S. Cohen1, C.H. van Dyck2, M. Gee3, T. Doherty3, M. Kanekiyo4, S. Dhadda4, D. Li4, S. Hersch4, M. Irizarry4, L.D. Kramer4
1. Toronto Memory Program, Toronto, ON, Canada; 2. Yale School of Medicine, New Haven, CT, USA; 3. Eisai Co., Ltd, Hatfield, UK; 4. Eisai Inc. Nutley, NJ, USA
Corresponding Author: Sharon Cohen, MD, FRCPC, Medical Director & Site Principal Investigator, 1 Valleybrook Drive, Suite 400, Toronto, Canada M3B 2S7, Tel: 416-386-9761; Fax: 416-386-0458, cohen@memorydisorders.ca
J Prev Alz Dis 2023;4(10):771-777
Published online September 29, 2023, http://dx.doi.org/10.14283/jpad.2023.123
Abstract
BACKGROUND: Lecanemab is a humanized IgG1 monoclonal antibody binding with high affinity to amyloid-beta protein protofibrils. In phase 3 development, lecanemab has been shown to reduce markers of amyloid in early Alzheimer’s disease and reduce decline on clinical endpoints of cognition and function at 18 months.
OBJECTIVES: To describe the health-related quality-of-life (HRQoL) results from Clarity AD which were exploratory outcomes in this trial.
DESIGN: Clarity AD was an 18-month, multi-center, double-blind, phase 3 trial.
SETTING: Early Alzheimer’s disease.
PARTICIPANTS: Individuals 50-90 years of age with a diagnosis of mild cognitive impairment or mild dementia due to Alzheimer’s disease and positron emission tomography or cerebrospinal fluid evidence of cerebral amyloid accumulation.
INTERVENTION: Placebo or lecanemab 10-mg/kg IV biweekly.
MEASUREMENTS: HRQoL was measured at baseline and every 6 months using the European Quality of Life–5 Dimensions (EQ-5D-5L; by subject) and Quality of Life in AD (QOL-AD; by subject and proxy). Study partner burden was measured using the Zarit Burden Interview (ZBI).
RESULTS: A total of 1795 participants were enrolled (lecanemab:898; placebo:897). At month 18, adjusted mean change from baseline in EQ-5D-5L and QOL-AD by subject showed 49% and 56% less decline, respectively. QOL-AD rated by study partner as proxy resulted in 23% less decline. ZBI adjusted mean change from baseline at 18 months resulted in 38% less increase of care partner burden. Individual HRQoL test items and dimensions also showed lecanemab benefit.
CONCLUSIONS: Lecanemab was associated with a relative preservation of HRQoL and less increase in caregiver burden, with consistent benefits seen across different quality of life scales and within scale subdomains. These benefits provide valuable patient reported outcomes which, together with previously reported benefits of lecanemab across multiple measures of cognition, function, disease progression, and biomarkers, demonstrate that lecanemab treatment may offer meaningful benefits to patients, care partners, and society.
Key words: Health-related quality of life, early Alzheimer’s disease, lecanemab.
Introduction
Alzheimer’s disease (AD) is a chronic and progressive neurodegenerative disorder (1-4), which impacts cognition, daily function, and neuropsychiatric behavior and leads to increasing loss of autonomy and relentless deterioration in quality of life. AD significantly affects the daily lives not only of patients, but also of their families and care partners. Optimal management of AD should include maintaining the patient’s well-being and their quality of life (QOL) (5), as well as limiting the degree of burden experienced by those providing care.
Standard clinical efficacy assessments evaluate the effect of the disease on measures of cognition and daily function. However, health-related quality of life (HRQoL) assessments provide unique perspectives from the patient and care partner with respect to their own perceptions of how the disease affects them (6-7). QOL is the perception of one’s position in life in the context of one’s culture, values, goals, expectations, standards, and concerns; it includes emotional, social, and physical aspects of one’s life (8). HRQoL is a broad concept that encompasses physical and mental health, autonomy, social interactions, and the relationship between a subject and their environment.
The perception of how one’s well-being is affected by a disease, disability, or disorder is not interchangeable with health status (9). Furthermore, HRQoL is broader than activities of daily living (ADL), although it may correlate with ADL measures due to the high value that individuals place on independence. HRQoL should ideally be rated by the person directly affected (i.e., the patient on behalf of the patient and the care partner on behalf of the care partner), and measured in relation to their personal expectations, which can vary over time and with disease. Patient-reported outcomes are essential to understanding the value of a treatment. HRQoL questionnaires may be multidimensional, covering physical, social, emotional, cognitive, work/role-related aspects, and/or disease-related covering such aspects as symptoms, side effects, and financial impact of disease. The European Quality of Life–5 Dimensions (EQ-5D-5L) is a commonly used general HRQoL scale while the Quality of Life in AD (QOL-AD) and the Zarit Burden Interview (ZBI) are commonly used AD-specific HRQoL scales (10-12).
