M. Kiraly1, J.F. Foss1, T. Giordano1
1. NeuroTherapia, Inc., 10000 Cedar Ave., GCIC Building, Cleveland, OH 44106
Corresponding Author: Tony Giordano, NeuroTherapia, Inc. 10000 Cedar Ave., GCIC Building, Cleveland, OH 44106, USA, tony@neurotherapia.com, phone: 440-228-4089, fax: 440-389-4501
J Prev Alz Dis 2023;4(10):686-698
Published online October 24, 2023, http://dx.doi.org/10.14283/jpad.2023.109
Abstract
Neuroinflammation precedes the clinical onset of various neurodegenerative diseases, including Alzheimer’s disease (AD), by years or frequently even decades (1-3). In terms of the underlying physiology, there is a great need for understanding and controlling interactions between the central nervous system (CNS) and the immune system in an attempt to develop approaches to prevent or delay the disease’s progression. Nerve cells have limited motion capability, whereas immune cells can migrate freely via circulation. This difference raises a variety of questions in the context of senile plaque formation and phagocytosis. Broad-scale unbiased genomic studies bring several genetic variants such as sialic acid binding Ig-like lectin 3 (CD33), triggering receptor expressed on myeloid cells 2 (TREM2) or complement receptor type 1 (CR1) into the focus of researchers’ attention as potential risk factors for neuroinflammation. In addition, advanced proteomic analyses have been revealing links between these genetic contributors and complex, malfunctioning signaling pathways (including the upregulation of factors like tumor necrosis factor TNF-α, tumor growth factor TGF-β and interleukin IL-1α) that promote proinflammatory mechanisms via intracellular signaling and trafficking, synaptic function, and cell metabolism/ proliferation. In AD, the brain’s microglia and astrocytes, which are normally responsible for maintaining the homeostasis of synaptic transmission and its remodeling by pruning, are the initiators of neuroinflammation and toxic tau and amyloid-β (Aβ) accumulation. Thus, they drive the CNS into a state of sustained or even self-accelerated deterioration. Here we aim to review the cell types and mediators involved in neuroinflammation and AD, the symptom manifestation in clinical settings, and potential candidates for improving diagnosis and treatment.
Key words: Neuroinflammation, therapeutics, microglia.
The main hallmarks of AD
AD affects nearly 10% of the 65+ year-old and over 30% of the 85+ year-old populations worldwide and has been known to be the leading cause of dementia (~70% of cases, (4)). The gradual loss of cognitive and memory functions correlates with the accumulation of Aβ and tau deposits and fibrils. Briefly, amyloid precursor proteins (APP) are cleaved to Aβ peptide units of various sizes. Aβ oligomers can then aggregate with each other or with single-peptide monomers, forming insoluble polymers and large plaques. Aβ oligomers are known to exert the most significant pathological damage to neurons (5). In addition to Aβ, aggregated tau proteins that detach from microtubules and thereby cause a loss in synaptic activity and neuronal function also significantly contribute to the disease pathology (6). Surprisingly however, treatments in clinical trials that aim to eliminate senile plaques in the brains of AD patients showed controversial results: the volume of protein aggregates decreased in certain settings, and yet the clearance did not lead to an improvement in cognitive function (7, 8). However, most recent studies showed that treatment with monoclonal antibody therapeutics such as aducanumab, lecanemab, and most recently, donanemab can slow the progression of the disease significantly in early stages of AD and can clear amyloid deposition to up to 90%. The manufacturer of donanemab, Eli Lilly, published encouraging data from their 1,736-subject trial on July 17th, 2023, at the Alzheimer’s Association International Conference in Amsterdam (9). According to their presentation, progression of cognitive decline was not observed in 47% of the patients, vs. the equivalent percentage of 29% in those who took a placebo. Nevertheless, amyloid-related imaging abnormalities (ARIA) remain a serious side-effect of treatment with antibody therapeutics. In Eli Lilly’s Phase III clinical trial, nearly 25% of the participants developed ARIA, and three died with this condition (9). Screening for ARIA will be expensive and challenging, thus there is a great need to further investigate the importance of neuroinflammation in AD progression. In addition, leaders from each of the company’s development teams, during a round table discussion, stressed the likely importance of combination therapies for treating such a complex disease, with one of the presenters citing how antithrombotics, anti-hypertensives and lipid-lowering drugs in various combinations have greatly improved cardiovascular care. Thus, the development of neuroinflammatory inhibitors might not only protect against some of the deleterious effects of ARIA but may also provide synergistic benefits to patients on antibody therapies.
The role of microglia, astrocytes, and cell mediators in AD neuroinflammation
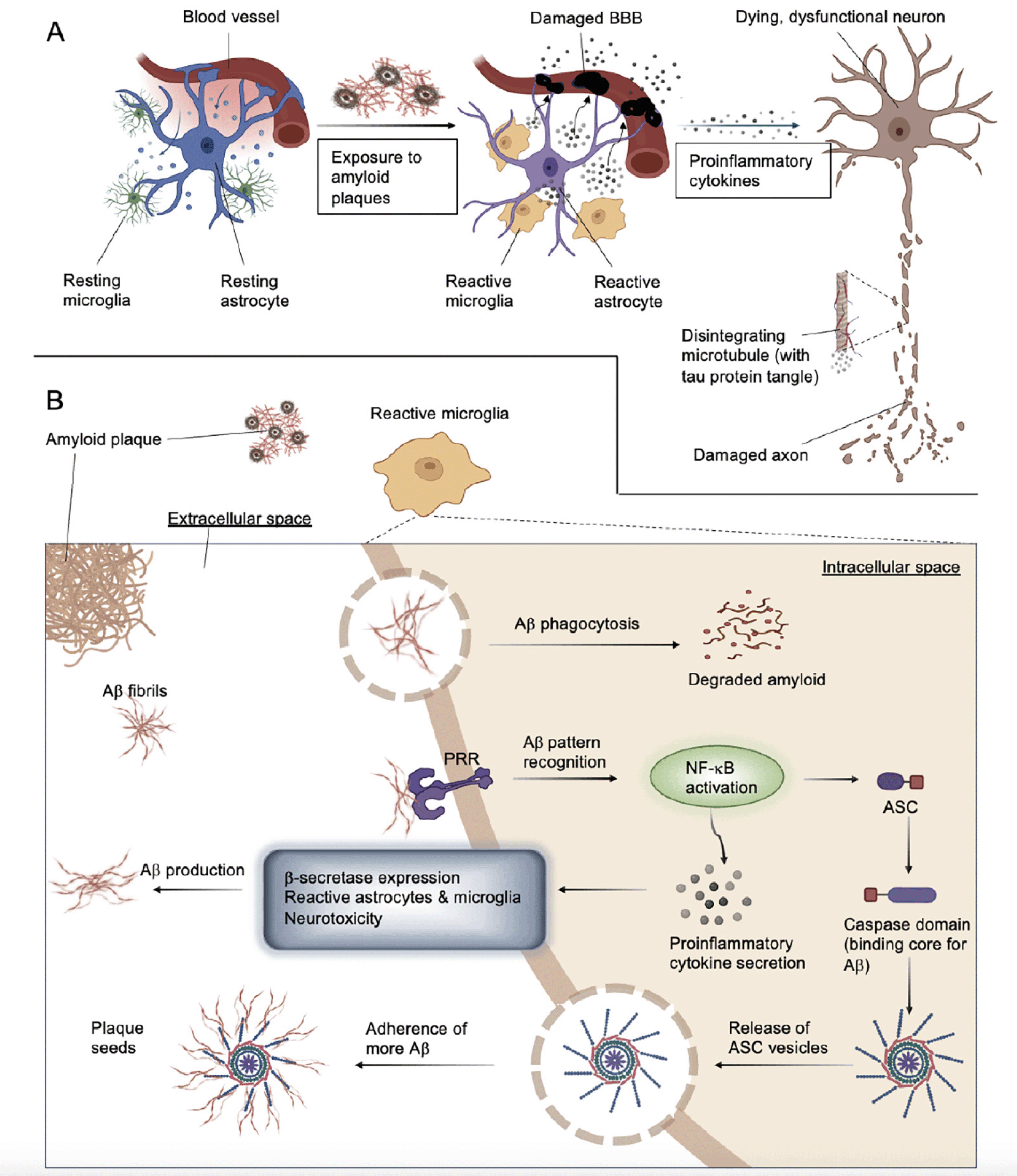
Neuroinflammation evolved to be a primary mechanism to protect the homeostasis of the brain. Its purpose and function in protecting and restoring synaptic functions against traumatic or infectious damage is highly conserved. Nevertheless, the physiological outcome greatly depends on the responses evoked in the individual participatory cell types of the system, including but not limited to neurons, glial cells, and astrocytes. The process begins with the secretion of chemokines, tumor necrosis factors, small molecule messengers, proinflammatory cytokines, and reactive oxygen species produced by astrocytes and microglia. Systemic inflammation and blood cells infiltrating through the compromised blood-brain barrier (BBB) may further escalate the destruction. The process is considered chronic, and even when the anti-inflammatory system is activated, and neuroprotective interleukins are being produced, it is not likely possible to tame without intervention (Figure 1A, (10)).
