M. Riessland1,2, M.E. Orr3,4
1. Department of Neurobiology and Behavior, Stony Brook University, Stony Brook, NY, USA; 2. Center for Nervous System Disorders, Stony Brook University, Stony Brook, NY, USA; 3. Department of Internal Medicine Section on Gerontology and Geriatric Medicine, Wake Forest University School of Medicine, Winston-Salem, NC, USA; 4. Salisbury VA Medical Center, Salisbury, NC, 28144, USA.
Corresponding Author: Miranda E. Orr, 575 Patterson Ave, Winston-Salem, NC 27101, Telephone Number: (336)716-7804, morr@wakehealth.edu
J Prev Alz Dis 2023;4(10):633-646
Published online October 24, 2023, http://dx.doi.org/10.14283/jpad.2023.104
Abstract
The recent FDA-approval for amyloid lowering therapies reflects an unwavering commitment from the Alzheimer’s disease (AD) research community to identify treatments for this leading cause of dementia. The clinical benefits achieved by reducing amyloid, though modest, provide evidence that disease modification is possible. Expanding the same tenacity to interventions targeting upstream drivers of AD pathogenesis could significantly impact the disease course. Advanced age is the greatest risk factor for developing AD. Interventions targeting biological aging offer the possibility of disrupting a foundational cause of AD. Senescent cells accumulate with age and contribute to inflammation and age-related diseases like AD. Senolytic drugs that clear senescent cells improve healthy aging, halt AD disease progression in animal models and are undergoing clinical testing. This review explores the biology of aging, the role of senescent cells in AD pathology, and various senotherapeutic approaches such as senolytics, dampening the SASP (senescence associated secretory phenotype), senescence pathway inhibition, vaccines, and prodrugs. We highlight ongoing clinical trials evaluating the safety and efficacy of the most advanced senolytic approach, dasatinib and quercetin (D+Q), including an ongoing Phase II senolytic trial supported by the Alzheimer’s Drug Discovery Foundation (ADDF). Challenges in the field of senotherapy for AD, including target engagement and biomarker development, are addressed. Ultimately, this research pursuit may lead to an effective treatment for AD and provide the field with another disease-modifying therapy to be used, alone or in combination, with other emerging treatment options.
Key words: Alzheimer’s disease, biology of aging, cellular senescence, senolytics, tau.
Abbreviations: Aβ: Amyloid beta; AD: Alzheimer’s disease; ADDF: Alzheimer’s Drug Discovery Foundation; ADRD: Alzheimer’s disease and related dementias; CDR: Clinical Dementia Rating; CNS: Central nervous system; CSF: Cerebrospinal fluid; COX-2: Cyclooxygenase 2; D: Dasatinib; D+Q: Senolytic therapy including the combination of Dasatinib and Quercetin; FDA: Food and Drug Administration; FOXO4: Forkhead box protein O4; FOXO4-DRI: FOXO4-D-Retro-Inverso; PET: Positron emission tomography; MAPK: Mitogen-activated protein kinas; MDM2: Murine double minute 2; mTOR: Mammalian target of rapamycin; MRI: Magnetic resonance imaging; NFT: Neurofibrillary tangle; OPC: Oligodendrocyte precursor cell; PI3Kδ: Phosphatidylinositol 3-kinase-delta; PL: Piperlongumine; Q: Quercetin; ROS: Reactive oxygen species; SToMP-AD: Senolytic Therapy to Modulate the Progression of Alzheimer’s Disease, acronym for clinical trial; USP7: Ubiquitin-specific peptidase 7; TBI: Traumatic brain injury; uPAR: Urokinase-type plasminogen activator receptor; WAT: White adipose tissue.
Introduction: Biology of Aging and Alzheimer’s disease
Older adults fear developing dementia more than any other health condition (1-3). The World Alzheimer Report (2021) estimates that over 55 million individuals live with dementia (4); Alzheimer’s disease (AD) is the leading cause. AD is histologically defined by the accumulation of Aβ plaques (extracellular) and tau protein aggregates (intracellular) in the brain (5). Clinical trials that successfully reduce these abnormal protein deposits have demonstrated only modest disease-modifying effects (6, 7) highlighting the continued need for additional therapeutic approaches to prevent, slow and treat AD.
Historically, research focused on identifying AD drug targets and interventions have experienced significant failures with a ~1% success rate (8). One major hurdle in drug development is an incomplete understanding of the underlying mechanisms that drive Aβ plaques and tau-containing neurofibrillary tangles (NFTs) in the brain which may begin decades prior to cognitive decline (9-11). The greatest risk factor for developing AD is advanced age (12). While chronological aging is inevitable, research using laboratory organisms has identified cellular and molecular processes that directly influence successful biological aging (13). These “hallmarks of aging” satisfy three conditions: (i) they become apparent with advancing age, (ii) experimentally perturbing them causes poor aging (iii) therapeutically intervening upon them prevents, delays or reverses aging phenotypes.
Initially identified as nine fundamental biological drivers of aging (13), the list has been updated to include twelve hallmarks: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, disabled macroautophagy, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, altered intercellular communication, chronic inflammation, and dysbiosis (14). Their organization into broad categories represents an interconnected hierarchy where the primary hallmarks reflect macromolecular damage (i.e., genome, proteome, organelles) which lead to antagonistic hallmarks, e.g., response to the damage. Integrative hallmarks refer to the downstream consequences of the persistent, irreversible stress.
Experimentally manipulating aging biology influences AD pathogenesis which provides a mechanistic link between increasing age and elevated risk of developing AD (reviewed, (15)). Moreover, many proposed AD hypotheses overlap with biology of aging hallmarks. For example, the amyloid hypothesis (16, 17) aligns with the decline in proteostasis (a primary hallmark); the cholinergic hypothesis (18) reflects deficits in intercellular communication; the mitochondrial cascade hypothesis (19) falls under an antagonistic hallmark; while the inflammatory hypothesis (20) represents one of the integrative hallmarks. Given the interconnected relationship among biological aging hallmarks, manipulating one has potential to impact others. For example, cellular senescence, classified as an antagonistic/secondary hallmark of aging, results as a consequence of persistent DNA and/or proteomic damage (primary hallmarks) that drives integrative hallmarks such as chronic inflammation and altered intracellular signaling, which further perpetuate other aging hallmarks. Targeting cellular senescence has emerged as an especially appealing therapeutic target given its contribution to persistent inflammation and functional decline across tissues and organ systems (reviewed, (21)).
Cellular Senescence
The cellular senescence fate is initiated and sustained through an elaborate cellular stress response. Instead of reacting to stressors by uncontrolled proliferation (cancer) or apoptosis (degeneration), senescent cells survive in a stable cell cycle arrest. They undergo profound chromatin changes, metabolic alterations, cellular remodeling, and resistance to apoptosis (22-28). This switch in cell fate may be advantageous in tissues with limited replicative potential, like neurons in the brain and myocytes of the heart; however, senescent cells infamously secrete high levels of pro-inflammatory cytokines, chemokines, and extracellular matrix-degrading proteins that negatively impact the extracellular environment and induce spread of senescence to non-senescent cells (29-32). This toxic secretome, referred to as senescence-associated secretory phenotype (SASP), contributes to tissue destruction and chronic cell death.
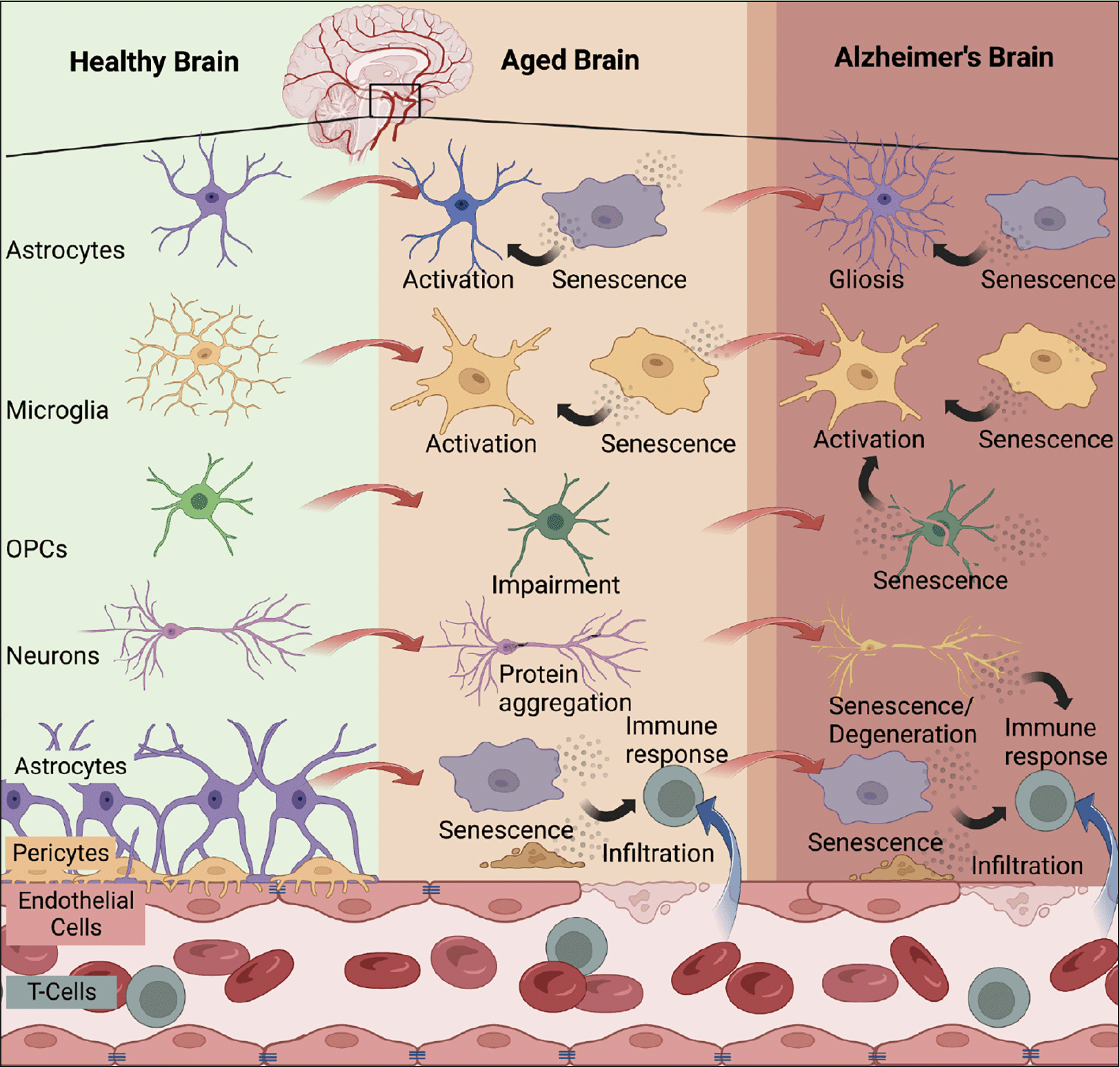
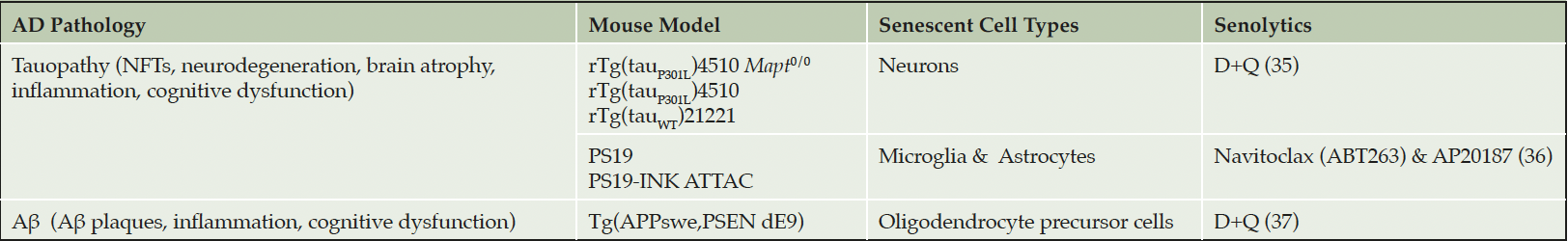
Preclinical studies indicate that senescent cell burden is low in young rodents and increases with chronological age in several tissues (33, 34). Tau (35, 36) and Aβ (37) accumulation induces premature cellular senescence in transgenic mouse models of AD neuropathology. Similarly, various senescent cell types have been identified in postmortem AD brain tissue including astrocytes (38), neurons (35, 39), microglia (40), oligodendrocyte precursor cells (37), and endothelial cells (41). We analyzed single-cell transcriptomic data generated from the dorsolateral prefrontal cortex of individuals with various levels of AD neuropathology; approximately 2% of brain cells were senescent, with excitatory neurons as the main contributing cell type (39). The cell cycle inhibitor, CDKN2D/p19INK4d, and NFT molecular signatures significantly overlapped with the senescent neuron population (39). Interestingly, bioinformatic analyses conducted on bulk tissue data obtained from healthy human brain tissue donors revealed that endothelial cells and microglia were the predominant senescent cell types, driven by CDKN1A/p21WAF1/CIP1 (42) revealing potential differences in senescent cell types between healthy and diseased states. Nevertheless, data generated from multiple groups have demonstrated the presence of senescent cells in postmortem human brain (Figure 1).