Herein, we describe the HRQoL results from Clarity AD, a phase 3 trial evaluating lecanemab, a novel humanized immunoglobulin G1 monoclonal antibody targeting both neurotoxic Aβ protofibrils and Aβ plaques (13-14). EQ-5D-5L, QOL-AD, and ZBI served as prespecified exploratory endpoints in this study. As previously reported for Clarity AD, lecanemab substantially reduced markers of amyloid and significantly slowed clinical decline on multiple measures of cognition and function in early AD (i.e., mild cognitive impairment (MCI) or mild dementia due to Alzheimer’s disease) at 18 months (15). Lecanemab was generally well tolerated but was associated with an increase in amyloid-related imaging abnormalities (ARIA) and infusion reactions (15).
Methods
Trial design and oversight
Methods and primary results for the Clarity AD double-blind phase have been published (15). Briefly, Clarity AD was an 18-month, multicenter, double-blind, placebo-controlled, parallel-group trial in individuals with early AD. Eligible participants were randomly assigned to receive placebo or lecanemab 10 mg/kg IV biweekly in a 1:1 ratio. Participants were required to be 50 to 90 years of age, with either MCI due to AD or mild AD dementia based on National Institute of Aging–Alzheimer’s Association (NIA-AA) criteria (16-17).
All participants were required to have positron emission tomography or cerebrospinal fluid evidence of amyloid as well as an objective impairment in episodic memory as indicated by ≥1 standard deviation below the age-adjusted mean on the Wechsler Memory Scale IV-Logical Memory (subscale) II. The trial was conducted in accordance with International Conference on Harmonisation guidelines and ethical principles of the Declaration of Helsinki. Clarity AD was approved by the institutional review board or independent ethics committee at each center, and all participants provided written informed consent.
HRQoL Objectives and endpoints
HRQoL assessments employed in Clarity AD are summarized in Table S1. The objective of this analysis was to evaluate the effects of lecanemab 10 mg/kg biweekly compared to placebo on HRQoL in subjects with early AD at 18 months of treatment as measured by the EQ-5D-5L and QOL-AD. In addition, the effects of lecanemab compared to placebo on study partner burden were evaluated by the ZBI. Change from baseline at 18 months was the main endpoint for each HRQoL assessment. However, each scale was also administered at 6 and 12 months, allowing additional timepoint assessments in relationship to baseline.
EQ-5D-5L measures 5 dimensions of health (mobility, self-care, usual activities, pain or discomfort, and anxiety or depression) with 5 levels of severity in each dimension (no problems, slight problems, moderate problems, severe problems, and unable to perform or extreme problems). The overall current health is scored as Health Today by a visual analog scale (VAS; 0 [worst imaginable health state] to 100 [best imaginable health state]). QOL-AD is a 13-item questionnaire designed to provide an assessment of QOL of patients with AD. Each of 13 items are assessed on a scale of 1-4 (poor, fair, good, or excellent). In addition to direct reporting from the patient, the QOL-AD scales provide the opportunity for separate reporting by the study care partner as a proxy for the patient. ZBI is a 22-item instrument used in dementia caregiving research to assess the stresses experienced by study partners of patients with dementia. The total score range is 0-88 (0-21: no to mild burden; 21-40: mild to moderate burden; 41-60: moderate to severe burden; 61-88: severe burden). The ZBI is completed solely by the care partner.
Statistical methodology
Change from baseline in EQ-5D-5L, QOL-AD, and ZBI at 18 months were analyzed on a modified intent-to-treat population using the mixed model for repeated measures (MMRM; as described in van Dyck 2023). MMRM analysis included baseline value corresponding to the response variable as a covariate, with treatment group, visit, stratification variables (i.e., clinical subgroup [MCI, mild dementia], use of AD symptomatic medication at baseline [yes, no], ApoE4 carrier status [carriers, noncarriers], and geographical region [North America, Europe, and Asia Pacific]), baseline value-by-visit interaction and treatment group-by-visit interaction in the model. Prespecified subgroup analyses included evaluating HRQoL assessments by sex, race/ethnicity, and ApoE4 carrier status.