Neurotoxicity, synaptic dysfunction, and the suppression of neurogenesis are induced by proinflammatory mediators released in the damaged CNS (11). TNF and IL-1β overexpression via prostaglandin E2 release leads to excitotoxicity and loss of synapses (12, 13). Furthermore, macrophage-attracting proteins, the complement system (including but not limited to C1q, C3b, C3c, C3d, and C4 within the cascade), factors of coagulation, proteases, pentraxins, and many other molecules play critical roles in causing functional impairment and neuronal death (14). Despite our understanding of how various contributing components alter AD pathophysiology on a cellular and molecular level, the full picture is yet to be completed.
Astrocytes serve as the support system of the CNS; their purpose in regulating neurotransmitter balance, supporting the BBB to remain intact, and supplying existing and newly formed synapses, is indispensable (Figure 1A, (15)). They also help remove cell debris, tau, and amyloid particles and respond to ischemia, infection, protein deposits, or other brain abnormalities via scar formation and reactive gliosis (15). Their core structural protein, the glial fibrillary acidic protein (GFAP), has emerged as a marker of the reactive astrocyte population. Activated astrocytes can adopt one of the two typical phenotypes. A1 cells are generated through nuclear factor kappa B (NF-κB) signaling, they express proinflammatory molecules, and they can induce neuronal apoptosis. A2 types are converted from A1 or resting states via the signal transducers and activators of the transcription 3 (STAT3) pathway and secrete anti-inflammatory and neuroprotective factors (16). It is unclear whether astrocytes can realistically be categorized with such dichotomy or whether they rather represent unique states within a continuum between the two distinct characteristics (15). Controversial studies have debated the benevolence of activated astrocytes in AD: on the one hand, they demonstrate the capability to locally clear protein deposits in the brain (17, 18). On the other hand, however, large populations of proinflammatory astrocytes have been shown to be present in postmortem brain tissue dissected from AD patients, thereby suggesting a defective function of A1 cells (16). In addition, reactive A1 astrocytes have also been shown to alter the normal functions of the BBB and blood supply in the CNS, thus contributing to the initiation and progression of the disease (15).
Microglia are the brain’s primary immune cells, responsible for the phagocytosis of cellular debris and pathogens, as well as secreting molecules to support the homeostasis of local cells. Coincidentally, microglia also play an important role in maintaining healthy synaptic plasticity (19). Recent studies suggest that high cytokine levels in the cerebrospinal fluid (CSF) reduce the capacity of Aβ uptake in the microglia (20). Moreover, new evidence shows that an uncommon mutation of the extracellular unit of TREM2 increases the probability of AD development to a similar extent as the occurrence of apolipoprotein unit apoEα4 (21).
TREM2 is abundantly expressed in microglial cells and has been shown to facilitate phagocytosis (22-24). As a consequence of pathogenic overstimulation, microglia activated to an abnormal level can change their gene expression and morphology to an amoeboid structure with a reduced number of processes and shorter surveillance radius (15). The intensity and duration of an environmental insult may change the morphology of the microglia in correlation with the severity of the damage (15). Exposure to the hyperphosphorylated soluble tau protein has also been reported to alter the phenotype of microglia, resulting in the loss of normal cell functions and thus contributing to the further accumulation of local protein deposits (15, 25). According to the classic terminology of categorizing microglia, M1 represents the proinflammatory, and M2 is known as the anti-inflammatory phenotype (15). Similar to the characterization of activated astrocytes, it is possible that considering a spectrum between the two dominant characteristics is a more realistic approach than assuming the existence of only two extreme versions. Transition to activated microglia states is generally associated with the upregulation of proteins like TREM2, apolipoprotein E (APOE), and TYRO protein tyrosine kinase-binding protein TYROBP. Proliferation-related gene expression is more typical in the early stages of neuroinflammation. In contrast, at more advanced states, the expression of immune response-related genes as well as the downregulation of homeostasis genes such as structural (cytoskeleton), cell adhesion, and external receptor encoding genes, is more representative (15, 26, 27). This finding is consistent with the diversity of microglia observed in postmortem brain tissue samples of AD patients (27). Details on the role of microglia in AD, alternative pathways that contribute to their activation to various degrees in between the M1 and M2 states (including NF-κB, mTOR, MAPK, proinflammatory mediators, interleukins, anti-inflammatory cytokines, complement proteins, chemokines, caspases, prostanoids, neuroprotection D1 reactive oxygen species and nitric oxide, local blood flow and various genetic components) have been extensively reviewed for example by Heneka et al. (10), Hampel et al. (28), and Thakur et al. (29).
A: Activated by exposure to Aβ, reactive microglia and astrocytes lose their homeostatic balance, compromise their ability to produce sustainable levels of neurotrophic factors, release increased amounts of proinflammatory cytokines and consequently, damage the blood-brain barrier (BBB) and trigger neuronal death and tau accumulation. Reactive microglia can directly damage and reduce the number of synaptic connections. B: Microglial activation following exposure to Aβ. Amyloid fibers adhere to pattern recognition receptors (PRRs, TLRs, for example), triggering proinflammatory pathways and promoting phagocytosis. This process can lead either to Aβ degradation or NF-κB activation and proinflammatory cytokine release combined with inflammasome assembly. NLRP3 activation leads to the production of ASC specks that can seed further plaques when released into the extracellular space.
Microglial activation induced by Aβ and pharmaceutical candidates for intervention
Reactive microglia have been described to colocalize with amyloid plaques, and a strong correlation between local tau deposition and microglial tau content has also been established (reviewed in (15)). According to trending hypotheses, reactive microglia induced by Aβ oligomers contribute to the emergence of abnormally hyperphosphorylated tau aggregates (15). First, Aβ peptides are recognized by cell surface pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), integrins, and scavenger receptors (e.g., CD36). Subsequently, IL-1β, nitric oxide (NO), IL-8, and TNF production is upregulated via the activation of mitogen-activated kinase (MAPK), N-terminal kinase (JNK), NF-κB and c-Jun pathways (15). CD36-integrin complexes enhance the effectivity of phagocytosis. The secreted interleukins, TNFs and other molecules then elevate the expression level of β-secretase, the enzyme responsible for generating toxic Aβ species from APP via the NF-κB pathway, thus creating a pathological feedback loop (15).