Proof of principle experiments utilizing genetically engineered progeroid mouse models conclusively demonstrated that senescent cells contribute to poor aging (43). Genetic ablation of senescent cells ameliorated the progeroid phenotypes and produced overall health benefits. A later study using the same genetic approach to clear senescent cells, but this time in naturally aging mice, provided further evidence that removing senescent cells improves healthy aging (44). The discovery of compounds that selectively remove senescent cells eliminated the need for genetic models and catapulted this field of study (24, 45). The initial discovery of senolytics involved bioinformatics and transcriptomics to evaluate RNA and protein expression profiles of senescent cells. The approach revealed that the anti-apoptotic survival of senescent cells relied on six senescent-cell anti-apoptotic pathways (SCAPs) (45, 46). These pathways include BCL-XL, the cell cycle inhibitor p21, the ephrins EFNB1 and EFNB3, serpin PAI1, the oxygen sensor HIF-1α and phosphatidylinositol 3-kinase-delta (PI3Kδ). Decreasing the expression of key proteins in SCAP pathways induced senescent cell death, promoting the discovery of small molecules and compounds that target these SCAPs, referred to as “senolytics”.
Multiple brain cell types have been identified to enter cellular senescence with advanced aging and/or AD including astrocytes (38), microglia (40), OPCs (37), neurons (35, 39), endothelial cells (41), and smooth muscle vascular cells (42). The accumulation of senescent cells with age leads to immune reactions that have detrimental effects on the brain. Persistent senescent cells can propagate the senescent phenotype, resulting in blood-brain barrier breakdown, infiltration of systemic immune cells, neurodegeneration, and gliosis, all of which are observed in the context of AD. Created with BioRender.com (Agreement no: ED25XPZLUF).
Senolytics
Among the first senolytic agents discovered was a combination therapy consisting of Dasatinib (D; Sprycel®, Bristol Myers Squibb), a tyrosine kinase inhibitor that is FDA approved for use in humans for the treatment of leukemias, and Quercetin (Q), a flavonoid (45). Despite its ability to induce cancer cell apoptosis, D did not show strong capacity to clear senescent cells when used as a monotherapy. Similarly, the naturally occurring flavonoid Q possesses various biological activities such anti-inflammatory and relatively unspecific kinase inhibitory properties, but as a stand-alone-treatment displays only marginal senolytic effects. The D+Q combination targets a greater number of SCAPs (45), leading to effective senescent cell clearance across tissues and positively impacts several age-associated conditions and diseases. These include neurodegeneration, frailty, osteoporosis, vasomotor dysfunction, hepatic steatosis, insulin resistance, pulmonary fibrosis, chronic kidney disease, and skeletal muscle dysfunction (47).
Natural compounds
Similar to Q, fisetin is a naturally occurring flavonoid that is present in various fruits, vegetables, and teas (48, 49). It is widely used as a supplement and possesses diverse pharmacological effects, including antioxidant, antidiabetic, anti-inflammatory, anti-cancer, antibacterial, antiviral, and neuroprotective activities (48-50). On the molecular level, fisetin acts on multiple signaling pathways, including BCL-2, PI3K/AKT, p53, and NF- κB, all of which are critical for senescence. Consequently, research has demonstrated that fisetin exhibits senolytic activity, which was discovered in a screen of flavonoid polyphenols in senescent human and murine fibroblasts (51, 52). It has been shown that fisetin reduced senescence in a cell type-specific manner: in white adipose tissue (WAT), it reduced mesenchymal stem/progenitor, immune, and endothelial cells, while senescent dendritic cells and macrophages were not changed. Interestingly, fisetin has been reported to restore tissue homeostasis, reduce age-related pathology, and extended median and maximum lifespan in wild-type mice (51). In the context of AD, fisetin has been shown to modulate inflammatory pathways and maintain cognitive function in AD transgenic mice (53) and to enhance memory and induce hippocampal long-term potentiation in rats (54). These findings suggest that fisetin can cross the blood brain barrier and that it has beneficial effects on the hippocampus and can ameliorate AD symptoms in vivo. Given the positive results across tissues and diseases of aging, fisetin has entered clinical testing. Currently around 20 clinical trials are ongoing using fisetin. These clinical trials target diverse age-related diseases including osteoarthritis (NCT05276895), frailty (NCT03675724), mild cognitive impairment (NCT02741804), aging (NCT04994561) and others (55).
In addition to fisetin and Q, other natural substances derived from plants also exhibit senolytic properties, such as piperlongumine (PL) and its analogues (56), or the curcumin analog EF24 (57). Both compounds have been shown to ablate senescent cells in a ROS (reactive oxygen species)-independent manner and are able to synergize with other senolytic compounds like navitoclax (ABT-263) (56, 58, 59). These natural senolytic compounds offer potential therapeutic strategies for combating age-related diseases and improving health-span. Due to the lack of comprehensive studies on natural compounds, little is known about their dosing and side effect profiles. The initial clinical studies on fisetin, and others, will be critical to inform the field on the safe use of natural compounds as senolytic therapies.
BCL-2 family inhibitors
Senescent cells enter a near-permanent cell cycle arrest and activate an anti-apoptotic program to persist in the tissue where they contribute to inflammation. An important anti-apoptotic pathway that that allows senescent cell survival involves expression of members of the BCL-2 family of proteins, including BCL-2, BCL-XL, and BCL-W (60). Inhibition of the BCL-2 pathway induced apoptosis of some senescent cell types, such as mouse and human fibroblasts, HUVECs, as well as cells of the lung and epidermis, but not primary human preadipocytes (60, 61). Consequently, BCL-2 family inhibitors have been identified to function as senolytics, including ABT-737, ABT-263 (Navitoclax), A-1331852, and A-1155463. The first characterization of ABT-737, an inhibitor of BCL-W, BCL-XL, and BCL-2, has been shown to specifically induce apoptosis in senescent cells in a BCL family-dependent manner (60). In mice, ABT-737 efficiently removed senescent cells in the lungs as well as in the epidermis, leading to an increased hair-follicle stem cell proliferation (60). However, ABT-737 has limited oral bioavailability and aqueous solubility. To overcome these limitations, ABT-263 (Navitoclax), a derivative of ABT-737, was developed as an orally bioavailable pan-BCL inhibitor (62). In addition to its in vitro senolytic activity, ABT-263 has been shown to rejuvenate aged hematopoietic stem cells and effectively clear senescent cells in irradiated or aged mice (63). Unfortunately, significant side effects such as thrombocytopenia and transient thrombocytopathy have been reported for ABT-263 (64). In addition to systemic adverse effects, inhibiting BCL-2 family members could be problematic for diseases and conditions of the CNS. In our in vitro model, we found that treatment with ABT-737 is toxic to stem cell derived dopaminergic neurons (65). The underlying reason for this toxicity is most likely because neuronal development and survival is based on BCL-XL activity (66). Additionally, navitoclax is almost impenetrable to the blood brain barrier, thus, the current drugs are not able to reach the brain parenchyma (67). Thus, despite the demonstrated senolytic effects of BCL-2 family inhibitors, their clinical translation is limited due to potential side effects, off-target effects on platelets and poor central nervous system (CNS) penetrance. Further research is needed to develop more specific, safe and brain penetrant BCL-2 family inhibitors for senolytic therapies.
P53 pathway modulators
P53 controls senescence and apoptosis in part via regulation of BCL-2 (68, 69). In line with this, p53 modulators have been investigated as potential senolytic agents. Forkhead box protein O4 (FOXO4) is an anti-apoptotic transcription factor which is upregulated in some senescent cells and prevents cell death by sequestering the nuclear protein p53 (70). A senolytic peptide called FOXO4-D-Retro-Inverso (FOXO4-DRI) has been reported to disrupt the interaction between FOXO4 and p53, allowing p53 to translocate to the cytosol and induce apoptosis. FOXO4-DRI selectively removed senescent cells and has shown benefits in alleviating age-related symptoms in animal models, including hypogonadism, skin phenotypes, fur density, and renal function.