Results
Baseline characteristics
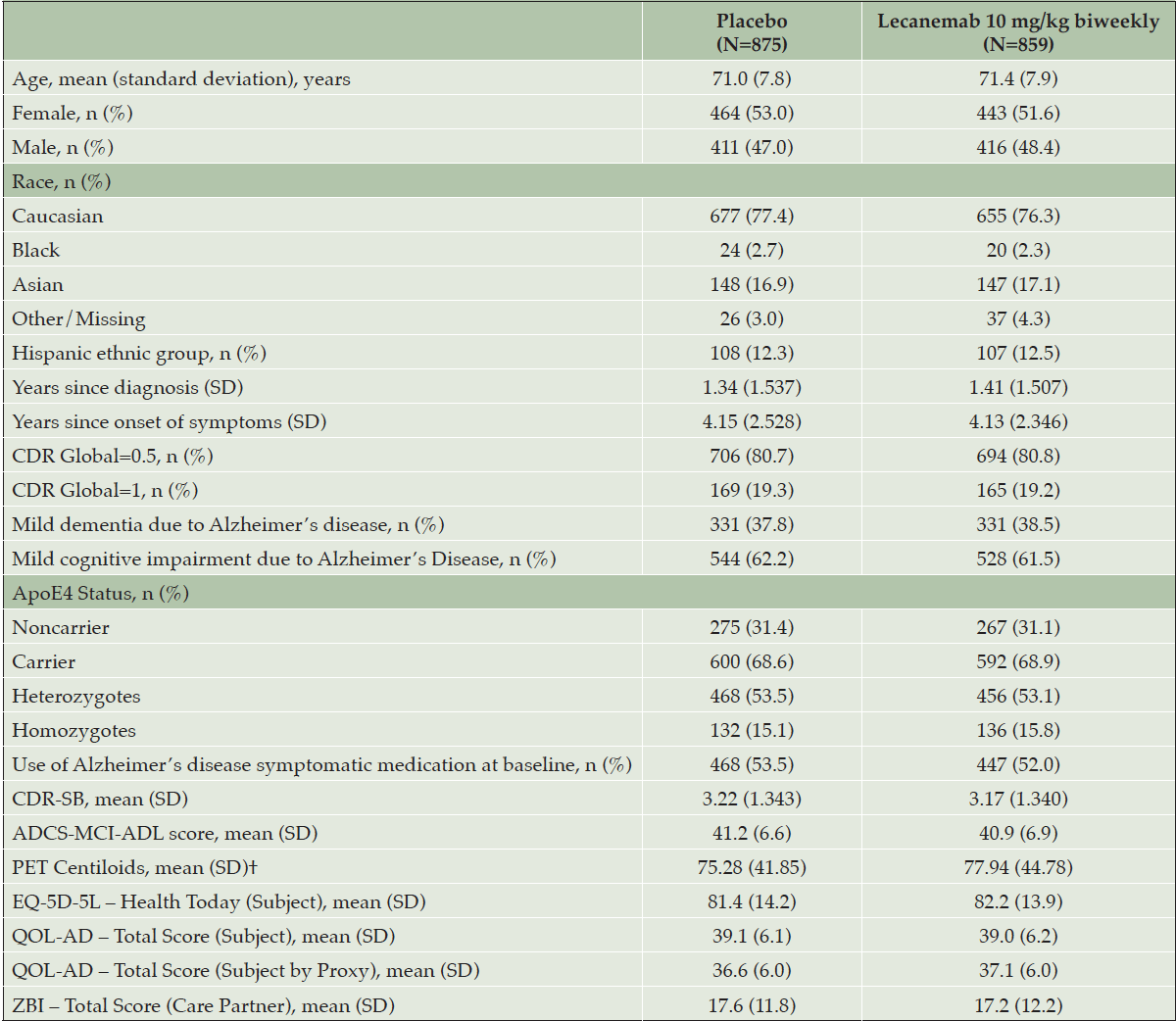
Baseline characteristics are summarized in Table 1. A total of 1795 (898 assigned to lecanemab, and 897 assigned to placebo) were randomized at 235 sites in North America, Europe, and Asia from March 2019 to March 2021. Of those randomized, 729 (81.2%) and 757 (84.4%) completed treatment in the lecanemab and placebo groups, respectively. The modified intention-to-treat population (randomly assigned participants who received at least one dose of lecanemab or placebo and underwent assessment for the primary end point) included 1734 participants (lecanemab:859 placebo:875; missing 3.4% of participants). The majority of participants had MCI (62.2% placebo; 61.5% lecanemab) and the remainder had mild dementia due to AD (37.8% placebo; 38.5% lecanemab). Baseline characteristics were generally similar across treatment groups and baseline HRQoL scores were consistent with an early AD population. Mean baseline EQ-5D-5L was 81.4 and 82.2 for placebo and lecanemab groups respectively, reflecting “slight problems” as rated by subjects. Baseline QOL-AD scores were 39.1 and 39 for placebo and lecanemab respectively, reflecting “good” QOL as rated by subjects. Baseline ZBI scores were 17.6 and 17.2 for placebo and lecanemab respectively, reflecting “no to mild” care partner burden. Study partner baseline characteristics were generally balanced between the two treatment groups (Table S2). None of the participants, sites, or sponsor were unblinded to treatment allocation during the conduct of the study.
†Baseline PET is for the PET substudy population. ADCS MCI-ADL=Alzheimer’s Disease Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment; CDR=Clinical Dementia Rating; ApoE4= apolipoprotein E – e4; CDR-SB= Clinical Dementia Rating-Sum-of-Boxes; EQ-5D-5L=European Quality of Life–5 Dimensions; PET= positron emission tomography; QOL-AD=Quality of Life in AD; ZBI=Zarit Burden Interview.