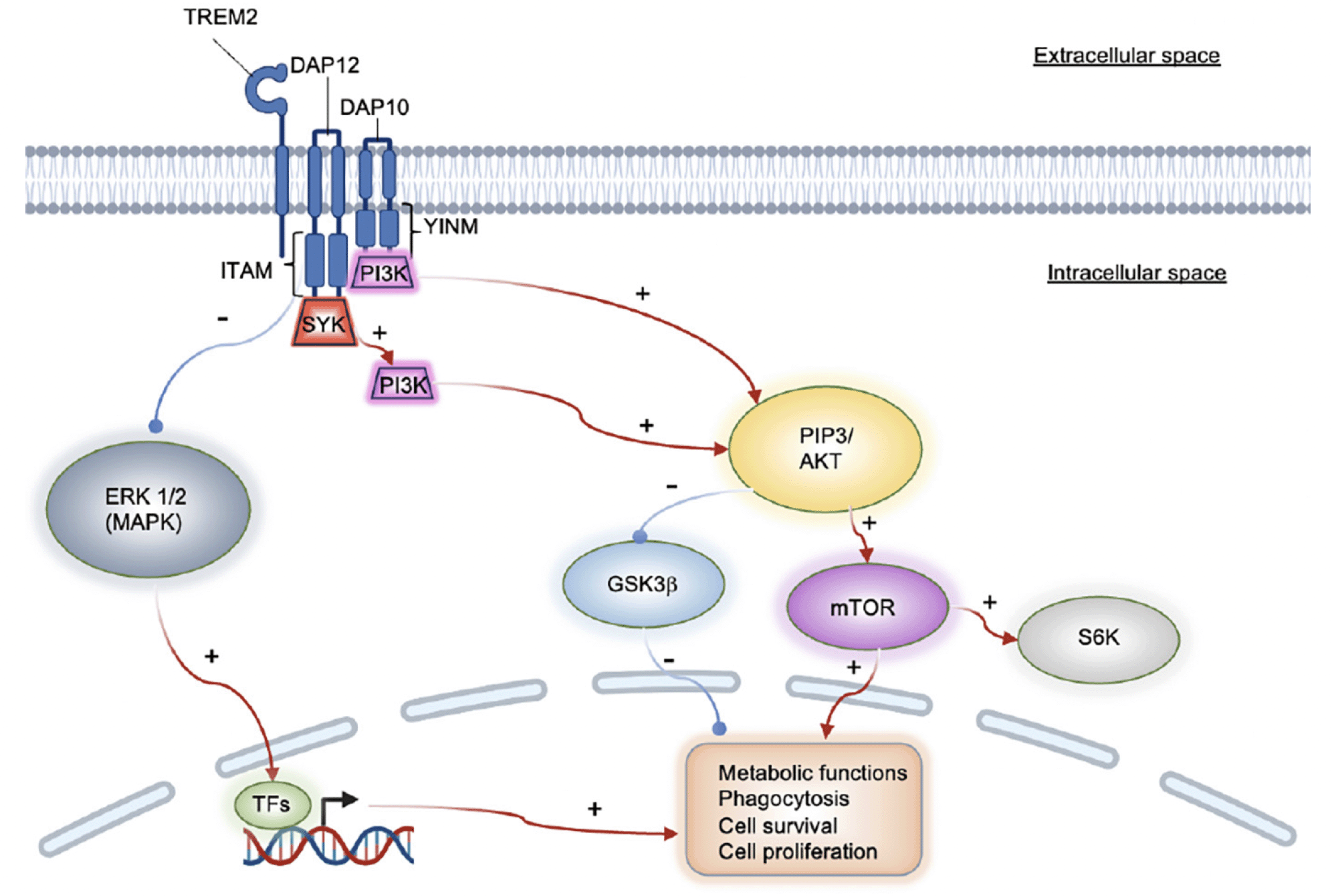
Two microglial target molecule candidates for pharmacological intervention are currently the focus of researchers’ interest: the transient receptor potential melastatin-related 2 (TRPM2) channel and TREM2 (26, 30, 31). Calcium currents mediated by TRPM2 are triggered by oxidative stress and induce microglial pyrin domain-containing 3 (NLRP3) inflammasome assembly via NF-κB activation. The adaptor molecule for NLRP3 inflammasome activation, the apoptosis-associated speck-like protein containing C-terminal caspase recruitment domain (ASC), can function as an adhesive core for Aβ peptide aggregation. Growing particles can then be passed on to other cells, thus seeding newly formed amyloid plaques (FIG 1B, (32)). Preventing this process pharmacologically could potentially slow down AD progression. TREM2 is a receptor that activates upon the stimulation of the immunoreceptor tyrosine-based motif (ITAM) pathway, thus causing microglia to become reactive (32). One signaling route is mediated via the DAP12 transmembrane adaptor and the protein tyrosine kinase SYK. The SYK enzyme regulates many downstream processes via phosphorylation, including the PLCγ2 Ca2+ and PI3K-AKT-mTOR cascades, thus, suppressing GSK3β function and stimulating cell proliferation (Figure 2, (33)). A recent study shows that SYK deficiency corrupts the microglial phagocytosis of Aβ deposits and leads to neurotoxicity and cognitive impairment. Aβ accumulation-related pathophysiology in SYK-deficient animals was demonstrated to be similarly severe as in TREM2-knockout animals; thus, TREM2 and SYK appear to have a similar impact on disease progression. However, TREM2 can activate microglia in a non-SYK-dependent manner as well, through the DAP10 signaling adapter and a cytoplasmic YxNM motif (YINM) (Figure 2, (33)). These discoveries suggest that small molecules and antibodies that act on microglial receptors and induce TREM2-YINM/ ITAM-SYK signaling may serve as novel drug candidates for the treatment of AD in the future.
TREM2 is currently assessed for antibody-mediated therapy (34, 35). However, optimizing strategic approaches for TREM2 pathway modulation will likely have to be disease-state-dependent. One study using an AD mouse model showed that TREM2 overexpression directly positively correlates with the expression of phagocytosis-associated genes. In contrast, it negatively correlates with immune response-related genes, thus resulting in a net neuroprotective effect (36). However, in another scenario, triggering the TREM2 pathway led to progressive neuroinflammation and the disruption of microglial homeostasis via APOE-dependent signaling (31). In addition, TREM2 is an essential regulator of key downstream functions related to the microglial cell cycle and phenotype adjustment. These data indicate that interpreting experimental results may highly depend on variables such as study design, animal model, or disease state. Translating preclinical discoveries into clinical treatments will require caution and a detailed understanding of each case.
TREM2 can transmit intracellular stimulation either via the DAP12 (ITAM), or the DAP10 (YINM) adaptors. ITAM recruits PI3K via SYK, whereas YINM accesses PI3K directly. Both pathways activate mTOR and inhibit GSK3β via AKT, thus impacting fundamental cell functions. The TREM2/ ITAM route inhibits ERK in an SYK-independent manner. TFs: transcription factors.