Protein levels of p53 are regulated through its ubiquitination by the E3 ubiquitin ligase, murine double minute 2 (MDM2). Inhibitors of the MDM2/p53 interaction, such as UBX0101 and RG7112 (RO5045337), and inhibition of the de-ubiquitinating ubiquitin-specific peptidase 7 (USP7) increase and stabilize p53 levels and have been investigated for their senolytic effects. RG7112 selectively cleared senescent intervertebral disc cells, reduced senescence-associated secretory phenotype (SASP) factors, and improved disc matrix homeostasis in human disc tissue. UBX0101 induced apoptosis in senescent chondrocytes in an osteoarthritis mouse model. USP7 inhibitors, such as P5091 and P22077, selectively ablated senescent cells by upregulating p53 (71).
While p53 modulators, including FOXO4-DRI, MDM2 inhibitors, and USP7 inhibitors have shown senolytic effects by selectively eliminating senescent cells or promoting their apoptosis in the laboratory, translating these findings to the clinic have so far been unsuccessful. A Phase II clinical study of UBX0101 for osteoarthritis did not show statistical significance compared to placebo. Given that the role of p53 in senescence regulation is complex, with both promoting and inhibitory effects depending on cell type and stress levels, manipulating the levels of p53 could have unwanted consequences. For example, activity of p53 can induce p21-dependent senescence. Moreover, some agents that have been used to interfere with p53 function are peptide-based, which would render them difficult to apply for brain senescence. In summary, additional research is needed to understand the intricate molecular mechanisms of p53 in regulating senescence in order to optimize the efficacy and safety for clinical applications.
Immune system modulation for senolysis
Using senescence immune surveillance, senescent cells are naturally removed by the body’s own immune cells such as macrophages, T-cells, natural killer cells (NK cells) (72) and microglia in the brain (73, 74). However, due to age-related immunosenescence and the capability of senescent cells to evade immune surveillance, senescent cells are spared and accumulate in diverse tissues (75). Consequently, increasing the efficiency of immune surveillance has become a novel strategy to target senescent cells. One strategy is the use of so-called chimeric antigen receptor T cells (CAR-T cells) which have been developed for cancer treatment (76). In brief, T cells derived from patients are primed by genetic manipulation to target and remove cells that express a specific antigen. Using urokinase-type plasminogen activator receptor (uPAR) as antigen, it has recently been shown that CAR-T cells can actively remove senescent cells and ameliorate diverse pathologies in mouse models (77). Similarly, an antibody-based approach to target senescent cell surface protein DPP4 has been utilized to ablate senescent cells in vitro (78). Moreover, an anti-senescence vaccine-like protocol has been recently reported. This approach focused on glycoprotein nonmetastatic melanoma protein B (GPNMB) as a molecular target for senolytic therapy. Suda et. al. showed immunization of mice against Gpnmb resulted in a reduction of Gpnmb-positive cells. Furthermore, it was shown that senolytic vaccination improved normal and pathological phenotypes associated with aging, and extended the male lifespan of progeroid mice (79).
The concept of using CAR-T cells as senolytic agents is still in its early stages, and research in this area is ongoing. Since immune cell trafficking into the CNS is tightly regulated by the blood brain barrier which selectively only allows entry of immune cell subsets required for immune surveillance (80), it remains unknown if CAR-T would efficiently reach senescent cells in the brain parenchyma. One additional challenge is identifying specific and reliable markers that are exclusively expressed on senescent cells to ensure accurate targeting by CAR-T cells or antibodies or to use for immunization. Moreover, immune system-based approaches remove senescent cells by an inflammatory response, which could be problematic in the context of AD where neuroinflammation is present. While harnessing the immune system offers promise to clear senescent cells, the potential impact of off-target effects and unintended immune responses needs to be carefully evaluated to minimize potential harm to healthy tissues.
Senomorphics
An alternative approach of the use of senolytics, which clear senescent cells, is the use of “senomorphics” to ameliorate their SASP (summarized in: (55)). This approach may be especially favorable when difficult to replace cells, like neurons in the brain, become senescent. It remains unknown whether senescent neurons, or other cells with a similar gene expression profile, should be actively removed from its tissue or if it would be advantageous to simply target their detrimental SASP and ameliorate the likelihood of senescence, and associated inflammation, spreading. Senomorphics are currently under development to lower this inflammatory burden. Numerous signaling pathways, such as cyclooxygenase 2 (COX-2), mammalian target of rapamycin (mTOR), mitogen-activated protein kinase (MAPK) signaling, phosphoinositide 3 kinase (PI3K), and the GATA4/p62-mediated autophagy pathways result in the stimulation of NF-κB and/or C/EBPβ pathways, which in turn regulate the SASP secretome (81, 82). This convergence renders the NF-κB and C/EBPβ pathways promising drug targets for the modulation of the SASP. Accordingly, a multitude of molecules and antibodies to interact either with NF-κB and C/EBPβ transcriptional activities at different levels have been developed (summarized in: 81).
mTOR inhibition
Rapamycin is a well-studied FDA approved immunosuppressive drug that has been shown to extend lifespan in yeast, worms, flies, and mice (83, 84). Furthermore, it has been reported to reduce cellular senescence and suppress the SASP. Rapamycin functions by inhibiting mTOR to stimulate autophagy, among other pathways. Even though a promising candidate for lifespan extension and SASP repression, multiple side effects including metabolic dysregulation, impaired wound healing, and hyperlipidemia have been reported (85). Given the positive effects in multiple animal models of aging and neurodegeneration, including models of AD, clinical trials for rapamycin in AD are underway (NCT04200911, NCT04629495).
NF-κB inhibition
One of the most central transcription factors for the regulation of immune responses and the regulation of the SASP is NF-κB. Genetically enhancing NF-κB activity causes neuroinflammation and increases senescent cell burden in the brain (86). In a basal state, the function of NF-κB is repressed by IκB inhibitors. During stress- or inflammatory-responses, IκB inhibitors are phosphorylated by the so-called IKK complex (87). Interestingly, inhibition of the binding to IKK by using a peptide inhibitor reduced NF-kB activity and thereby senescence in progeroid mice (88). Consequently, a small molecule was developed to inhibit the interaction between NF-κB and IKK. This molecule, SR12343, significantly reduced the release of SASP factors, senescence, and improved markers of senescence in progeroid and naturally aged mice (89). The inhibitory molecule SR12343 has been shown to cross the blood brain barrier positioning it as an interesting compound to test in the context of AD and other neurodegenerative/neurological conditions (90). However, given that NF-κB is involved in diverse biological processes related to inflammatory response, cell adhesion, growth control, and protection against apoptosis (87), its inhibition could lead to multiple unwanted side effects. It will require thorough characterization before it can be applied in humans. Of note, the treatment of mice with the nonsteroidal anti-inflammatory drug, ibuprofen, mitigated senescent cell accumulation and neuroinflammation and improved cognitive function (86). However, clinical trials using ibuprofen have not successfully modified AD disease progression or outcomes (91, 92), which may reflect a need for combination therapies to target multiple aspects of this complex disease.
P38MAPK inhibition
The p38MAPK inhibitor SB203580, by decreasing the transcriptional activity of NF-κB, has been shown to reduce the levels of mRNA of genes of SASP factors in human senescent cells (81). Similarly, next-generation p38MAPK inhibitors UR-13756 and BIRB 796 inhibited IL-6 expression in human senescent fibroblasts, demonstrating their effectiveness in reducing SASP-related effects (93). Interestingly, inhibition of JAK/STAT signaling by ruxolitinib suppressed C/EBPβ transcriptional activity, leading to a reduction in systemic inflammation by SASP repression and improvement in fitness in elderly mice (94). Reducing the activity of the stress kinase p38MAPK may be particularly beneficial for the neuroinflammation common to AD. Because of this interest, a brain permeable and orally available p38MAPK inhibitor has recently been developed (MW150, a.k.a. MW01-18-150SRM) and is currently in a clinical trial for AD (NCT05194163).
In summary, several approaches show promise as senolytic therapies. The combination of D+Q effectively clears senescent cells and positively impacts age-associated conditions and diseases. Natural compounds like fisetin have also exhibited senolytic activity and are being tested in clinical trials. BCL-2 family inhibitors, such as ABT-263 (Navitoclax), induce apoptosis in senescent cells but have limitations regarding side effects and CNS penetrance. Modulators of the p53 pathway, such as FOXO4-DRI and MDM2 inhibitors, selectively eliminate senescent cells but face challenges in clinical translation. Immune system modulation shows potential for enhancing immune surveillance and clearing senescent cells, but is in early stages. While several senolytic and senomorphic therapies show promise in clearing senescent cells and impacting age-related conditions, many lack data on CNS penetrance and efficacy in AD relevant systems. Among these approaches, the first-generation D+Q combination has accumulated the most experimental data regarding safety and efficacy, and CNS bioavailability (95-99). Currently, D+Q represents the most mature, and promising, senolytic therapy for CNS conditions.
Senolytics Improve AD Outcomes in Preclinical Studies
Multiple independent research groups have demonstrated that D+Q clear senescent cells across biological systems (model organisms), diseases and tissues. Senolytic target engagement does not require continuous receptor binding, enzyme modulation, or persistent modulation of biochemical pathways. Instead their mechanism of action is to temporarily disrupt anti-apoptotic pathways utilized by senescent cells for their survival. Moreover, senescent cells do not divide and accumulate slowly; as such, senolytics do not require continuous presence in the circulation. Preclinical studies in rodents have validated this approach; intermittent treatment with senolytics have conclusively reduced senescent cell burden in peripheral tissues (fat, lung, kidney, pancreas, cartilage, and muscle), improved pathologies in these tissues, and extended healthspan and lifespan (45, 46, 100-103).
We began exploring the efficacy of senolytics in tauopathy mouse models to determine the impact of senescent cells on brain structure and function relevant to AD neuropathology (35). In the study, we utilized four distinct tau transgenic mouse models including an aggressive, early age onset tauopathy (rTg(tauP301L)4510)Mapt0/0; late-onset slowly progressive tauopathy (rTg(tauWT)21221); tau knockout mice (Mapt0/0); mice with a combination of Aβ and NFT pathology (3xTgAD); and age-matched wild type mice. Additionally, we analyzed postmortem human brain tissue with histopathologically confirmed AD or progressive supranuclear palsy (PSP), an age-associated tauopathy that clinically manifests as parkinsonism with additional motor abnormalities and cognitive dysfunction, and is neuropathologically defined by NFTs, gliosis and neurodegeneration (104). Among the distinct tau transgenic mouse models, senescence was a robust and invariant response to tau accumulation that correlated with brain atrophy in mice and human PSP (35). Robust markers of cellular senescence including Cdkn2a, Cdkn1a and SASP (i.e., Tnfa, Il1b, Clxcl1, etc.,); tissue remodeling; and karyomegaly were observed in tau transgenic mouse brains (35). Transcriptomic analyses of laser capture microdissected neurons from postmortem human AD revealed that NFT-containing neurons expressed signaling pathways consistent with cellular senescence, including SCAPs, upreguled pro-survival and inflammatory pathways and downregulated cell death pathways (35).
