-specific pathological effects of apoE4 in vivo are prevented by a fish oil (DHA) diet and are modified by cholesterol. J Alzheimers Dis, 2012. 28(3): p. 667-83. 10.3233/JAD-2011-111265
31. Chouinard-Watkins, R., et al., Docosahexaenoic acid prevents cognitive deficits in human apolipoprotein E epsilon 4-targeted replacement mice. Neurobiol Aging, 2017. 57: p. 28-35. 10.1016/j.neurobiolaging.2017.05.003
32. Smith, A.D., et al., Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One, 2010. 5(9): p. e12244. 10.1371/journal.pone.0012244
33. Durga, J., et al., Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet, 2007. 369(9557): p. 208-16. 10.1016/S0140-6736(07)60109-3
34. Jernerén, F., et al., Brain atrophy in cognitively impaired elderly: the importance of long-chain ω-3 fatty acids and B vitamin status in a randomized controlled trial. Am J Clin Nutr, 2015. 102(1): p. 215-21. 10.3945/ajcn.114.103283
35. Yurko-Mauro, K., et al., Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement, 2010. 6(6): p. 456-64. 10.1016/j.jalz.2010.01.013
36. Yassine HN, R.V., Mack WJ, Quinn JF, Yurko-Mauro K, Bailey-Hall E, Aisen PS, Chui HC, Schneider LS, The effect of APOE genotype on the delivery of DHA to cerebrospinal fluid in Alzheimer’s disease. Alzheimer’s Reaserch and Therapy, 2016. 8: p. 25. 10.1186/s13195-016-0194-x
37. Burggren, A.C., et al., Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. Neuroimage, 2008. 41(4): p. 1177-83. 10.1016/j.neuroimage.2008.03.039
38. Daiello, L.A., et al., Association of fish oil supplement use with preservation of brain volume and cognitive function. Alzheimer’s & dementia : the journal of the Alzheimer’s Association, 2015. 11(2): p. 226-235. 10.1016/j.jalz.2014.02.005
39. Weiser, M.J., C.M. Butt, and M.H. Mohajeri, Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients, 2016. 8(2): p. 99-99. 10.3390/nu8020099
40. Calderon, F. and H.Y. Kim, Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem, 2004. 90(4): p. 979-88. 10.1111/j.1471-4159.2004.02520.x
41. Dyall, S.C., G.J. Michael, and A.T. Michael-Titus, Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats. J Neurosci Res, 2010. 88(10): p. 2091-102. 10.1002/jnr.22390
42. Goustard-Langelier, B., et al., n-3 and n-6 fatty acid enrichment by dietary fish oil and phospholipid sources in brain cortical areas and nonneural tissues of formula-fed piglets. Lipids, 1999. 34(1): p. 5-16. 10.1007/s11745-999-331-6
43. Anderson, V., et al., Attentional skills following traumatic brain injury in childhood: a componential analysis. Brain Inj, 1998. 12(11): p. 937-49. 10.1080/026990598121990
44. Barkley, R.A., The executive functions and self-regulation: an evolutionary neuropsychological perspective. Neuropsychol Rev, 2001. 11(1): p. 1-29. 10.1023/a:1009085417776
45. Umhau, J.C., et al., Brain docosahexaenoic acid [DHA] incorporation and blood flow are increased in chronic alcoholics: A positron emission tomography study corrected for cerebral atrophy. PLoS ONE [Electronic Resource], 2013. 8: p. e75333. https://doi.org/10.1371/journal.pone.0075333
46. Garcia, A.D. and E.A. Buffalo, Anatomy and Function of the Primate Entorhinal Cortex. Annu Rev Vis Sci, 2020. 6: p. 411-432. 10.1146/annurev-vision-030320-041115
47. Sala-Vila, A., et al., DHA intake relates to better cerebrovascular and neurodegeneration neuroimaging phenotypes in middle-aged adults at increased genetic risk of Alzheimer disease. Am J Clin Nutr, 2021. 113(6): p. 1627-1635. 10.1093/ajcn/nqab016
48. Persson, J., et al., Altered brain white matter integrity in healthy carriers of the APOE epsilon4 allele: a risk for AD? Neurology, 2006. 66(7): p. 1029-33. 10.1212/01.wnl.0000204180.25361.48
49. Tsiknia, A.A., J. Bergstrom, and E.T. Reas, Midlife omega-3 fatty acid intake predicts later life white matter microstructure in an age- and APOE-dependent manner. Cerebral Cortex, 2022: p. bhac196. 10.1093/cercor/bhac196
50. Zamroziewicz, M.K., et al., Predictors of Memory in Healthy Aging: Polyunsaturated Fatty Acid Balance and Fornix White Matter Integrity. Aging and disease, 2017. 8(4): p. 372-383. 10.14336/AD.2017.0501