HRQoL Results
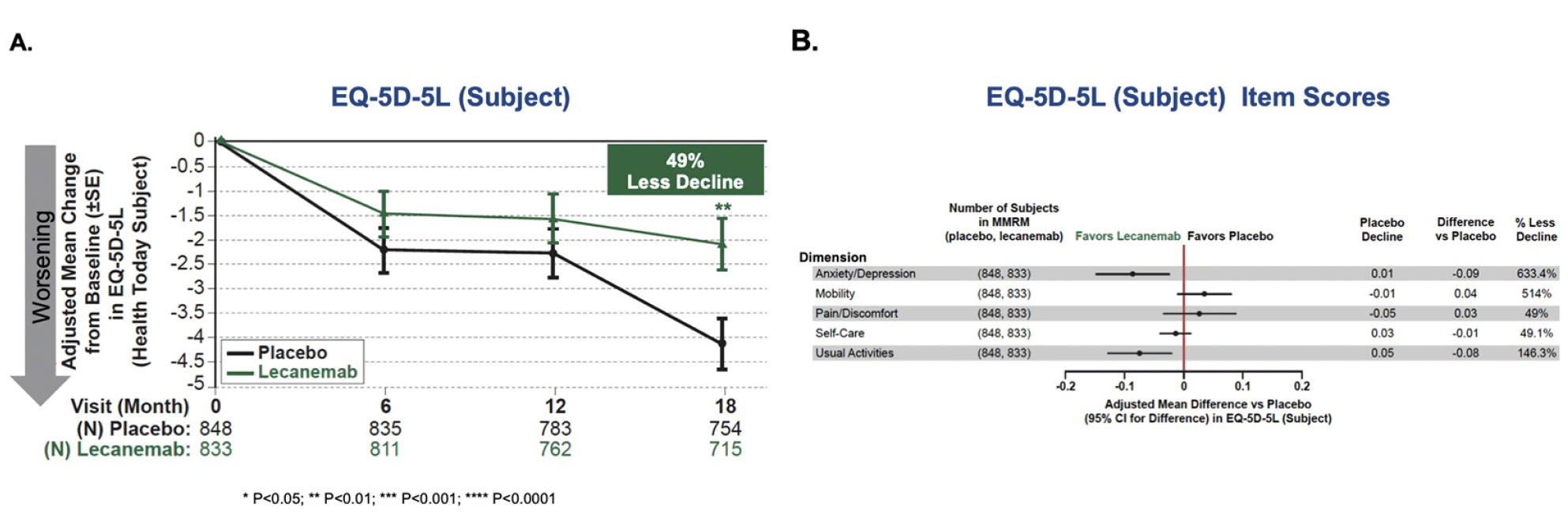
There was a highly statistically significant difference between placebo and lecanemab on change from baseline in EQ-5D-5L Health Today by subject at 18 months in the Subject´s Survey (Figure 1). The adjusted mean treatment difference was 2.017, representing 49.1% less decline, P=0.00383. Separation of results in favor of lecanemab began at 6 months with statistical significance reached by 18 months. Subject scores for the five dimensions are summarized in Figure 1B. The effect favored lecanemab on the dimensions of usual activities, anxiety/depression, and self-care, although did not reach statistical significance for self-care. For mobility and pain/discomfort, the placebo group improved so the percentage less decline shows percentage of less improvement. The EQ-5D-5L results were consistent across APOE genotypes, clinical subgroups of MCI and mild dementia, and the range of randomization strata (Figure S1).
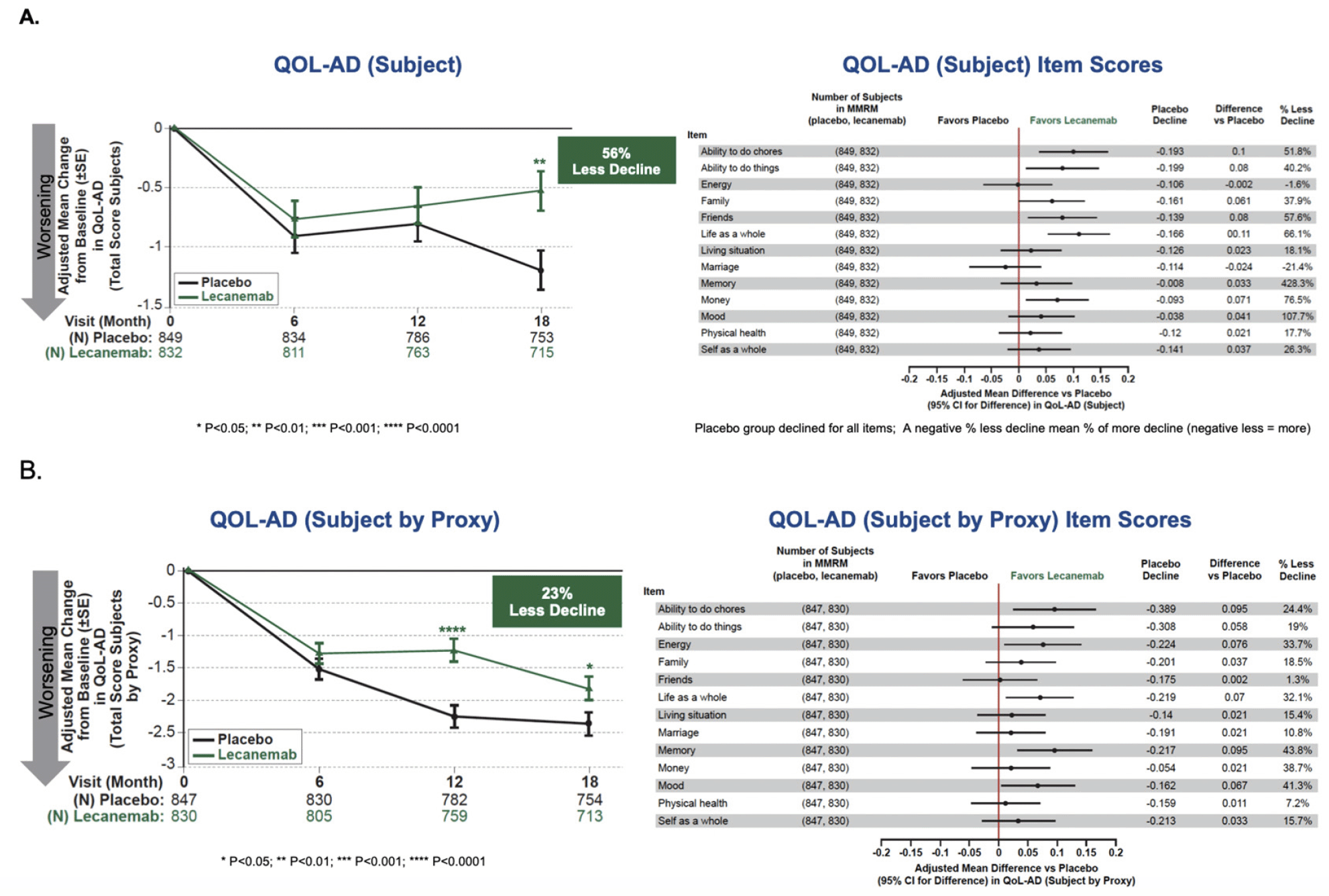
For QOL-AD total score in the Subject´s Survey (Figure 2) there was a highly statistically significant difference between placebo and lecanemab on change from baseline, with an adjusted mean treatment difference of 0.657, and 55.6% less decline, P=0.00231. Separation of results in favor of lecanemab began at 6 months with statistical significance reached by 18 months. In the QOL-AD