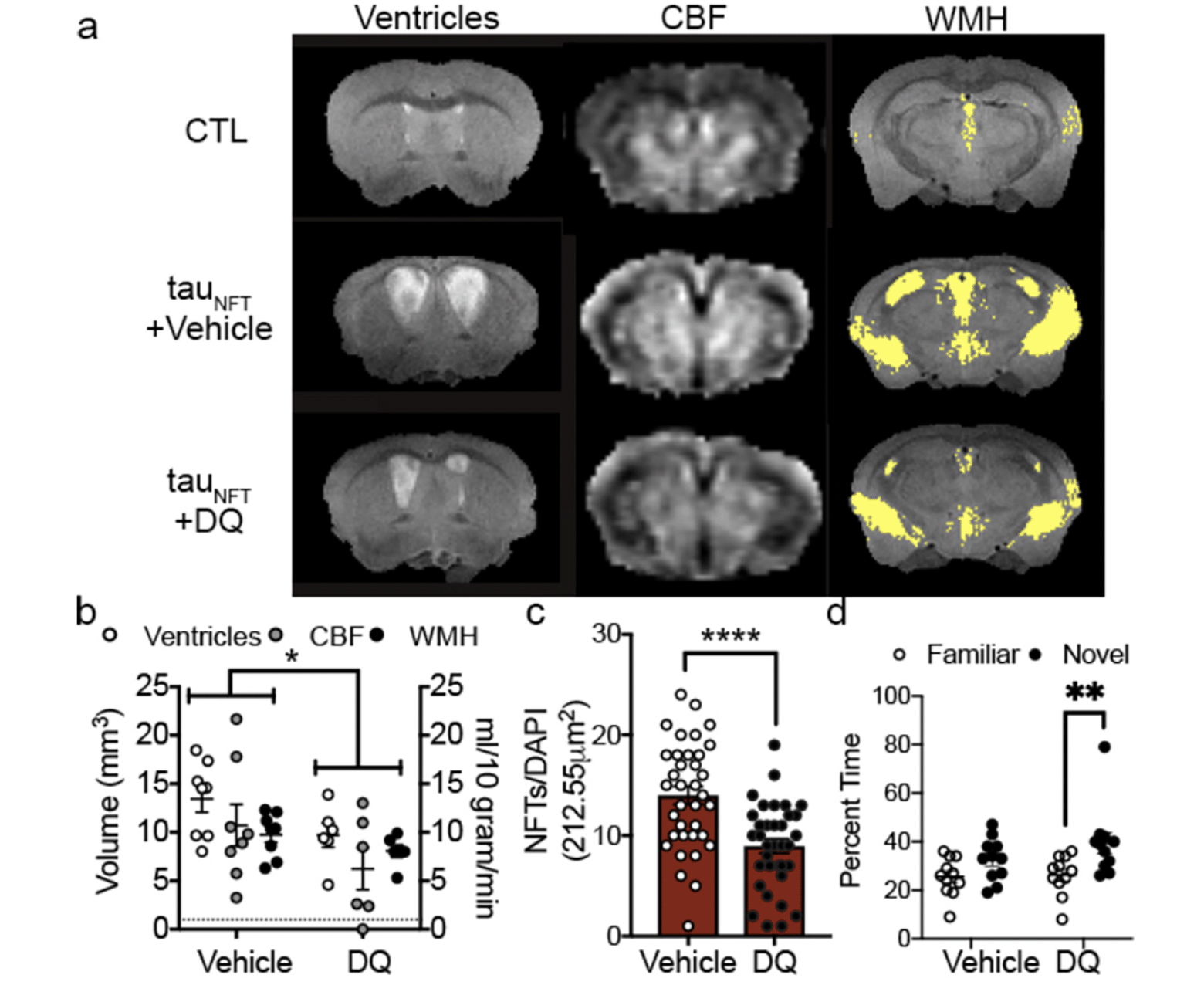
To determine a causal role between senescent cells and tauopathy, we administered intermittent (once every two weeks) senolytic therapy (D+Q) to tau transgenic mice between the ages of 20-23 months old with already present tau pathology, neurodegeneration, inflammation and brain atrophy (35). The goal was to determine if senolytic therapy could effectively clear senescent cells in the CNS and thereby stop the progression of tau-associated pathogenesis in advanced disease stages. After three months of cyclic senescent cell removal using D+Q, we assessed cerebral blood flow, brain volume and ventricle volume with MRI to determine if removing senescent cells produced global effects on brain function and structure. D+Q treatment mitigated pathogenic ventricle enlargement, aberrant cerebral blood flow and white matter hyperintensity pathology (Figure 2a-c, adapted from (21)). We repeated these experiments in a separate cohort of tau transgenic mice. Behavior analyses indicated that D+Q treatment improved tau transgenic mouse discrimination index in the y-maze, providing additional evidence for improved brain function with D+Q treatment (Figure 2d).
Target engagement was confirmed by decreased phospho-tyrosine and NFT burden. We found that the improvements were mechanistically attributed to a significant reduction in NFTs and inflammation. We predicted that off-target effects of D+Q on non-senescent neurons would be evidenced by a decrease in total neuron density; however, we observed an overall increase in neuronal protein expression (e.g., NeuN, synaptophysin and PSD95). Combined with the D+Q associated reduction in ventricle size as assessed by MRI, a surrogate measure of neurodegeneration, the data suggested that clearing senescent cells was beneficial for neuronal preservation. Though we did not observe differences in total astrocyte or myelin protein expression, we noted an increase in Iba1, reflective of increased microglia. In peripheral tissue, senescent cells are cleared by macrophages. Microglia are the resident macrophage cells of the brain. Our interpretation was that elevated Iba1 may have reflected an increase in microglia-mediated clearance of cellular debris after senolytic treatment (35). Data from Dr. Maria Grazia Spillantini’s laboratory reported that microglia engulf NFT-bearing neurons that display phosphatidylserine on their cells surface (73). Subsequent studies by the same research team found that microglia develop a senescence-like phenotype after engulfing NFT-containing neurons, and they release toxic forms of partially digested tau (74). Given the downstream consequences of senescent cells, even when efficiently cleared by microglia, eliminating them with therapeutic interventions may potentially mitigate their abundance, reduce inflammation, and alter disease progression.
Collectively, work across multiple independent laboratories have reported on both amyloid and tau transgenic AD mouse models, postmortem human brain tissue from two distinct tau-expressing neurodegenerative conditions, and three senolytic compounds (Table 1). The results convincingly demonstrate that protein aggregation induces senescence of many cell types in the brain, and that senolytics effectively reduce pathogenesis (35-37). Experimental evidences indicate that neurons are a primary source of senescence in AD. While therapeutically clearing senescent neurons may not be an appealing approach, the brain’s immune system is already attempting to rid these harmful cells. Once the microglia engulf the senescent neurons, they become senescent, dysfunctional and amplify pathogenesis through inflammation, releasing toxic tau and failure to survey the brain microenvironment. In this way, therapeutically clearing senescent cells, even neurons, may prove beneficial as supported by experimental data in transgenic mice (35-37).
(a) MRI on advanced aged control (CTL) and transgenic (tauNFT) mice revealed abnormal ventricles, cerebral blood flow (CBF) and white matter hyperintensity (WMH) pathologies that were mitigated by (b) treatment with DQ. Two-way ANOVA, significant treatment effect p<0.05. (c) Quantification of the number of senescence neurons with NFTs between vehicle and DQ treated mice. Two-tailed t-test, **** p < 0.0001. (d) Cognitive behavior was assessed by time spent in familiar or novel arm of the y-maze. Two-way ANOVA, Sidak post-hoc. **: p = 0.0083. n=11/group. (Adopted from (122)).
Clinical Trials Using Senolytics
The enthusiasm for senescent cell clearance across diseases of aging, along with a therapeutic approach that could be translated to patients, led to the first clinical trial of senolytic therapy (D+Q) in adults with idiopathic pulmonary fibrosis (IPF). The intermittent treatment strategy was well-tolerated and improved physical function (gait speed) (105). A second Phase I study in adults with diabetic kidney disease reported a D+Q dependent clearance of senescent adipose and skin cells and reduced SASP within 11 days of treatment (106). Using the safety and feasibility data from these first-in-human trials demonstrating that intermittent D+Q was well tolerated in 50 subjects (NCT 02874989 and NCT 02848131), combined with the efficacy data in mouse models, we initiated a Phase I study of senolytic therapy in older adults with early-stage symptomatic AD, SToMP-AD (Senolytic Therapy to Modulate the Progression of Alzheimer’s Disease, NCT04063124) (107).
Phase I Trial: D+Q in Early Alzheimer’s Disease, SToMP-AD
The primary objective of the vanguard SToMP-AD trial was to evaluate CNS penetrance of D and Q and collect data relevant to safety of intermittent orally delivered senolytic therapy. The secondary endpoints included collecting data on cognition, neuroimaging, and plasma and cerebrospinal fluid (CSF) biomarkers before and after treatment. The intermittent treatment design follows preclinical evidence that demonstrated senescent cells require 2-4 weeks to develop and complete senescent cell clearance is not required for disease modifying effects. In tau transgenic mice we saw improvements in brain structure and function by reducing senescent cell burden by 30% with an intermittent treatment strategy (35), which is consistent with preclinical studies on other models of aging and/or disease (37, 45, 46, 101-103, 108-112). D, a chemotherapy, is a powerful drug and can have serious side effects. However, the intermittent, not continuous, administration of senolytics reduces concerns of toxicity, and effectively circumvents potential off-target effects due to continuous receptor occupancy or modulation of an enzyme or biochemical pathway. Given that the Phase I clinical trial in IPF demonstrated a reduction of senescent cells and SASP within eleven days after a single treatment of D+Q, and improvements in physiological outcomes after 10 weeks (105), we reasoned that a three-month period would provide important feasibility, compliance and efficacy data, as well as potentially reveal other cognitive and biomarker effects useful for designing a larger study.