Cannabinoid receptor type 2 (CB2) as a target for microglial phenotype shift and neuroprotection
The cannabinoid receptor (CB) family includes two cloned metabotropic receptors: CB1 (found predominantly in the brain, (37)) and CB2 (found primarily in the peripheral immune system, (38)) and to a lesser degree in the CNS and microglia, (39)). Healthy brain tissue (except for a small population of neurons in the brain stem and the cerebellum) does not express CB2 receptors (40). However, CB2 receptors are upregulated in reactive microglial cells in AD, Huntington’s disease, HIV encephalitis, and multiple sclerosis (41, 42). Results from research in our laboratory demonstrate that microglial activation is present in murine AD models, and this activation is associated with increases in CB2 expression and the subsequent release of proinflammatory agents leading to neuronal injury (43-45). Activation of the CB2 receptor can blunt neuroinflammatory responses in different CNS disorders (46-48), including Alzheimer’s disease (49). Strong induction of CB2 results in controlling amyloid-related pathology in C6 rat astroglioma cells challenged with Aβ fibrils as well (50). Figure 3 illustrates the complexity of effects downstream of CB2 receptor activation.
For example, CB2 stimulation was shown to regulate microglial pro- and anti-inflammatory phenotype polarization (and thereby suppressing neuroinflammation) through the cyclic adenosine monophosphate (cAMP) / cyclic-AMP dependent protein kinase/protein kinase A (PKA) pathway in a rat germinal matrix hemorrhage model (51), as well as in vitro in BV2 cells (52). Modulating protein kinases (53) or K+ channels (54) alone is promising for taming microglial phenotypes by modifying gene expression and cytokine secretion patterns, thereby preventing neuronal apoptosis and synapse loss. β-arrestin recruitment (55) and prevention of the increase of intracellular Ca2+ with chelators resulted in suppressed lipopolysaccharide (LPS)-stimulated microglial NO, cytokine, and chemokine production (56). A reduction in ceramide accumulation decreases the activation of the extracellular signal-regulated kinase (Erk) pathway and increases cell survival (57). Altogether, CB2-activated cAMP, cAMP-response element binding protein (CREB), and p38-MAPK cascades are necessary for microglial TLR function, and TLR receptors are well-known for their key role in phagocytosis, uptake, and clearance of amyloid (58). In addition, the neuroprotective microglial phenotype has been demonstrated to reduce brain atrophy (characterized by loss of neurons and synapses, and dystrophic neuritis in AD (59). Our lead compound, 1-((3-benzyl-3-methyl-2,3-dihydro-1-benzofuran-6-yl)carbonyl) piperidine (NTRX-07, formerly known as MDA7), exerts its effect both on the canonical (inhibition of adenylate cyclase and Erk 1/2, EC50 <20 nM) and non-canonical CB2 pathways (EC50 <25 nM) as our data collected from β-arrestin assays demonstrate; thus, exerts its therapeutic effects in a uniquely complex manner.
Studies in rodent models have shown that increased neuroinflammation, promoted by the overexpression of proinflammatory cytokines, can lead to increased hyperphosphorylated tau and decreased hippocampal function (60). Decreasing microglial activation and neuroinflammation in rodent models via CB2 receptor activation increased clearance of amyloid plaques, improved hippocampal plasticity and glutamatergic signaling, and enhanced memory performance in the Morris water maze (44, 45). NTRX-07 is a selective and potent CB2 receptor agonist. NTRX-07 binding to activated microglia engaged the CB2 pathway leading to the transformation of activated microglia, which are proinflammatory, to their normal anti-inflammatory states. Administration of NTRX-07 decreased Iba1 (ionized calcium-binding adapter molecule 1, a microglia/macrophage-specific calcium binding protein) immunoreactivity and CB2 receptor expression in the hippocampal dentate gyrus and entorhinal cortex areas in mouse AD models, as demonstrated via quantitative fluorescence immunohistochemistry, and colocalization of the Iba1 and CB2 markers. No substantial CB2 expression was seen in the wild type mice, but the increased expression of CB2 (n = 20 sections from 5 mice per group, F3,16 = 25.6, P <0.0001) observed for example in the APP/PS1 mice was significantly attenuated after 15 mg/kg NTRX-07 treatment i.p. at alternate days for 5 months. Statistical significance was determined by one-way ANOVA test followed by Student-Newman-Keuls test. Presumably, due to this effect, NTRX-07 restored synaptic plasticity as well as functional memory in a rat model of AD (43-45).
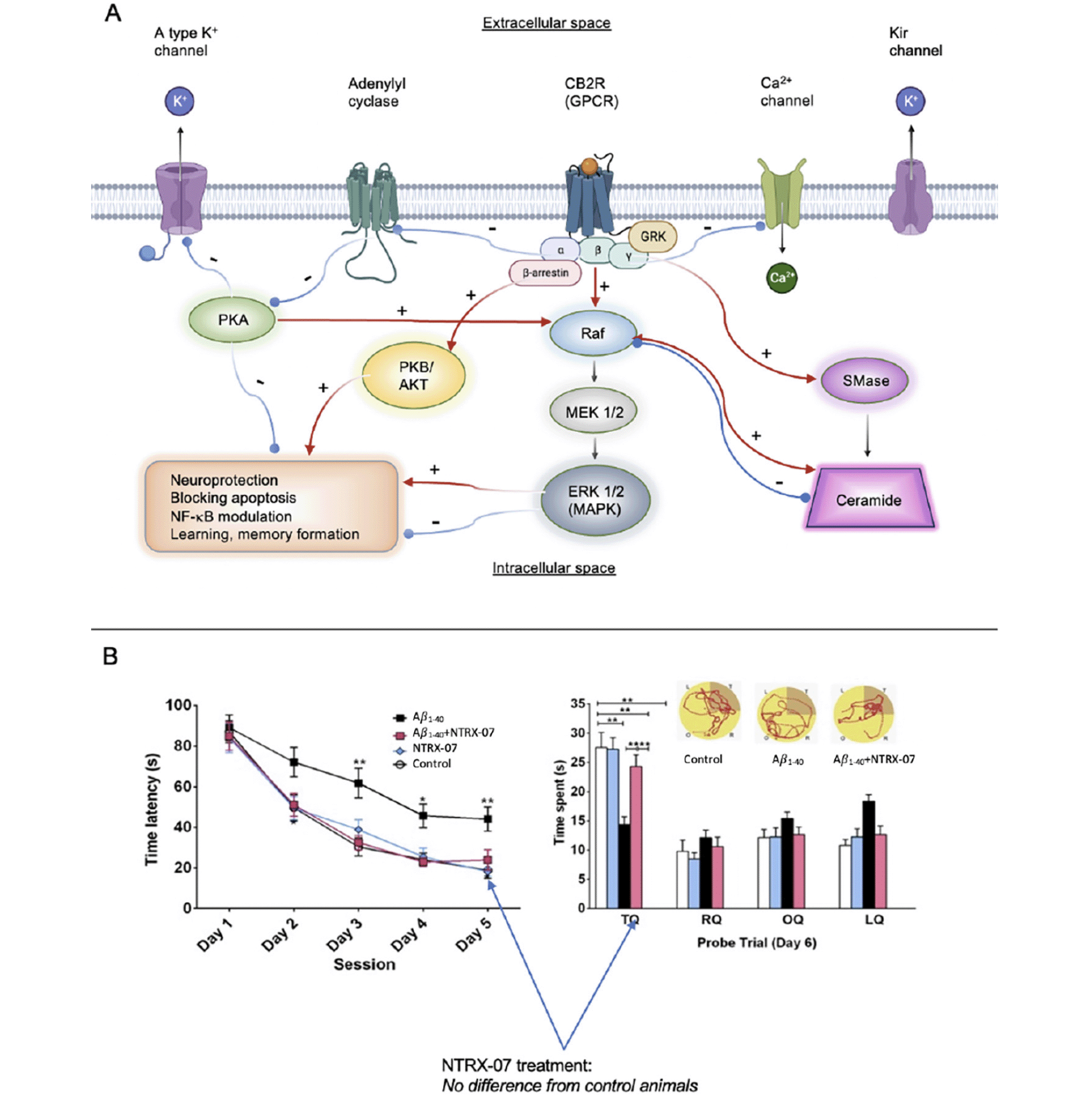
Figure 3. A: Signaling cascades mediated via CB2 G-protein coupled receptor (GPCR) receptor activation. CB2 agonists may activate the canonical (cAMP) or non-canonical (β-arrestin recruitment) pathways to various degrees to achieve therapeutic effects. We have shown that, unlike other compounds, NTRX-07 can exert a therapeutic impact by manipulating both downstream routes. B: Administration of NTRX-07 attenuated amyloid fibril-impaired performance in the Morris water maze test. Rats injected with bilateral intracerebral (i.c.) microinjection Aβ1–40 fibrils and treated with saline intraperitoneally (i.p.) for 14 days had a significantly extended escape latency in the Morris water maze test compared with that of the rats that received bilateral i.c. microinjection of artificial cerebrospinal fluid and treated with saline i.p. (controls) or animals injected with Aβ1–40 and treated with 15 mg/kg NTRX-07 i.p. for 14 days at days 3 to 5. During the probe trial at day 6, to determine the time spent in the target quadrant (TQ or platform quadrant) compared with right quadrant (RQ), opposite quadrant (OQ), and left quadrant (LQ), the rats injected with Aβ1–40 fibrils and treated with NTRX-07 15 mg/kg i.p. for 14 days spent the longest time in the Target Quadrant (TQ) than animals injected with Abeta1–40 and treated for 14 days with saline i.p., (p <0.01)
Statistical significance was determined by repeated measures analysis of variance followed by Student–Newman–Keuls multiple range test. Each point represents the mean + standard error of the mean of each group (n = 10 per group). * p <0.05, ** p < 0.01. Figure 3b adapted from European Journal of Pharmacology with Permission, reference #45.