Partner as a Proxy Survey, the adjusted mean treatment difference was 0.535, representing 22.9% less decline, P=0.02558. Subject scores for individual items are summarized in Figure 2. The observed effect was consistent across QOL-AD items (by subject and by proxy) with almost all the 13 items numerically favoring lecanemab and the placebo group showing decline on all items. Subscores for which differences were in fact significant based on 95% confidence intervals for ratings by subject included: ability to do chores; ability to do things; family; friends; and life as whole, whereas for ratings by proxy significant differences were observed for: ability to do chores; energy; life as whole; memory; and mood. These results were consistent across subgroups (Figure S2).
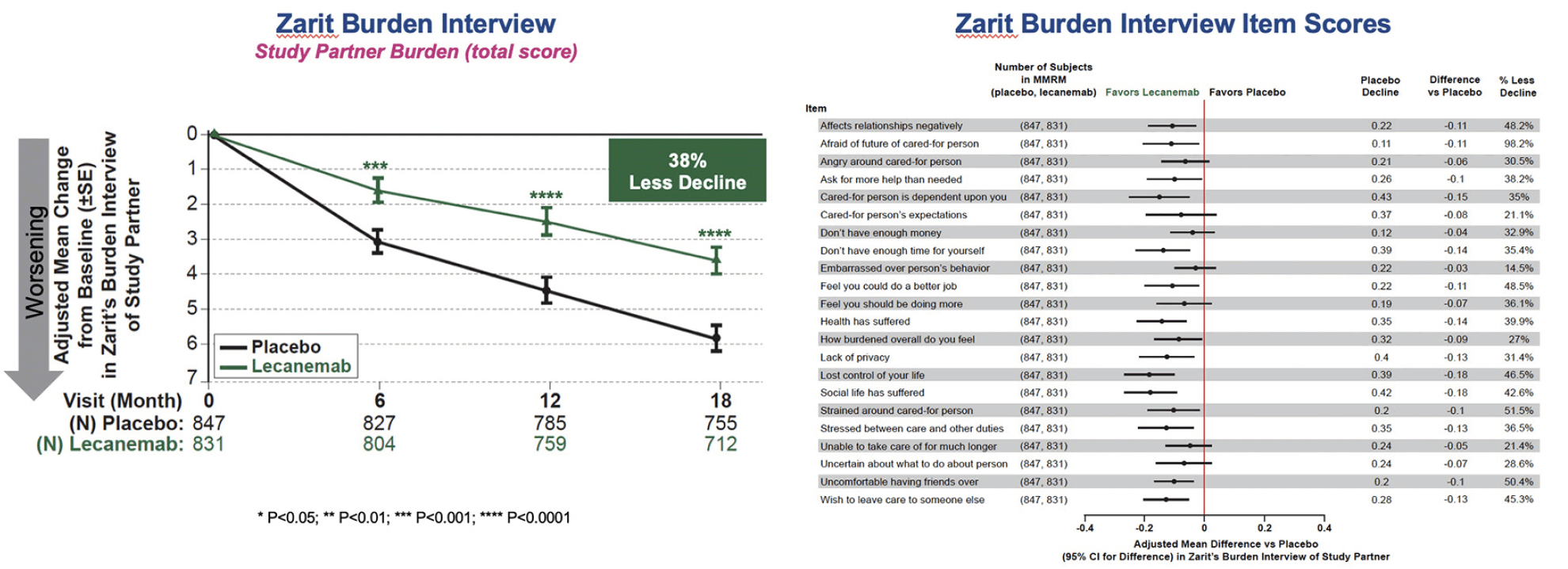
For the ZBI study partner total score, there was a highly statistically significant difference between placebo and lecanemab on change from baseline, with an adjusted mean treatment difference of -2.211, and 38.4% less progression, P=0.00002. The separation between lecanemab and placebo was statistically significant as early as 6 months and increased numerically thereafter. Study partner scores for individual items are summarized in Figure 3. The results show consistent benefit with all 22 items favoring lecanemab over placebo. Results were consistent across subgroups (Figure S3).