A total of five participants (mean age: 76+5 years; 40% female) completed the SToMP-AD vanguard trial. To determine CNS penetrance, the levels of D and Q in CSF were measured using high-performance liquid chromatography with tandem mass spectrometry. Safety evaluation involved continuous monitoring and reporting of adverse events, as well as tracking vitals and laboratory work. The treatment increased levels of D and Q in the blood for all participants. In CSF, D levels were detected in four participants (80%) with a CSF to plasma ratio ranging from 0.422% to 0.919%, while Q was not detected. The treatment was well-tolerated, with no early discontinuations. Six mild to moderate adverse events occurred during the study, but no serious events were reported. Cognitive and neuroimaging endpoints did not show significant differences between baseline and post-treatment. Analysis of CSF biomarkers revealed that IL-6 and GFAP levels increased from baseline to post-treatment, coinciding with a trending decrease in cytokines and chemokines associated with senescence. Additionally, there was a trend toward higher (beneficial) levels of Aβ42 (99). Thus, while the study was not specifically designed to assess efficacy, the data indicate potential treatment-related changes in markers associated with cellular senescence and AD pathology.
As other Phase I studies using D+Q in the context of AD report out, we will be positioned to begin determining whether there is a convergence of data for specific disease states (e.g., NCT04785300 and NCT05422885 are both AD Phase I trials using D+Q), or study/site-specific changes are noted. For example, participants with chronic kidney disease reported changes in IL-1α, IL-6, and MMPs-9 and -12 (106), while we observed changes in IL-6, TARC/CCL17, IL-17A, I-TAC/CXCL11, Eotaxin-2/CCL24, Eotaxin/CCL11, and MIP-1a in our AD study participants (99). The low CSF-to-plasma ratio of compound D and the absence of compound Q in CSF may suggest the need for Phase Ib dosing studies to explore novel strategies for increasing Q bioavailability and D levels in the CNS. Nevertheless, given that clearing senescent cells in peripheral tissues has systemic benefits, including the reduction of systemic inflammation, we cannot rule out the possibility that D+Q in the periphery may positively impact the brain (113). While we advise caution in interpreting the secondary and exploratory outcomes data from Phase I trials, our initial study demonstrated the safety and tolerability of compounds D and Q in older adults with AD (99). Collectively the prior work support moving forward with a Phase II, placebo-controlled study of D+Q (NCT04685590).
Phase II SToMP-AD
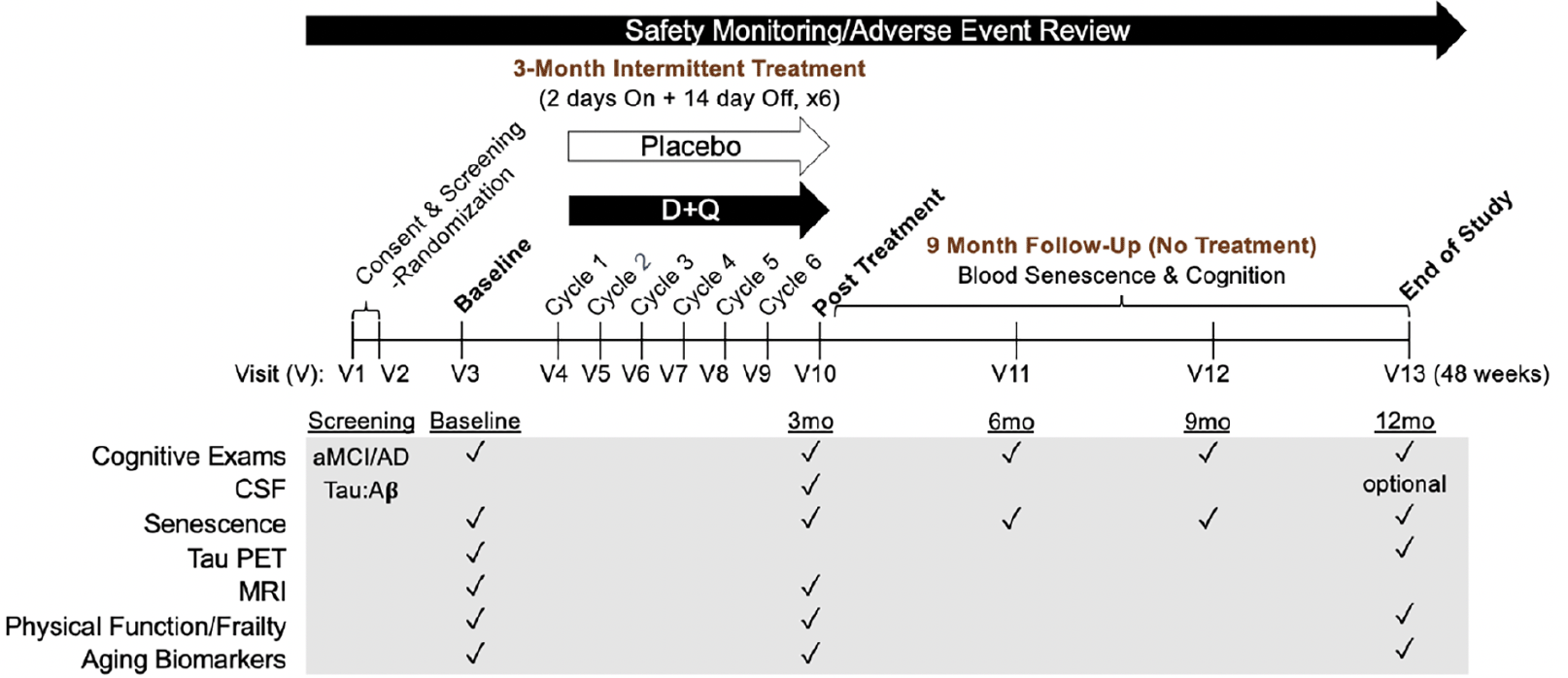
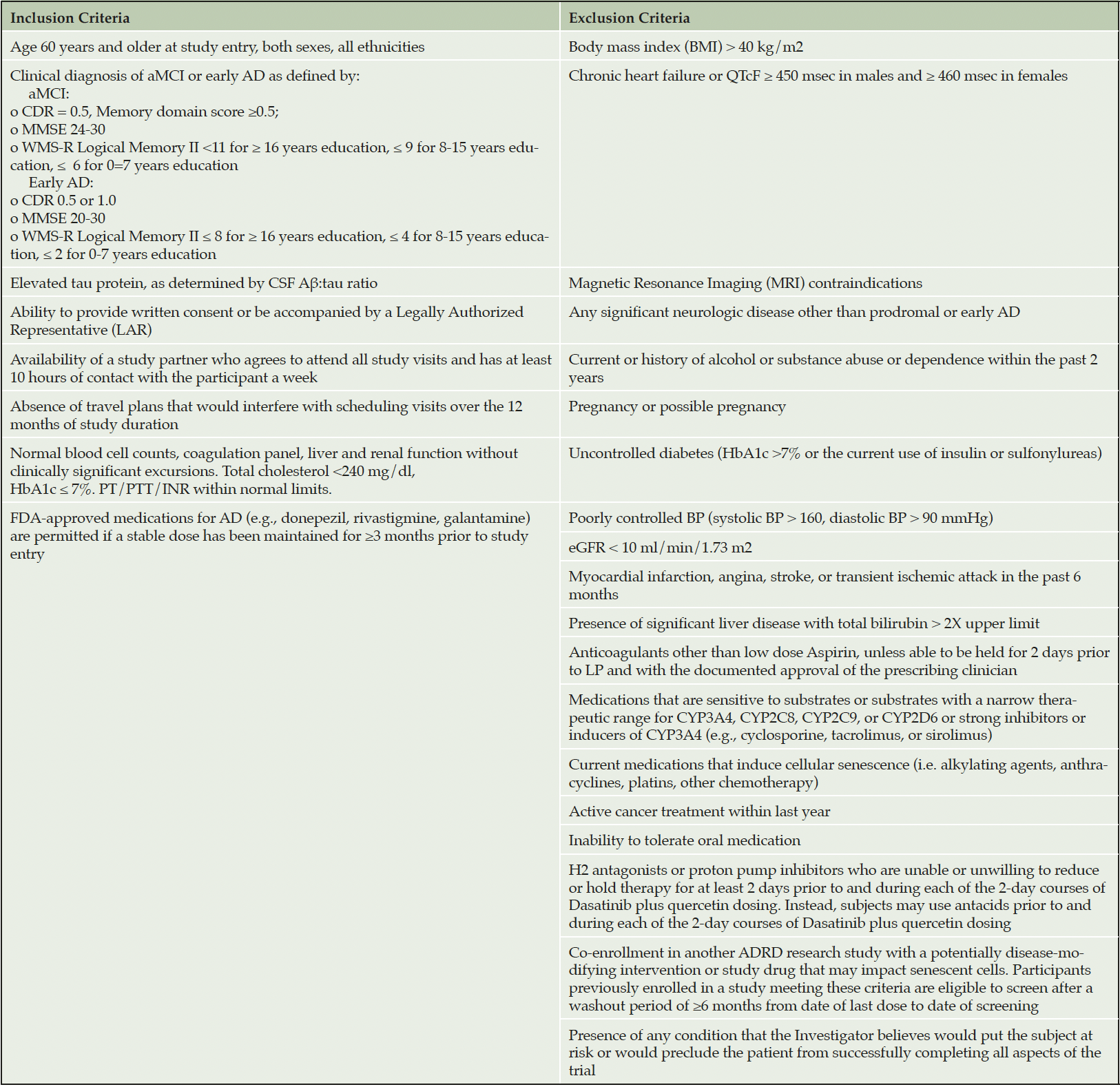
To rigorously test D+Q in the context of AD, we have initiated a Phase II multi-site, randomized, double-blind placebo-controlled trial (Phase II SToMP-AD, NCT04685590). The primary objective is to determine safety through adverse event (AE) monitoring, vitals, and laboratory assessments. Forty-eight participants, ≥60-years old, with amnestic MCI or early AD (CDR=0.5 or 1; NIA-Alzheimer’s Association criteria) and elevated tau protein, as determined by CSF Aβ:tau ratio, are eligible (Table 2). The study is powered to determine target engagement of senolytics in peripheral blood based on data generated from a Phase I D+Q trial in IPF (106). Participants are randomized 1:1 to receive D+Q or placebo administered for two consecutive days out of every fifteen days across three months with an extended nine-month follow-up after the intervention. The 12-month trial design enables us to assess senolytic-associated changes before and after treatment during the 3-month active treatment phase, track the reappearance of senescent cells after their removal during the 9-month post-treatment period, and evaluate disease trajectory differences over a one-year period (Figure 3). Our primary outcome is change in senescent cell burden in blood as measured by CD3+ p16INK4A+ T cells and SASP factors. Plasma will be assessed pre- and post-treatment (baseline and three months) as well as every three months during the 9-month no-treatment follow-up period. We will also collect necessary data to evaluate target engagement in the CNS and disease-modifying effects in aMCI/early AD. CNS target engagement will be assessed by measuring levels of key senescence markers in CSF at baseline and after three months treatment with senolytics. As such, secondary outcomes include changes in cognition, disease severity and function before and after treatment and across the one-year study period and measures of senescence and AD biomarkers in CSF pre- and post-treatment (baseline and three months) with an optional end of study collection. We are also collecting data on brain structure and function through magnetic resonance imaging (MRI) and tau deposition as assessed by tau positron emission tomography (PET) changes between baseline and end-of-study. Although we may not have sufficient power to detect significant changes in our secondary outcomes (tau PET, MRIs, CSF AD biomarkers and cognition), including a nine-month follow-up will help provide preliminary evidence of successful modification of disease trajectory over a one-year period. The Wake Forest University School of Medicine Alzheimer’s Disease Research Center is coordinating the study, which is sponsored by the Alzheimer’s Drug Discovery Foundation (ADDF). The study is anticipated to complete in 2025.