Neuroinflammation biomarkers in Alzheimer’s disease
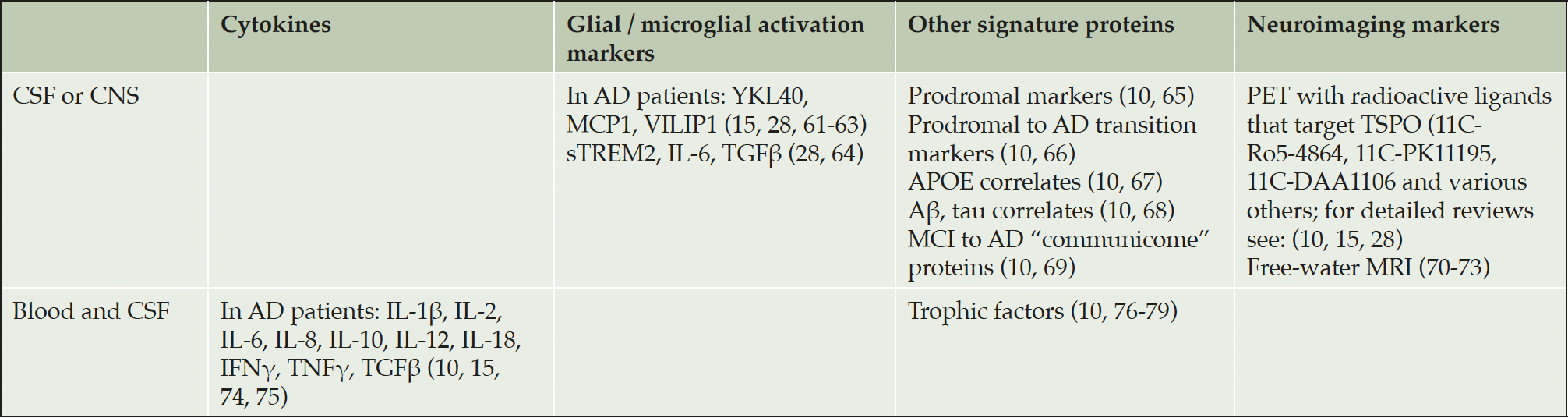
There has been ongoing discussion and research into neuroinflammation’s timing, source, and contribution to the neural injury and cognitive decline seen in AD. Over the past decades, a large number of studies attempted to identify reliable biomarkers associated with neurodegeneration in order to make the diagnosis more accurate and propose appropriate therapeutic strategies for each patient individually. Table 1 summarizes the most promising biomarker candidates’ current state of the art.
Ishii et al. reported the identification of the components of immunoglobulins in senile plaques. They hypothesized that immunological factors may be involved in the pathogenesis of the plaques and Alzheimer’s disease (80). The activation of the complement system has been associated with the defense of the innate immune system against pathogens. In the brain, microglia play a major role in the production of elements of the complement system, with astrocytes also contributing to a lesser degree (10). Eikelenboom et al. demonstrated in 1982 that senile plaques contain the complement factors C1q, C3b, C3c, C3d and C4, (81) again supporting the hypothesis that the complement system and inflammation play a role in AD.
Positron emission tomography (PET) imaging using the mitochondrial 18 kDa translocator protein (TSPO) has been employed to study neuroinflammation in vivo. [11C]PK-11195 has been used in preclinical and clinical studies but is limited by poor blood-brain barrier permeability and high non-specific binding. However, it has been seen to be increased in MCI and AD patients with increased Aβ, and it has been used to demonstrate increased microglial activation matched to increased tau accumulation as demonstrated by [18F]flortaucipir (82). Fan et al. have demonstrated a biphasic pattern in AD patients studied longitudinally suggesting there may be two phases of microglial activation (83). Second- and third-generation TSPO tracers are under development, but TSPO is limited by the fact that it is not exclusively expressed in glia. New targets of interest include colony-stimulating factor-1, cyclooxygenase-1 and -2, cannabinoid receptor type 2 and the purinergic P2X7 receptor with a goal of achieving higher specificity and an improved signal-to-noise ratio (82).
In a comparison of healthy controls, patients with MCI and patients with AD utilizing PET for TSPO and tau, and MRI for cortical thickness, neuroinflammation was demonstrated to play a significant role in AD pathogenesis and was not a byproduct of tau pathology. Fusion mapping of the imaging correlated tau and microglial spatially with gray matter atrophy. Furthermore, ongoing neuroinflammation has been demonstrated in a longitudinal study of AD patients with MRI and PET for neuroinflammation, tau, and glucose metabolism. Persistent neuroinflammation was observed and was associated with localized amyloid deposition, synaptic dysfunction, and decreased glucose metabolism (83).
Systemic inflammation, such as that associated with obesity, has also been implicated in neuroinflammation and damage to limbic structures, which are known to be involved in AD, demonstrated by MRI changes in asymptomatic obese subjects (84). Neuroinflammation has been linked to other factors that interact with the gut microbiota-brain axis, an emerging area of research in Alzheimer’s disease. This axis involves the bidirectional communication between the gut microbiota and the central nervous system, and it has been suggested that alterations in the gut microbiota may contribute to neuroinflammation and cognitive decline in Alzheimer’s disease (85).