* P<0.05; ** P<0.01; *** P<0.001; **** P<0.0001
Discussion
Preserving QOL is an important goal in the treatment of AD. At early stages of disease, namely the MCI and mild dementia stages, QOL is only mildly impacted as evidenced by the baseline scores on HRQoL measures in this trial (11). However, deterioration in QOLand worsening of care partner burden is an integral part of AD progression, and this worsening can be detected and quantified even at early stages of AD and over the 18-month timeframe of this trial.
Lecanemab was associated with a relative preservation of HRQoL and less worsening of caregiver burden. Consistent benefits were seen across different scales, within scales, and across randomization strata. At month 18, adjusted mean change from baseline in EQ-5D-5L and QOL-AD by subject showed 49% and 56% less decline, respectively. Study partner burden as measured by adjusted mean change from baseline at 18 months using the ZBI resulted in 38% less decline. For each HRQoL subject assessment, results separate in favor of lecanemab beginning at 6 months.
Although proxy measures were obtained in Clarity AD, HRQoL measures are ideally reported directly by patients on their own behalf rather than being inferred by a proxy. The concept of QOL by proxy in AD may be reasonable and necessary when patients reach stages of disease in which worsening cognitive impairment limits their insight and/or their ability to communicate their views. However, in early symptomatic stages of AD, patients are the more credible respondents regarding their own QOL as they are able to relay firsthand their frustrations, concerns, limitations, aspirations, and successes, while proxy measures are subject to considerable bias and assumptions about what someone else feels and values. It is instructive that the proxy results for the EQ-5D-5L in this trial (not statistically significant) and QOL-AD (23% less decline) do not reflect the benefit expressed by subjects themselves. This serves to reinforce the importance of patient reported outcomes in early AD populations.
The HRQoL by subject results are consistent with the previously reported clinical outcomes from Clarity AD (15), which demonstrated 26% to 37% less decline on cognitive, global, and functional measures. The magnitude of benefit on subject reported QOL measures (49% to 56% less decline) most closely corresponds to the results on the functional scale, the Alzheimer’s Disease Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment (ADCS-MCI-ADL) which showed an adjusted mean change from baseline at 18 months of 37% less decline (−3.5 in the lecanemab group and −5.5 in the placebo group; difference, 2.0; 95% CI, 1.2 to 2.8; P<0.00001). Preservation of daily functional abilities is a key contributor to one’s quality of life, although HRQoL is broader than function alone.
Care partners face significant burden in caring for individuals with AD, and the severity of burden increases substantially as the disease progresses to more advanced stages (18- 24). At early stages of AD, care partners often assume the role of the supportive partner and trusted confidant. However, in later stages, the demands on care partners’ time along with the financial burden, and toll on the emotional, social, and physical health of care partners can be overwhelming and may lead to emergency room visits, hospitalizations, and institutionalization of patients. In Clarity AD, care partner burden is minimal at baseline. The reduced worsening of burden relative to placebo, seen as early as 6 months and attaining 38% less progression of burden by 18 months, is clinically meaningful.
HRQoL findings are seldom reported for AD pharmacological trials including those aimed at disease modification. Relevant HRQoL assessments for comparison with our findings are not readily available in the literature. The appropriate comparators would be other large pivotal trials of monoclonal antibody treatments for early AD. The lecanemab phase 2 study did not include a HRQoL assessment (25). Furthermore, there are no HRQoL assessments published from the phase 3 aducanumab or donanemab studies to date (26-28). A 2016 publication explores the characteristics of the QOL-AD assessment within the context of the EXPEDITION and EXPEDITION 2 solanezumab trials (29). In this publication, the authors noted that caregivers rated patients’ QOL worse than did patients themselves and that patients’ QOL was correlated, albeit modestly, with clinical/health measures.
Clinicians and patients often have differing perspectives on assessing the impact of treatment (30). Patients tend to value outcomes such as preservation of everyday functioning, maintaining relationships and social connections, enjoying their lives, preserving a sense of identity and alleviating symptoms (31). The concept of “meaningful benefits” is broader than that of “minimal clinically important differences” and can include benefits such as buying time/slowing disease and maintaining QOL (27, 32). The clinical and QOL benefits observed in Clarity AD could amount to long-term cumulative benefit in which the difference between the benefit on and off treatment could continue to grow in time beyond that observed in the 18-month clinical trial (15, 33).
The study population in Clarity AD is consistent with patients with early AD, as evidenced by the baseline mean Clinical Dementia Rating Sum-of-Boxes values as well as the fact that over 60% of individuals were in the MCI stages of AD when enrolling in the trial. QOL is affected early in AD and continues to decline even within the early stages. Its decline, and the impact of interventions, can be demonstrated in an early AD population and hence QOL considerations should not be reserved only for moderate to severe stages of disease.