Major measures collected at each study visit (V) are indicated by black checkmarks. Abbreviations: D: dasatinib; Q: quercetin; V: visit; CSF: cerebrospinal fluid; PET: positron emission tomography; MRI: magnetic resonance imaging; aMCI: amnestic mild cognitive impairment; AD: Alzheimer’s disease; mo: months.
Summary: Current Limitations and Optimism for the Future
Recent FDA-approved amyloid lowering therapies provide modest clinical effects. Considering their safety concerns and high costs, a significant need to identify additional treatment options remains. Advanced chronological age stands alone as the single common, and greatest, risk factor for developing AD. Regardless of genetic predisposition, lived experiences and disease heterogeneity, the risk of developing AD unvaryingly increases with age across the global population. Biological aging interventions, in isolation and/or in combination, hold potential for preventing, delaying and treating AD and other age-associated conditions. The “geroscience hypothesis” proposes this principle but necessitates experimental testing. Given the extensive experimental data demonstrating health benefits from senescent cell clearance and significant advances in cellular senescence drug discovery, senolytics have emerged as a leading translational geroscience therapy.
The SToMP-AD trial will test the geroscience hypothesis by investigating the impact of targeting cellular senescence, a secondary hallmark of aging, on AD pathogenesis and other biological aging hallmarks. The trial endpoints include both traditional AD outcomes (e.g., cognition, brain imaging, AD fluid biomarkers) and gold standard aging measures (e.g., frailty, physical function, epigenetic age, etc.). While the ultimate goal of the geroscience approach is to extend healthy lifespan by delaying or preventing disease, the lack of specific biomarkers for senescent cells (fluid, imaging, etc.) represents a major hurdle for identifying participants that may most benefit from senolytics. For example, in our evaluation of postmortem human brain tissue from individuals with various levels of AD neuropathology, we observed a wide range (0-15%) of senescent cell burden (39). The current limited knowledge of senescent cell burden and heterogeneity across ages, disease stages or ethnicities compels early trials, such as ours, to enroll symptomatic participants. This strategy is needed not only to provide foundational evidence for the safety and efficacy of senescent cell removal, but also to begin collecting much needed biomarker data that could be used as surrogate markers for senescent cell identification and tracking in pre-clinical/prodromal disease stages. For example, the neuroscience field has identified senescent cells in multiple CNS diseases and disorders, and emerging data highlight their critical importance in driving disease phenotypes. Specifically, beyond amyloid and tau, defective autophagy (114, 115); insulin resistance/obesity (110, 116); traumatic brain injury (TBI) (117); depression (118); alcohol use disorder (119), Parkinson’s disease (120) and pain (121) all reportedly increase CNS senescent cell burden. Given the epidemiology linking each of these independently to increased AD risk, targeting senescent cells in early disease stages (preclinical and/or prodromal) may hold promise to prevent chronic neurodegeneration and dementia.
Senescent cell biomarkers are also critical for designing senolytic therapies. Many molecules involved in establishing the senescence cell fate also play critical roles in cell survival or apoptosis pathways. Developing senolytics with high selectivity and efficacy in targeting senescent cells while minimizing off-target effects on healthy cells remains an ongoing challenge. In fact, since D+Q targets a range of biological pathways in diverse tissues, it is generally difficult to attribute cell type-specific biological effects to the senolysis itself (45, 103, 112). In response to the challenges faced, multiple approaches, pathways, and strategies are under investigation e.g., senolytics and senomorphics. With the increasing interest in senescent cell removal or manipulation, a working group to align study outcomes may be beneficial to better understand efficacy and, potentially, begin considering combination therapies for the treatment of ADRD and other CNS diseases. Given the early stages of this therapeutic approach senescence therapeutics will need to overcome challenges related to biomarker identification, target engagement, CNS penetrance and safety. The AD field successfully overcame similar challenges with amyloid lowering strategies; we are optimistic that AD researchers and trialists will apply the same tenacity and lessons learned to senolytics and other promising biology of aging interventions. Ultimately, we anticipate that the continued development of precise and effective senotherapeutic interventions will lead to the targeted elimination of senescent cells, their detrimental SASP, and improved health outcomes in age-related diseases such as AD.
Glossary: Amyloid hypothesis: proposes that the accumulation of amyloid-beta protein in the brain is the primary cause of Alzheimer’s disease, leading to the formation of plaques and subsequent neurodegeneration. CAR-T Therapy: Chimeric antigen receptor (CAR) T-cell therapy refers to an immunotherapeutic approach aimed at harnessing the anti-cancer potential of T cells. Through ex vivo genetic modification, T cells are engineered to express chimeric antigen receptors, enabling them to recognize and eliminate cancer cells with enhanced specificity and efficacy. Cholinergic hypothesis: the degeneration of cholinergic neurons and reduced acetylcholine levels in the brain play a critical role in the development of AD. Frailty: A medical and geriatric syndrome characterized by a decline in physiological reserves and increased vulnerability to stressors, leading to an increased risk of adverse health outcomes. Hallmarks of aging: A concept in the field of biology and gerontology that refers to a set of interconnected cellular and molecular processes and characteristics associated with the aging process. These hallmarks represent the underlying biological mechanisms that contribute to the aging of living organisms. The concept was initially proposed in 2013 (13) and was updated in 2023 (14). Healthspan: The portion of life spent in good health and functional independence in which an individual remains free from significant disease, illnesses or chronic health conditions that substantially impact daily living. Inflammatory hypothesis: Chronic inflammation, particularly in the brain, plays a significant role in the development and progression of various age-related diseases and neurodegenerative conditions. Intermittent Treatment: Administration of a therapeutic intervention at specific intervals rather than continuously. The approach involves alternating periods of treatment and non-treatment. MCI: Mild Cognitive Impairment refers to a condition that falls between the normal cognitive changes associated with aging and more severe cognitive decline seen in dementia. It is characterized by noticeable and measurable cognitive deficits that are greater than typical age-related changes but not severe enough to significantly interfere with daily activities or independence. Amnestic MCI (aMCI) primarily affects memory, and individuals may have difficulty remembering recent conversations, appointments, or events. Mitochondrial cascade hypothesis: Mitochondrial dysfunction and the accumulation of reactive oxygen species lead to neuronal damage and ultimately contribute to the pathogenesis of neurodegenerative diseases, particularly AD. Progeroid: A condition where young organisms appear much older than their chronological age/premature aging. Proteostasis: maintenance of protein homeostasis in cells, involving processes that regulate protein synthesis, folding, degradation, and clearance to ensure proper protein function and prevent cellular dysfunction and disease. SASP: The senescence-associated secretory phenotype refers to molecules released by senescent cells that both help them survive, and that signal immune cell clearance. SASP factors include cytokines, chemokines, extracellular remodeling proteins. SCAP: Senescent-cell anti-apoptotic pathways engaged by senescent cells to ensure survival/avoid apoptosis. Senolytics: A class of therapeutics that induce senescent cell death by targeting their pro-survival pathways. Senomorphics: A class of therapeutics that reduce the toxicity of senescent cells by dampening their secretome (e.g., SASP). Senotherapeutics: Term referring to the general therapeutic strategy of targeting and/or disrupting senescent cells. Tauopathy: A group of neurodegenerative diseases that arise concomitant with abnormal tau protein deposition in brain cells.
Conflict of Interest Statement: Dr. Orr reports grants from Alzheimer’s Drug Discovery Foundation, grants from NIA, grants from NINDS, grants from US Department of Veterans Affairs, grants from Cure Alzheimer’s Fund, grants from AFAR/Hevolution during the conduct of the study. In addition, Dr. Orr has a patent Biosignature and therapeutic approach for neuronal senescence pending. Dr. Riessland has no conflict of interest to declare.
Acknowledgments/Funding: The authors would like to thank Drs. Suzanne Craft, Mitzi Gonzales, James Kirkland, Nicolas Musi, Ronald Petersen, Sudha Seshadri and Tamara Tchkonia for their significant contributions to the Phase 2 study design of SToMP-AD, and their ongoing collaborative support as co-investigators on the trial (NCT04685590). MEO is supported by the Alzheimer’s Drug Discovery Foundation, GC-201908-2019443; the Coordinating Center for Claude D. Pepper Older Americans Independence Centers, U24AG059624; US Department of Veterans Affairs (I01BX005717), National Institute on Aging (R01AG068293, R01AG065839, U54AG079754, R24AG073199), National Institute of Neurological Disorders and Stroke (R21NS125171), Cure Alzheimer’s Fund and Hevolution Foundation/American Federation of Aging Research. MR is supported by the Thomas Hartman Foundation and the National Institute of Neurological Disorders and Stroke (1R01NS124735-01A1). The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Mehegan, L., and Chuck Rainville. 2021 AARP Survey on the Perceptions Related to a Dementia Diagnosis: Adults Age 40+. (Washington, DC: AARP Research, 2021).
2. Davy, C. Dementia overtakes cancer as the UK’s most feared condition for the 55+ age group. (2021).
3. Watson, R., Sanson-Fisher, R., Bryant, J. & Mansfield, E. Dementia is the second most feared condition among Australian health service consumers: results of a cross-sectional survey. BMC Public Health 2023;23, 876, doi:10.1186/s12889-023-15772-y.
4. Gauthier S, W. C., Servaes S, Morais JA, Rosa-Neto P. World Alzheimer Report 2022: Life after diagnosis: Navigating treatment, care and support., (2022).
5. Probst, A., Langui, D. & Ulrich, J. Alzheimer’s disease: a description of the structural lesions. Brain Pathol 1991;1 229-239.
6. Aisen, P. S. et al. The future of anti-amyloid trials. The Journal of Prevention of Alzheimer’s Disease 2020;7, 146-151.