The first row represents lists of biomarkers that have been used to detect neuroinflammation either in the tissue components of the CNS or in the CSF. The publications cited here, however, do not provide evidence that the expression levels of these biomarkers are also elevated peripherally (in blood samples of the same subjects). The table’s second row refers to biomarkers found to be overexpressed both in the CSF and the blood plasma samples of patients with acute neuroinflammation. Abbreviations: Aβ: amyloid beta, AD: Alzheimer’s disease, APOE: apolipoprotein E, IFN: interferon, IL: interleukin, PET: positron emission tomography, TNF: tumor necrosis factor, TGF: transforming growth factor, YKL40: chitinase 3-like protein-1, MCI: mild cognitive impairment, MCP1: monocyte chemotactic protein 1, MRI: magnetic resonance imaging, sTREM2: soluble Triggering Receptor Expressed on Myeloid Cells 2, TSPO: translocator protein (peripheral benzodiazepine receptor), VILIP1: visinin-like protein-1
Therapeutic strategies targeting neuroinflammation in Alzheimer’s disease
Given the importance of microglia in neuroinflammation, it is not surprising that many therapies targeting neuroinflammation focus on regulating microglia. ALZT-OP1 is one of the more advanced molecules targeting, in part, neuroinflammation. Being developed by AZTherapeutics, ALZT-OP1 is being tested in a 620-subject Phase 3 clinical trial (NCT02547818). ALZT-OP1 is a combination product consisting of two repurposed drugs, cromolyn, approved for treating arthritis, and ibuprofen, a non-steroidal anti-inflammatory. This combination was shown to be very effective in the Tg2576 model of AD in reducing levels of Aβ through enhancing phagocytosis by microglia, suggesting that treatment shifted the microglia from a proinflammatory/toxic state to a pro-phagocytic/neuroprotective state (86). The effects on inflammation were later confirmed when cromolyn was shown to directly affect microglia-mediated inflammation (87). Although the trial has been completed, there have been no publications or press releases concerning the study’s outcome. A good summary of the clinical development of ALZT-OP1 has been reported by Lozupone et al. (88).
In addition to microglia, mast cells play an important role in regulating the immune response in the brain. Mast cells are thought to initiate the immune response when presented with a toxin, such as Aβ, leading to the activation of microglia and initiation of neuroinflammation (89). AB Science completed enrollment of its ongoing Phase 3 AD clinical trial of Masitinab. This selective tyrosine kinase inhibitor is thought to act through mast cells to modulate neuroinflammation and neurodegenerative processes (NCT01872598). In a small Phase 2 clinical trial, twice daily administration for 24 weeks of Masitinab as an adjuvant to cholinesterase inhibitors or memantine led to a significant reduction in cognitive decline (90). However, in a subsequent preclinical study using APPswe/PSEN1dE9 transgenic mice, improvements in spatial learning were reported without any effect on neuroinflammation (91). Whether this is also true in AD patients remains to be seen when the data from the Phase 3 trial is available.
While not directly targeting microglia, Cassava Sciences, Inc. has recently started a large, 76-week Phase 3 trial with their drug simufilam, PTI-125 (NCT05026177). Simufilam binds to protein filamin A and restores its normal function. In its altered conformation, among other things, filamin A enables the activation of toll-like-receptor 4 by Aβ, resulting in increased neuroinflammation (92). Following 28-days of treatment of AD subjects in a Phase 2a clinical trial, a modest but significant reduction in both the CSF and plasma of YKL40, a protein associated with microglial activation, was observed along with reductions in the proinflammatory cytokines IL-6, IL-1b, and TNF-a, and disease-associated proteins, including NfL, total-tau, phospho-tau and neurogranin (93). The company is exploring changes in YKL40 and soluble TREM2 (sTREM) in the CSF from baseline to after 76 weeks of treatment, in addition to other biomarkers and cognitive improvement in the Phase 3 trial.
Other trials currently underway looking for changes in sTREM2, include Alzheon’s study of ALZ-801, an inhibitor of amyloid oligomerization (NCT04693520), and a study of high-dose Omega-3 therapy (NCT03926351). In addition to looking for changes in sTREM2 levels, given the importance of TREM2 in the microglia neuroinflammatory response, several companies are developing molecules targeting TREM2 directly. The leader in this field is Alector, who, in partnership with Abbvie, is testing their antibody against TREM2, AL002 in a Phase 2 clinical trial of subjects with early AD (NCT05744401). Their antibody acts as an agonist to activate TREM2 and, when used to treat R47H-transgenic mice, led to a reduction in neuroinflammation, reduced plaques, and improved neuronal function (35). The trial is a 49-week study of three different doses administered every week, intravenously. The study is scheduled to be completed by the end of 2025.
In partnership with Takeda, Denali Therapeutics is also developing an antibody, TAK-920/DNL919, designed to modulate TREM2 expression by increasing microglia activity (94). The company began a Phase 1 trial in healthy volunteers last year with expected completion in mid-2023 (NCT05450549). The company also has a second program focused on neuroinflammation with a RIPK1 inhibitor that has completed Phase 1 studies in subjects with AD or ALS (95).
A third company focusing on therapies targeting TREM2 is Vigil Neuro. Like Alector and Denali, their lead product, VGL101, is an antibody directed against human TREM2. Although they are not advancing the antibody for AD, Vigil is currently in a Phase 2 clinical trial for the treatment of axonal spheroids and pigmented glia that will incorporate imaging and biomarkers of disease progression (NCT05677659) and could develop this for AD in the future. The company is currently developing a small molecule TREM2 agonist for treating AD and has reported advancing their product into IND-enabling studies. The only data available on this program were presented at the 2023 Keystone Symposia on Molecular and Cellular Biology (96), where they showed that their molecule enhanced SYK activation in cells, while also reducing CSF levels of sTREM2 in non-human primates, suggesting the molecule is centrally active.
A more recent clinical strategy for targeting microglia-induced neuroinflammation is NeuroTherapia’s small molecule selective CB2 receptor agonist, which has been shown in animal models of AD to not only inhibit inflammation but also to enhance Aβ clearance and improve LTP (44, 45). With support, in part, from the Alzheimer’s Drug Discovery Foundation, the molecule has been shown to be safe in healthy volunteers and AD patients at doses that resulted in blood levels predicted to be efficacious based on the modeling of animal data. NeuroTherapia will initiate a Phase 2a study shortly with the intention of showing that 28-day treatment can lead to decreases in markers of neuroinflammation. Epidiolex (GW Pharma), a 99% pure cannabidiol (CBD) extract, has been studied in numerous clinical trials and approved as an anti-seizure medication, is also currently being investigated for its ability to reduce neuroinflammation in a 4-week (97), Phase 2 clinical trial (NCT05066308). Another study at Yale is recruiting healthy subjects to test the effects of CBD on brain microglial activation (NCT04398719), while in the Netherlands, the effect of CBD on microglia activation is being investigated in schizophrenia patients (NCT02932605) and a study at Massachusetts General Hospital is investigating CBD’s effect on neural inflammation in patients with lower back pain (NCT03891264). Together, these studies will provide valuable information on the importance of CB2 receptor regulation on microglia and inflammation in humans.