One limitation of this analysis is that the assessments in this trial are only for 18 months. However, future studies will look at longer term HRQoL. For example, the ongoing Clarity AD open-label extension study includes HRQoL assessments and will provide additional insights when data are available. In addition, unintended bias may always be a limitation in the conduct of clinical trials (e.g., attrition bias, observer bias, manufacturer bias, AD spectrum bias, etc.). Attempts were made to minimize any bias during the conduct of the trial (15). In summary, lecanemab in Clarity AD was associated with a relative preservation of HRQoL and less increase in caregiver burden, as reported by patients and their care partners, with consistent benefits seen across different scales, within items and subdomains of these scales, and across randomization strata. At month 18, adjusted mean change from baseline in in EQ-5D-5L and QOL-AD by subject showed 49% and 56% less decline, respectively. QOL-AD by proxy showed 23% less decline. Study partner burden measured by ZBI resulted in 38% less increase of burden at 18 months and was already evident and statistically significant at 6 months. Assessment results were consistent across APOE genotypes. The results of multiple QOL measures from Clarity AD add to the previously reported converging evidence across measures of cognition, function, disease progression, and biomarkers, demonstrating that lecanemab treatment may offer meaningful benefits to patients and care partners and support the view that QOL measures are highly informative in early AD, allowing the patients’ and care partners’ perspectives to be heard. Future publications will include additional analyses such as whether the benefits of lecanemab on these HRQoL outcomes correlated with cognitive and functional outcomes and whether results differed based on the amount of amyloid cleared and/or the rate of amyloid clearance.
Funding: This study was funded by Eisai Inc and Biogen.
Acknowledgements: The authors would like to acknowledge the subjects who participated in these studies and their families, as well as all the investigators and site staff who made these studies possible. The authors would also like to acknowledge editorial support from Lisa Yarenis (Eisai Inc) and JD Cox, PhD, and Mayville Medical Communications, funded by Eisai Inc. and in compliance with Good Publication Practice 4 ethical guidelines (DeTora et al., Ann Intern Med 2022; 30 August 2022 [epub ahead of print]). The authors did not receive remuneration for their participation in the preparation, review and approval of this manuscript. The sponsor, Eisai Inc., designed the studies and analyzed the data in collaboration with academic authors, provided trial drug, provided funding for medical writing, and aided in drafting the manuscript.
Conflict of interest: SC is a consultant (no personal fees received) to Alnylam, Biogen, Cassava Sciences, Cognivue, Cogstate, Eisai, Eli Lily, INmune Bio, ProMIS Neuroscience, Roche, and RetiSpec and receives research support (paid to institution) from AbbVie, AgeneBio, Alnylam, Anavex, Biogen, Cassava, Eisai, Eli Lilly, Genentech, Green Valley, Janssen, RetiSpec, Roche, and Vielight. CvD is a consultant for Roche, Eisai, Cerevel, and Ono and receives research support from Biogen, Eisai, Roche, Genentech, Eli Lilly, Janssen, UCB, Cerevel, and Biohaven. MG, TD, MK, SD, DL, SH, MI, AND LDK are employees of Eisai.
ClinicalTrials.gov identifier: NCT03887455.
Ethical standards: The trial was conducted in accordance with International Conference on Harmonisation guidelines and ethical principles of the Declaration of Helsinki. Clarity AD was approved by the institutional review board or independent ethics committee at each center, and all participants provided written informed consent.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577-1590. doi: 10.1016/S0140-6736(20)32205-4.
2. Masters C, Bateman R, Blennow K, et al. Alzheimer’s disease. Nature Reviews 2015;1:15056. doi: 10.1038/nrdp.2015.56.
3. Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4,
4. Burns A, Iliffe S. Alzheimer’s disease. BMJ. 2009;338 b158. Burns A, Iliffe S. Alzheimer’s disease. BMJ. 2009;338 b158. doi: 10.1136/bmj.b1349.
5. Weyerer S, Schäufele M. The assessment of quality of life in dementia. Int Psychogeriatr. 2003 Sep;15(3):213-8. PMID: 14756157.
6. Lawton MP. Quality of life in Alzheimer disease. Alzheimer Dis Assoc Disord. 1994;8 Suppl 3:138-50. PMID: 7999340.
7. Barbe C, Jolly D, Morrone I, Wolak-Thierry A, Dramé M, Novella JL, Mahmoudi R. Factors associated with quality of life in patients with Alzheimer’s disease. BMC Geriatr. 2018 Jul 9;18(1):159. doi: 10.1186/s12877-018-0855-7.
8. WHO. Programme on Mental Health, WHOQOL User Guide. 1998. Available at: https://www.who.int/toolkits/whoqol Accessed May 23, 2023.
9. Centers for Disease Control and Prevention. Measuring healthy days: Population assessment of health-related quality of life. Centers for Disease Control and Prevention, Atlanta, Georgia 2000. Available at: https://www.cdc.gov/hrqol/pdfs/mhd.pdf Accessed Julye 18, 2023.