7. Haass, C. & Selkoe, D. If amyloid drives Alzheimer disease, why have anti-amyloid therapies not yet slowed cognitive decline? PLoS biology 2022;20, e3001694.
8. Cummings, J., Ritter, A. & Zhong, K. Clinical trials for disease-modifying therapies in Alzheimer’s disease: a primer, lessons learned, and a blueprint for the future. Journal of Alzheimer’s Disease 2018;64, S3-S22.
9. Hof, P. R., Glannakopoulos, P. & Bouras, C. The neuropathological changes associated with normal brain aging. Histol Histopathol 1996;11, 1075-1088.
10. Perl, D. P. Neuropathology of Alzheimer’s disease. Mt Sinai J Med 2010;77, 32-42, doi:10.1002/msj.20157.
11. Bateman, R. J. et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 2012;367, 795-804, doi:10.1056/NEJMoa1202753.
12. Guerreiro, R. & Bras, J. The age factor in Alzheimer’s disease. Genome Med 2015;7, 106, doi:10.1186/s13073-015-0232-5.
13. Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 2013;153, 1194-1217, doi:10.1016/j.cell.2013.05.039.
14. Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023;186, 243-278, doi:10.1016/j.cell.2022.11.001.
15. Gonzales, M. M. et al. Biological aging processes underlying cognitive decline and neurodegenerative disease. J Clin Invest 2022;132, doi:10.1172/JCI158453.
16. Hardy, J. & Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci 1991;12, 383-388, doi:10.1016/0165-6147(91)90609-v.
17. Selkoe, D. J. The molecular pathology of Alzheimer’s disease. Neuron 1991;6, 487-498, doi:10.1016/0896-6273(91)90052-2.
18. Davies, P. & Maloney, A. J. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976;2, 1403, doi:10.1016/s0140-6736(76)91936-x.
19. Swerdlow, R. H. & Khan, S. M. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses 2004;63, 8-20, doi:10.1016/j.mehy.2003.12.045.
20. Kinney, J. W. et al. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y) 2018;4, 575-590, doi:10.1016/j.trci.2018.06.014.
21. Tchkonia, T. & Kirkland, J. L. Aging, Cell Senescence, and Chronic Disease: Emerging Therapeutic Strategies. JAMA 2018;320, 1319-1320, doi:10.1001/jama.2018.12440.
22. Tchkonia, T., Zhu, Y., van Deursen, J., Campisi, J. & Kirkland, J. L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 2013;123, 966-972, doi:10.1172/JCI64098.
23. Wang, E. Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl2 is involved. Cancer Research 1995;55, 2284-2292.
24. Kirkland, J. L. & Tchkonia, T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017, doi:10.1016/j.ebiom.2017.04.013.
25. LeBrasseur, N. K., Tchkonia, T. & Kirkland, J. L. Cellular Senescence and the Biology of Aging, Disease, and Frailty. Nestle Nutr Inst Workshop Ser 2015;83, 11-18, doi:10.1159/000382054.
26. Zhu, Y., Armstrong, J. L., Tchkonia, T. & Kirkland, J. L. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care 2014;17, 324-328, doi:10.1097/MCO.0000000000000065.
27. Swanson, E. C., Manning, B., Zhang, H. & Lawrence, J. B. Higher-order unfolding of satellite heterochromatin is a consistent and early event in cell senescence. The Journal of cell biology 2013;203, 929-942, doi:10.1083/jcb.201306073
28. Kirkland, J. L., Tchkonia, T., Zhu, Y., Niedernhofer, L. J. & Robbins, P. D. The Clinical Potential of Senolytic Drugs. J Am Geriatr Soc 2017;65, 2297-2301, doi:10.1111/jgs.14969.
29. Coppé, J. P. et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biology 2008;6, 2853-2868.
30. Acosta, J. C. et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nature cell biology 2013;15, 978-990, doi:10.1038/ncb2784.
31. Kuilman, T. & Peeper, D. S. Senescence-messaging secretome: SMS-ing cellular stress. Nature reviews. Cancer 2009;9, 81-94, doi:10.1038/nrc2560.
32. Coppe, J. P., Desprez, P. Y., Krtolica, A. & Campisi, J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010;5, 99-118, doi:10.1146/annurev-pathol-121808-102144.
33. Herbig, U., Ferreira, M., Condel, L., Carey, D. & Sedivy, J. M. Cellular senescence in aging primates. Science 2006;311, 1257
34. Tchkonia, T. et al. Fat tissue, aging, and cellular senescence. Aging cell 2010;9, 667-684.
35. Musi, N. et al. Tau protein aggregation is associated with cellular senescence in the brain. Aging cell 2018;17, e12840, doi:10.1111/acel.12840.
36. Bussian, T. J. et al. Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature 2018;562, 578-582, doi:10.1038/s41586-018-0543-y.
37. Zhang, P. et al. Senolytic therapy alleviates Abeta-associated oligodendrocyte progenitor cell senescence and cognitive deficits in an Alzheimer’s disease model. Nature neuroscience 2019;22, 719-728, doi:10.1038/s41593-019-0372-9.
38. Bhat, R. et al. Astrocyte senescence as a component of Alzheimer’s disease. PloS one 2012;7, e45069, doi:10.1371/journal.pone.0045069.
39. Dehkordi, S. K. et al. Profiling senescent cells in human brains reveals neurons with CDKN2D/p19 and tau neuropathology. Nat Aging 2021;1, 1107-1116, doi:10.1038/s43587-021-00142-3.
40. Streit, W. J. & Xue, Q. S. Human CNS immune senescence and neurodegeneration. Curr Opin Immunol 2014;29, 93-96, doi:10.1016/j.coi.2014.05.005.
41. Bryant, A. G. et al. Cerebrovascular Senescence Is Associated With Tau Pathology in Alzheimer’s Disease. Front Neurol 2020;11, 575953, doi:10.3389/fneur.2020.575953.
42. Xu, P. et al. The landscape of human tissue and cell type specific expression and co-regulation of senescence genes. Molecular Neurodegeneration 2022;17, 5, doi:10.1186/s13024-021-00507-7.
43. Baker, D. J. et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011;479, 232-236, doi:10.1038/nature10600.
44. Baker, D. J. et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 2016;530, 184-189, doi:10.1038/nature16932.
45. Zhu, Y. et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging cell 2015;14, 644-658, doi:10.1111/acel.12344.
46. Fuhrmann-Stroissnigg, H. et al. Identification of HSP90 inhibitors as senolytics for extending healthspan. Nature communications In press (2017).
47. Wissler Gerdes, E. O., Zhu, Y., Tchkonia, T. & Kirkland, J. L. Discovery, development, and future application of senolytics: theories and predictions. Febs j 2020;287, 2418-2427, doi:10.1111/febs.15264
48. Kumar, R. M. et al. Fisetin in Cancer: Attributes, Developmental Aspects, and Nanotherapeutics. Pharmaceuticals 2023;16, 196.
49. Viñas, P., Martínez-Castillo, N., Campillo, N. & Hernández-Córdoba, M. Directly suspended droplet microextraction with in injection-port derivatization coupled to gas chromatography–mass spectrometry for the analysis of polyphenols in herbal infusions, fruits and functional foods. Journal of Chromatography A 2011;1218, 639-646, doi:https://doi.org/10.1016/j.chroma.2010.12.026.
50. Sundarraj, K., Raghunath, A. & Perumal, E. A review on the chemotherapeutic potential of fisetin: In vitro evidences. Biomedicine & Pharmacotherapy 2018;97, 928-940, doi:https://doi.org/10.1016/j.biopha.2017.10.164.
51. Yousefzadeh, M. J. et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018;36, 18-28, doi:10.1016/j.ebiom.2018.09.015.
52. Zhu, Y. et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-X(L) inhibitors, A1331852 and A1155463. Aging (Albany NY) 2017;9, 955-963, doi:10.18632/aging.101202.
53. Currais, A. et al. Modulation of p25 and inflammatory pathways by fisetin maintains cognitive function in Alzheimer’s disease transgenic mice. Aging Cell 2014;13, 379-390, doi:10.1111/acel.12185.
54. He, W. B., Abe, K. & Akaishi, T. Oral administration of fisetin promotes the induction of hippocampal long-term potentiation in vivo. J Pharmacol Sci 2018;136, 42-45, doi:10.1016/j.jphs.2017.12.008.
55. Zhang, L., Pitcher, L. E., Prahalad, V., Niedernhofer, L. J. & Robbins, P. D. Targeting cellular senescence with senotherapeutics: senolytics and senomorphics. The FEBS Journal 2023;290, 1362-1383, doi:https://doi.org/10.1111/febs.16350.
56. Liu, X. et al. Senolytic activity of piperlongumine analogues: Synthesis and biological evaluation. Bioorg Med Chem 2018;26, 3925-3938, doi:10.1016/j.bmc.2018.06.013.
57. Li, W., He, Y., Zhang, R., Zheng, G. & Zhou, D. The curcumin analog EF24 is a novel senolytic agent. Aging (Albany NY) 2019;11, 771-782, doi:10.18632/aging.101787.
58. Zhang, X. et al. Oxidation resistance 1 is a novel senolytic target. Aging cell 2018;17, e12780, doi:10.1111/acel.12780.
59. Wang, Y. et al. Discovery of piperlongumine as a potential novel lead for the development of senolytic agents. Aging (Albany NY) 2016;8, 2915-2926, doi:10.18632/aging.101100.
60. Yosef, R. et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nature Communications 2016;7, 11190, doi:10.1038/ncomms11190.
61. Zhu, Y. et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 2016;15, 428-435, doi:10.1111/acel.12445.
62. Tse, C. et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 2008;68, 3421-3428, doi:10.1158/0008-5472.Can-07-5836.
63. Chang, J. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nature medicine 2016;22, 78-83, doi:10.1038/nm.4010.
64. Schoenwaelder, S. M. et al. Bcl-xL–inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood 2011;118, 1663-1674, doi:10.1182/blood-2011-04-347849.
65. Riessland, M. et al. Loss of SATB1 Induces p21-Dependent Cellular Senescence in Post-mitotic Dopaminergic Neurons. Cell Stem Cell, 2019 doi:10.1016/j.stem.2019.08.013.
66. Nakamura, A. et al. Bcl-xL Is Essential for the Survival and Function of Differentiated Neurons in the Cortex That Control Complex Behaviors. The Journal of neuroscience : the official journal of the Society for Neuroscience 2016;36, 5448-5461, doi:10.1523/jneurosci.4247-15.2016.
67. Koessinger, A. L. et al. Increased apoptotic priming of glioblastoma enables therapeutic targeting by BH3-mimetics. bioRxiv, 2021;2021.2006.2013.448232, doi:10.1101/2021.06.13.448232.