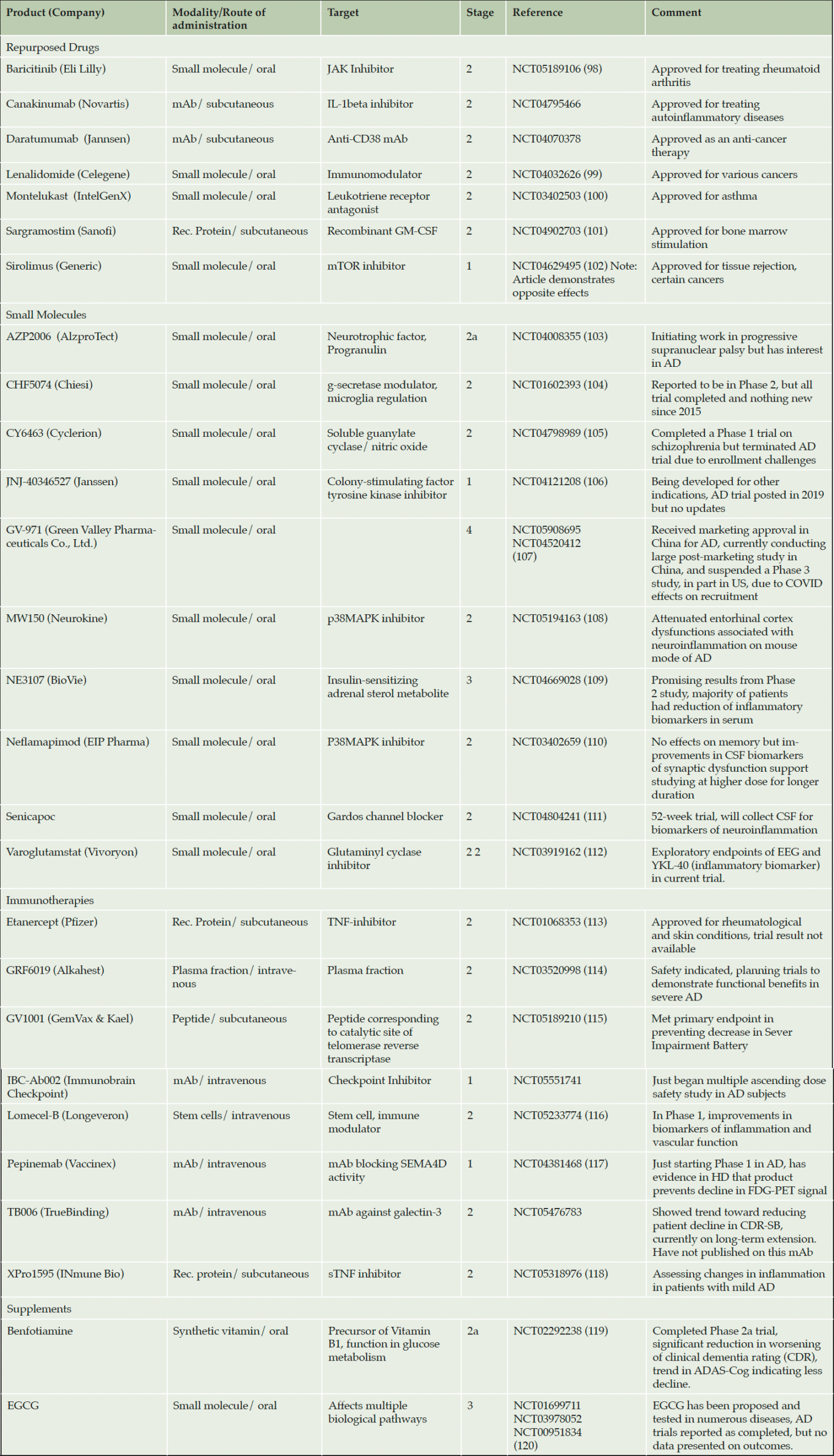
The above represents a number of promising approaches targeting neuroinflammation in AD. Several other products in clinical trials target inflammation, including repurposed drugs, immunotherapies and small molecules. Some of these are summarized in Table 2 below:
Summary
The role of neuroinflammation in brain homeostasis is both critical and complex. Activation of microglia cells has been reported to promote Aβ clearance while also increasing neuronal damage and inflammation in the brain. However, CB2 receptor agonists have been shown to reduce neuroinflammation and improve synaptic function while also promoting Aβ clearance. The ongoing clinical trials targeting neuroinflammation have the potential to be disease-modifying, addressing both the pathology (Aβ plaques, tau tangles, and neuroinflammation) while also improving clinical symptoms through improved synaptic function. Many of these clinical trials incorporate analysis of various biomarkers, which may be useful in the future in identifying AD patients who might respond best to a particular neuroinflammatory inhibitor.
Funding: The work on NTRX-07 was funded by the Alzheimer’s Drug Discovery Foundation, the Alzheimer’s Association and investors in NeuroTherapia.
Acknowledgements: Figures created with BioRender.
Conflict of Interest: Drs. Kiraly, Foss and Giordano are all employed by NeuroTherapia and have stock options in the Company.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Cuello, A.C., Early and Late CNS Inflammation in Alzheimer’s Disease: Two Extremes of a Continuum? Trends Pharmacol Sci, 2017. 38(11): p. 956-966.
2. Iulita, M.F., et al., Identification and Preliminary Validation of a Plasma Profile Associated with Cognitive Decline in Dementia and At-Risk Individuals: A Retrospective Cohort Analysis. J Alzheimers Dis, 2019. 67(1): p. 327-341.
3. Rogers, J., Principles for central nervous system inflammation research: A call for a consortium approach. Alzheimers Dement, 2018. 14(11): p. 1553-1559.
4. 2023 Alzheimer’s disease facts and figures. Alzheimers Dement, 2023. 19(4): p. 1598-1695.
5. Selkoe, D.J., Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res, 2008. 192(1): p. 106-13.
6. Wang, Y. and E. Mandelkow, Tau in physiology and pathology. Nat Rev Neurosci, 2016. 17(1): p. 5-21.
7. Lannfelt, L., N.R. Relkin, and E.R. Siemers, Amyloid-ss-directed immunotherapy for Alzheimer’s disease. J Intern Med, 2014. 275(3): p. 284-95.
8. Small, S.A. and K. Duff, Linking Abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron, 2008. 60(4): p. 534-42.
9. Reardon, S., Alzheimer’s drug donanemab helps most when taken at earliest disease stage, study finds. Nature, 2023. 619(7971): p. 682-683.
10. Heneka, M.T., et al., Neuroinflammation in Alzheimer’s disease. Lancet Neurol, 2015. 14(4): p. 388-405.
11. Lyman, M., et al., Neuroinflammation: the role and consequences. Neurosci Res, 2014. 79: p. 1-12.
12. Micheau, O. and J. Tschopp, Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell, 2003. 114(2): p. 181-90.
13. Mishra, A., et al., Synapse loss induced by interleukin-1beta requires pre- and post-synaptic mechanisms. J Neuroimmune Pharmacol, 2012. 7(3): p. 571-8.
14. Yang, S.H., Cellular and Molecular Mediators of Neuroinflammation in Alzheimer Disease. Int Neurourol J, 2019. 23(Suppl 2): p. S54-62.
15. Leng, F. and P. Edison, Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol, 2021. 17(3): p. 157-172.
16. Liddelow, S.A., et al., Neurotoxic reactive astrocytes are induced by activated microglia. Nature, 2017. 541(7638): p. 481-487.
17. Funato, H., et al., Astrocytes containing amyloid beta-protein (Abeta)-positive granules are associated with Abeta40-positive diffuse plaques in the aged human brain. Am J Pathol, 1998. 152(4): p. 983-92.
18. Thal, D.R., et al., Amyloid beta-protein (Abeta)-containing astrocytes are located preferentially near N-terminal-truncated Abeta deposits in the human entorhinal cortex. Acta Neuropathol, 2000. 100(6): p. 608-17.
19. Ji, K., et al., Microglia actively regulate the number of functional synapses. PLoS One, 2013. 8(2): p. e56293.
20. Hickman, S.E., E.K. Allison, and J. El Khoury, Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci, 2008. 28(33): p. 8354-60.