10. EQ-5D-5L. Available at: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/.
11. Kahle-Wrobleski K, Ye W, Henley D, Hake AM, Siemers E, Chen YF, Liu-Seifert H. Assessing quality of life in Alzheimer’s disease: Implications for clinical trials. Alzheimers Dement (Amst). 2016 Dec 13;6:82-90. doi: 10.1016/j.dadm.2016.11.004.
12. Bédard M, Molloy DW, Squire L, Dubois S, Lever JA, O’Donnell M. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41:652-7. doi: 10.1093/geront/41.5.652.
13. Nilsberth C, Westlind-Danielsson A, Eckman CB, et al. The ‘Arctic’ APP mutation (E693G) causes Alzheimer’s disease by enhanced Abeta protofibril formation. Nat Neurosci. 2001;4:887-93. doi: 10.1038/nn0901-887.
14. Tucker S, Möller C, Tegerstedt K, et al. The murine version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-ArcSwe mice. J Alzheimers Dis. 2015;43:575-588. doi: 10.3233/JAD-140741.
15. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023;388:9-21. doi: 10.1056/NEJMoa2212948.
16. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-9. doi: 10.1016/j.jalz.2011.03.008.
17. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011 May;7(3):263-9. doi: 10.1016/j.jalz.2011.03.005.
18. Kim B, Noh GO, Kim K. Behavioural and psychological symptoms of dementia in patients with Alzheimer’s disease and family caregiver burden: a path analysis. BMC Geriatr. 2021 Mar 5;21(1):160. doi: 10.1186/s12877-021-02109-w.
19. Yu H, Wang X, He R, Liang R, Zhou L. Measuring the Caregiver Burden of Caring for Community-Residing People with Alzheimer’s Disease. PLoS One. 2015 Jul 8;10(7):e0132168. doi: 10.1371/journal.pone.0132168.
20. Schulz R, Mendelsohn AB, Haley WE, et al. End-of-Life care and the effects of bereavement on family caregivers of persons with dementia. The New England Journal of Medicine. 2003; 349:1936–1942. doi: 10.1056/NEJMsa035373.
21. Schulz R, O’Brien A, Czaja S, et al. Dementia caregiver intervention research: In search of clinical significance. The Gerontologist. 2002; 42:589–602. doi: 10.1093/geront/42.5.589.
22. Tanji H, Ootsuki M, Matsui T, et al. Dementia caregivers’ burdens and use of public services. Geriatrics and Gerontology International. 2005; 5:94–98. doi: 10.1111/j.1447-0594.2005.00274.x
23. Huang SS, Lee MC, Liao YC, Wang WF, Lai TJ. Caregiver burden associated with behavioral and psychological symptoms of dementia (BPSD) in Taiwanese elderly. Archives of Gerontology and Geriatrics. 2012; 55:55–59. 10.1016/j.archger.2011.04.009
24. Beeri MS, Werner P, Davidson M, Noy S. The cost of behavioral and psychological symptoms of dementia in community dwelling Alzheimer’s disease patients. Int J of Geriatr Psychiatry. 2002;17: 403–408. doi: 10.1002/gps.490.
25. Swanson CJ, Zhang Y, Dhadda S, et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res Ther. 2021;13:80. doi: 10.1186/s13195-021-00813-8.
26. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J Prev Alzheimers Dis. 2022;9:197-210. doi: 10.14283/jpad.2022.30.
27. Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in Early Alzheimer’s Disease. N Engl J Med. 2021;384:1691-1704. doi: 10.1056/NEJMoa2100708.
28. Sims JR, Zimmer JA, Evans CD, et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA. 2023 Jul 17. doi: 10.1001/jama.2023.13239. Epub ahead of print.
29. Kahle-Wrobleski K, Ye W, Henley D, et al. Assessing quality of life in Alzheimer’s disease: Implications for clinical trials. Alzheimers Dement (Amst). 2016 Dec 13;6:82-90. doi: 10.1016/j.dadm.2016.11.004.
30. Assunção SS, Sperling RA, Ritchie C, K, et al. Meaningful benefits: a framework to assess disease-modifying therapies in preclinical and early Alzheimer’s disease. Alzheimers Res Ther. 2022;14:54. doi: 10.1186/s13195-022-00984-y.
31. Watson J, Saunders S, Muniz Terrera G, et al. What matters to people with memory problems, healthy volunteers and health and social care professionals in the context of developing treatment to prevent Alzheimer’s dementia? A qualitative study. Health Expect. 2019;22:504-517. doi: 10.1111/hex.12876.
32. Andrews JS, Desai U, Kirson NY, Zichlin ML, Ball DE, Matthews BR. Disease severity and minimal clinically important differences in clinical outcome assessments for Alzheimer’s disease clinical trials. Alzheimers Dement (N Y). 2019;5:354-363. doi: 10.1016/j.trci.2019.06.005.
33. Petersen RC, Aisen PS, Andrews JS, Atri A, Matthews BR, Rentz DM, Siemers ER, Weber CJ, Carrillo MC. Expectations and clinical meaningfulness of randomized controlled trials. Alzheimers Dement. 2023 Jun;19(6):2730-2736. doi: 10.1002/alz.12959.
© The Authors 2023