68. Hemann, M. T. & Lowe, S. W. The p53–Bcl-2 connection. Cell Death & Differentiation 2006;13, 1256-1259, doi:10.1038/sj.cdd.4401962.
69. Kruiswijk, F., Labuschagne, C. F. & Vousden, K. H. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol 2015;16, 393-405, doi:10.1038/nrm4007.
70. Baar, M. P. et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell 2017;169, 132-147.e116, doi:10.1016/j.cell.2017.02.031.
71. He, Y. et al. Inhibition of USP7 activity selectively eliminates senescent cells in part via restoration of p53 activity. Aging cell 2020;19, e13117, doi:https://doi.org/10.1111/acel.13117.
72. Marin, I., Serrano, M. & Pietrocola, F. Recent insights into the crosstalk between senescent cells and CD8 T lymphocytes. npj Aging 2023;9, 8, doi:10.1038/s41514-023-00105-5.
73. Brelstaff, J., Tolkovsky, A. M., Ghetti, B., Goedert, M. & Spillantini, M. G. Living Neurons with Tau Filaments Aberrantly Expose Phosphatidylserine and Are Phagocytosed by Microglia. Cell Rep 2018;24, 1939-1948 e1934, doi:10.1016/j.celrep.2018.07.072.
74. Brelstaff, J. H. et al. Microglia become hypofunctional and release metalloproteases and tau seeds when phagocytosing live neurons with P301S tau aggregates. Sci Adv 2021;7, eabg4980, doi:10.1126/sciadv.abg4980.
75. Lee, S. et al. A guide to senolytic intervention in neurodegenerative disease. Mechanisms of ageing and development 2021;200, 111585, doi:10.1016/j.mad.2021.111585.
76. Lim, W. A. & June, C. H. The Principles of Engineering Immune Cells to Treat Cancer. Cell 2017;168, 724-740, doi:10.1016/j.cell.2017.01.016.
77. Amor, C. et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020; 583, 127-132, doi:10.1038/s41586-020-2403-9.
78. Kim, K. M. et al. Identification of senescent cell surface targetable protein DPP4. Genes Dev 2017;31, 1529-1534, doi:10.1101/gad.302570.117.
79. Suda, M. et al. Senolytic vaccination improves normal and pathological age-related phenotypes and increases lifespan in progeroid mice. Nature Aging 2021;1, 1117-1126, doi:10.1038/s43587-021-00151-2.
80. Marchetti, L. & Engelhardt, B. Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc Biol 2020;2, H1-h18, doi:10.1530/vb-19-0033.
81. Khalil, R., Diab-Assaf, M. & Lemaitre, J. M. Emerging Therapeutic Approaches to Target the Dark Side of Senescent Cells: New Hopes to Treat Aging as a Disease and to Delay Age-Related Pathologies. Cells 2023;12, doi:10.3390/cells12060915.
82. Gonçalves, S. et al. COX2 regulates senescence secretome composition and senescence surveillance through PGE(2). Cell Rep 2021;34, 108860, doi:10.1016/j.celrep.2021.108860.
83. Lamming, D. W., Ye, L., Sabatini, D. M. & Baur, J. A. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest 2013;123, 980-989, doi:10.1172/jci64099.
84. Harrison, D. E. et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009;460, 392-395, doi:10.1038/nature08221.
85. Li, J., Kim, S. G. & Blenis, J. Rapamycin: one drug, many effects. Cell Metab 2014;19, 373-379, doi:10.1016/j.cmet.2014.01.001.
86. Fielder, E. et al. Anti-inflammatory treatment rescues memory deficits during aging in nfkb1(-/-) mice. Aging cell 2020;19, e13188, doi:10.1111/acel.13188.
87. Israël, A. The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol 2010;2, a000158, doi:10.1101/cshperspect.a000158.
88. Tilstra, J. S. et al. NF-κB inhibition delays DNA damage-induced senescence and aging in mice. J Clin Invest 2012;122, 2601-2612, doi:10.1172/jci45785.
89. Zhang, L. et al. Novel small molecule inhibition of IKK/NF-κB activation reduces markers of senescence and improves healthspan in mouse models of aging. Aging cell 2021;20, e13486, doi:10.1111/acel.13486.
90. Zhao, J. et al. Development of novel NEMO-binding domain mimetics for inhibiting IKK/NF-κB activation. PLoS Biol 2018;16, e2004663, doi:10.1371/journal.pbio.2004663.
91. Pasqualetti, P. et al. A randomized controlled study on effects of ibuprofen on cognitive progression of Alzheimer’s disease. Aging Clin Exp Res 2009;21, 102-110, doi:10.1007/BF03325217.
92. Tabet, N. & Feldmand, H. Ibuprofen for Alzheimer’s disease. Cochrane Database Syst Rev, 2003;CD004031, doi:10.1002/14651858.CD004031.
93. Alimbetov, D. et al. Suppression of the senescence-associated secretory phenotype (SASP) in human fibroblasts using small molecule inhibitors of p38 MAP kinase and MK2. Biogerontology 2016;17, 305-315, doi:10.1007/s10522-015-9610-z.
94. Xu, M. et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proceedings of the National Academy of Sciences of the United States of America 2015;112, E6301-6310, doi:10.1073/pnas.1515386112.
95. Ishisaka, A. et al. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radical Biology and Medicine 2011;51, 1329-1336, doi:https://doi.org/10.1016/j.freeradbiomed.2011.06.017.
96. Wiczkowski, W. et al. Quercetin and isorhamnetin aglycones are the main metabolites of dietary quercetin in cerebrospinal fluid. Mol Nutr Food Res 2015;59, 1088-1094, doi:10.1002/mnfr.201400567.
97. Porkka, K. et al. Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome–positive leukemia. Blood 2008;112, 1005-1012, doi:10.1182/blood-2008-02-140665.
98. Gong, X. et al. A Higher Dose of Dasatinib May Increase the Possibility of Crossing the Blood–brain Barrier in the Treatment of Patients With Philadelphia Chromosome–positive Acute Lymphoblastic Leukemia. Clinical Therapeutics 2021;43, 1265-1271.e1261, doi:https://doi.org/10.1016/j.clinthera.2021.05.009.
99. Gonzales, MM. et al. Senolytic therapy in mild Alzheimer’s disease: a phase 1 feasibility trial. Nat Med. 2023 Sep 7; doi: 10.1038/s41591-023-02543-w. Online ahead of print.
100. Palmer, A. K. et al. Cellular senescence: At the nexus between obesity and metabolic dysfunction. Aging cell, doi:10.1111/acel.12950 (2019 (in press)).
101. Farr, J. N. et al. Targeting cellular senescence prevents age-related bone loss in mice. Nature medicine, 2017 doi:10.1038/nm.4385 .
102. Roos, C. M. et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging cell, 2016;doi:10.1111/acel.12458.
103. Schafer, M. J. et al. Cellular senescence mediates fibrotic pulmonary disease. Nature Communications 2017;8, 14532, doi:10.1038/ncomms14532.
104. Flament, S., Delacourte, A., Verny, M., Hauw, J. J. & Javoy-Agid, F. Abnormal Tau proteins in progressive supranuclear palsy. Similarities and differences with the neurofibrillary degeneration of the Alzheimer type. Acta neuropathologica 1991;81, 591-596.
105. Justice, J. N. et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine (In press) (2019).
106. Hickson, L. J. et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine, 2019;doi:10.1016/j.ebiom.2019.08.069.
107. Gonzales, M. M. et al. Senolytic Therapy to Modulate the Progression of Alzheimer’s Disease (SToMP-AD): A Pilot Clinical Trial. J Prev Alzheimers Dis 2022;9, 22-29, doi:10.14283/jpad.2021.62.
108. Xu, M. et al. Senolytics improve physical function and increase lifespan in old age. Nature medicine, 2018;doi:10.1038/s41591-018-0092-9.
109. Ogrodnik, M. et al. Cellular senescence drives age-dependent hepatic steatosis. Nature communications 2017;8, 15691, doi:10.1038/ncomms15691.
110. Ogrodnik, M. et al. Obesity-Induced Cellular Senescence Drives Anxiety and Impairs Neurogenesis. Cell Metabolism, 2019 doi:10.1016/j.cmet.2018.12.008.
111. Nath, K. A. et al. The Murine Dialysis Fistula Model Exhibits a Senescence Phenotype: Pathobiologic Mechanisms and Therapeutic Potential. Am J Physiol Renal Physiol, 2018 doi:10.1152/ajprenal.00308.2018.
112. Lewis-McDougall, F. C. et al. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell 2019;18, e12931, doi:10.1111/acel.12931.
113. Yousefzadeh, M. J. et al. An aged immune system drives senescence and ageing of solid organs. Nature 2021;594, 100-105, doi:10.1038/s41586-021-03547-7.
114. Choi, I. et al. Autophagy enables microglia to engage amyloid plaques and prevents microglial senescence. Nature cell biology, 2023 doi:10.1038/s41556-023-01158-0.
115. Fujimaki, K. et al. Graded regulation of cellular quiescence depth between proliferation and senescence by a lysosomal dimmer switch. Proceedings of the National Academy of Sciences of the United States of America 2019;116, 22624-22634, doi:10.1073/pnas.1915905116.
116. Chow, H. M. et al. Age-related hyperinsulinemia leads to insulin resistance in neurons and cell-cycle-induced senescence. Nature neuroscience 2019;22, 1806-1819, doi:10.1038/s41593-019-0505-1.
117. Schwab, N. et al. Neurons and glial cells acquire a senescent signature after repeated mild traumatic brain injury in a sex-dependent manner. Front Neurosci 2022;16, 1027116, doi:10.3389/fnins.2022.1027116.
118. Diniz, B. S., Reynolds Iii, C. F., Sibille, E., Bot, M. & Penninx, B. Major depression and enhanced molecular senescence abnormalities in young and middle-aged adults. Transl Psychiatry 2019;9, 198, doi:10.1038/s41398-019-0541-3.
119. Sun, J. K. et al. Chronic alcohol metabolism results in DNA repair infidelity and cell cycle-induced senescence in neurons. Aging cell 2023;22, e13772, doi:10.1111/acel.13772.
120. Russo, T. & Riessland, M. Age-Related Midbrain Inflammation and Senescence in Parkinson’s Disease. Front Aging Neurosci 2022;14, 917797, doi:10.3389/fnagi.2022.917797.
121. Muralidharan, A. et al. Long-term male-specific chronic pain via telomere- and p53‑mediated spinal cord cellular senescence. J Clin Invest 2022;132, doi:10.1172/JCI151817.
122. Musi, N. et al. Tau protein aggregation induces cellular senescence in the brain. bioRxiv, 2018;369074, doi:10.1101/369074.
© The Authors 2023