51. Kerchner, G.A., et al., Cognitive Processing Speed in Older Adults: Relationship with White Matter Integrity. PLOS ONE, 2012. 7(11): p. e50425. 10.1371/journal.pone.0050425
52. Caselli, R.J., et al., Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med, 2009. 361(3): p. 255-63. 10.1056/NEJMoa0809437
53. Kosti, R.I., et al., Fish intake, n-3 fatty acid body status, and risk of cognitive decline: a systematic review and a dose–response meta-analysis of observational and experimental studies. Nutrition Reviews, 2022. 80(6): p. 1445-1458. 10.1093/nutrit/nuab078
54. Jackson, P.A., et al., No effect of 12 weeks’ supplementation with 1 g DHA-rich or EPA-rich fish oil on cognitive function or mood in healthy young adults aged 18-35 years. Br J Nutr, 2012. 107(8): p. 1232-43. 10.1017/S000711451100403X
55. Li, L., et al., A gene-environment interplay between omega-3 supplementation and APOE ε4 provides insights for Alzheimer’s disease precise prevention amongst high-genetic-risk population. Eur J Neurol, 2022. 29(2): p. 422-431. 10.1111/ene.15160
56. Walker, C.G., et al., Age and sex differences in the incorporation of EPA and DHA into plasma fractions, cells and adipose tissue in humans. Br J Nutr, 2014. 111(4): p. 679-89. 0.1017/S0007114513002985
57. Sibbons, C.M., et al., Effect of sex hormones on n-3 polyunsaturated fatty acid biosynthesis in HepG2 cells and in human primary hepatocytes. Prostaglandins Leukot Essent Fatty Acids, 2014. 90(2-3): p. 47-54. 10.1016/j.plefa.2013.12.006
58. Steffen, B.T., et al., Ethnicity, plasma phospholipid fatty acid composition and inflammatory/endothelial activation biomarkers in the Multi-Ethnic Study of Atherosclerosis (MESA). European Journal of Clinical Nutrition, 2012. 66(5): p. 600-605. 10.1038/ejcn.2011.215
59. Scaglia, N., et al., The relationship between omega-3 and smoking habit: a cross-sectional study. Lipids Health Dis, 2016. 15: p. 61. 10.1186/s12944-016-0220-9
60. Vandal, M., et al., Reduction in DHA transport to the brain of mice expressing human APOE4 compared to APOE2. J Neurochem, 2014. 129(3): p. 516-26. 10.1111/jnc.12640
61. Glaser, C., et al., Genetic variation in polyunsaturated fatty acid metabolism and its potential relevance for human development and health. Matern Child Nutr, 2011. 7 Suppl 2(Suppl 2): p. 27-40. 10.1111/j.1740-8709.2011.00319.x
62. Chalil, A., et al., PEMT, Δ6 desaturase, and palmitoyldocosahexaenoyl phosphatidylcholine are increased in rats during pregnancy. J Lipid Res, 2018. 59(1): p. 123-136. 10.1194/jlr.M080309
63. Umhau, J.C., et al., The relationship between folate and docosahexaenoic acid in men. European Journal of Clinical Nutrition, 2006. 60(3): p. 352-357. 0.1038/sj.ejcn.1602321
64. di Giuseppe, R., et al., Alcohol consumption and n-3 polyunsaturated fatty acids in healthy men and women from 3 European populations. Am J Clin Nutr, 2009. 89(1): p. 354-62. 10.3945/ajcn.2008.26661
65. Umhau, J.C., et al., Brain docosahexaenoic acid [DHA] incorporation and blood flow are increased in chronic alcoholics: a positron emission tomography study corrected for cerebral atrophy. PLoS One, 2013. 8(10): p. e75333. ttps://doi.org/10.1371/journal.pone.0075333
66. Galduróz, J.C.F., et al., OMEGA-3 Interventions in Alcohol Dependence and Related Outcomes: A Systematic Review and Propositions. Curr Neuropharmacol, 2020. 18(5): p. 456-462. 10.2174/1570159X18666200128120729
67. Li, J., et al., Dietary supplementation of α-linolenic acid induced conversion of n-3 LCPUFAs and reduced prostate cancer growth in a mouse model. Lipids Health Dis, 2017. 16(1): p. 136. 10.1186/s12944-017-0529-z
68. Ounnas, F., et al., Rye polyphenols and the metabolism of n-3 fatty acids in rats: a dose dependent fatty fish-like effect. Scientific Reports, 2017. 7(1): p. 40162. https://doi.org/10.1038/srep40162
69. Wu, A., Z. Ying, and F. Gomez-Pinilla, Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience, 2008. 155(3): p. 751-9. 10.1016/j.neuroscience.2008.05.061
70. Flock, M.R., et al., Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose-response randomized controlled trial. J Am Heart Assoc, 2013. 2(6): p. e000513. 0.1161/JAHA.113.000513
© The Authors 2023