21. Guerreiro, R., et al., TREM2 variants in Alzheimer’s disease. N Engl J Med, 2013. 368(2): p. 117-27.
22. Frank, S., et al., TREM2 is upregulated in amyloid plaque-associated microglia in aged APP23 transgenic mice. Glia, 2008. 56(13): p. 1438-47.
23. Hickman, S.E., et al., The microglial sensome revealed by direct RNA sequencing. Nat Neurosci, 2013. 16(12): p. 1896-905.
24. Hsieh, C.L., et al., A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem, 2009. 109(4): p. 1144-56.
25. Sanchez-Mejias, E., et al., Soluble phospho-tau from Alzheimer’s disease hippocampus drives microglial degeneration. Acta Neuropathol, 2016. 132(6): p. 897-916.
26. Keren-Shaul, H., et al., A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell, 2017. 169(7): p. 1276-1290 e17.
27. Mathys, H., et al., Temporal Tracking of Microglia Activation in Neurodegeneration at Single-Cell Resolution. Cell Rep, 2017. 21(2): p. 366-380.
28. Hampel, H., et al., A Path Toward Precision Medicine for Neuroinflammatory Mechanisms in Alzheimer’s Disease. Front Immunol, 2020. 11: p. 456.
29. Thakur, S., et al., Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation, 2023. 46(1): p. 1-17.
30. Malko, P., et al., TRPM2 Channel in Microglia as a New Player in Neuroinflammation Associated With a Spectrum of Central Nervous System Pathologies. Front Pharmacol, 2019. 10: p. 239.
31. Krasemann, S., et al., The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity, 2017. 47(3): p. 566-581 e9.
32. Venegas, C., et al., Microglia-derived ASC specks cross-seed amyloid-beta in Alzheimer’s disease. Nature, 2017. 552(7685): p. 355-361.
33. Wang, S. and M. Colonna, The microglial immunoreceptor tyrosine-based motif-Syk signaling pathway is a promising target of immunotherapy for Alzheimer’s disease. Clin Transl Med, 2023. 13(2): p. e1200.
34. Ellwanger, D.C., et al., Prior activation state shapes the microglia response to antihuman TREM2 in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A, 2021. 118(3).
35. Wang, S., et al., Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J Exp Med, 2020. 217(9).
36. Lee, C.Y.D., et al., Elevated TREM2 Gene Dosage Reprograms Microglia Responsivity and Ameliorates Pathological Phenotypes in Alzheimer’s Disease Models. Neuron, 2018. 97(5): p. 1032-1048 e5.
37. Matsuda, L.A., et al., Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature, 1990. 346(6284): p. 561-4.
38. Munro, S., K.L. Thomas, and M. Abu-Shaar, Molecular characterization of a peripheral receptor for cannabinoids. Nature, 1993. 365(6441): p. 61-5.
39. Nunez, E., et al., Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synapse, 2004. 53(4): p. 208-13.
40. Van Sickle, M.D., et al., Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science, 2005. 310(5746): p. 329-32.
41. Benito, C., et al., Cannabinoid CB2 receptors in human brain inflammation. Br J Pharmacol, 2008. 153(2): p. 277-85.
42. Ramirez, B.G., et al., Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci, 2005. 25(8): p. 1904-13.
43. Bie, B., et al., An overview of the cannabinoid type 2 receptor system and its therapeutic potential. Curr Opin Anaesthesiol, 2018. 31(4): p. 407-414.
44. Wu, J., et al., activation of the CB2 receptor system reverses amyloid-induced memory deficiency. Neurobiol Aging, 2013. 34(3): p. 791-804.
45. Wu, J., et al., Activation of CB(2) receptor system restores cognitive capacity and hippocampal Sox2 expression in a transgenic mouse model of Alzheimer’s disease. Eur J Pharmacol, 2017. 811: p. 12-20.
46. Aso, E., et al., CB2 cannabinoid receptor agonist ameliorates Alzheimer-like phenotype in AbetaPP/PS1 mice. J Alzheimers Dis, 2013. 35(4): p. 847-58.
47. Naguib, M., et al., Prevention of paclitaxel-induced neuropathy through activation of the central cannabinoid type 2 receptor system. Anesth Analg, 2012. 114(5): p. 1104-20.
48. Xu, J.J., et al., Spinal gene expression profiling and pathways analysis of a CB2 agonist (MDA7)-targeted prevention of paclitaxel-induced neuropathy. Neuroscience, 2014. 260: p. 185-94.
49. Magham, S.V., et al., Cannabinoid receptor 2 selective agonists and Alzheimer’s disease: An insight into the therapeutic potentials. J Neurosci Res, 2021. 99(11): p. 2888-2905.
50. Esposito, G., et al., Opposing control of cannabinoid receptor stimulation on amyloid-beta-induced reactive gliosis: in vitro and in vivo evidence. J Pharmacol Exp Ther, 2007. 322(3): p. 1144-52.
51. Tao, Y., et al., Cannabinoid receptor-2 stimulation suppresses neuroinflammation by regulating microglial M1/M2 polarization through the cAMP/PKA pathway in an experimental GMH rat model. Brain Behav Immun, 2016. 58: p. 118-129.
52. Ghosh, M., Y. Xu, and D.D. Pearse, Cyclic AMP is a key regulator of M1 to M2a phenotypic conversion of microglia in the presence of Th2 cytokines. J Neuroinflammation, 2016. 13: p. 9.
53. Lee, S.H. and K. Suk, Kinase-Based Taming of Brain Microglia Toward Disease-Modifying Therapy. Front Cell Neurosci, 2018. 12: p. 474.
54. Cocozza, G., et al., Microglial Potassium Channels: From Homeostasis to Neurodegeneration. Biomolecules, 2021. 11(12).
55. Du, R.W., R.H. Du, and W.G. Bu, beta-Arrestin 2 mediates the anti-inflammatory effects of fluoxetine in lipopolysaccharide-stimulated microglial cells. J Neuroimmune Pharmacol, 2014. 9(4): p. 582-90.
56. Stebbing, M.J., J.M. Cottee, and I. Rana, The Role of Ion Channels in Microglial Activation and Proliferation – A Complex Interplay between Ligand-Gated Ion Channels, K(+) Channels, and Intracellular Ca(2.). Front Immunol, 2015. 6: p. 497.
57. Zou, S. and U. Kumar, Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int J Mol Sci, 2018. 19(3).
58. Ruiz de Martin Esteban, S., et al., Cannabinoid CB(2) Receptors Modulate Microglia Function and Amyloid Dynamics in a Mouse Model of Alzheimer’s Disease. Front Pharmacol, 2022. 13: p. 841766.
59. Guo, S., H. Wang, and Y. Yin, Microglia Polarization From M1 to M2 in Neurodegenerative Diseases. Front Aging Neurosci, 2022. 14: p. 815347.
60. Dionisio-Santos, D.A., J.A. Olschowka, and M.K. O’Banion, Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer’s disease. J Neuroinflammation, 2019. 16(1): p. 74.
61. Baldacci, F., et al., Diagnostic function of the neuroinflammatory biomarker YKL-40 in Alzheimer’s disease and other neurodegenerative diseases. Expert Rev Proteomics, 2017. 14(4): p. 285-299.
62. El Kadmiri, N., et al., Biomarkers for Alzheimer Disease: Classical and Novel Candidates’ Review. Neuroscience, 2018. 370: p. 181-190.
63. Sutphen, C.L., et al., Longitudinal Cerebrospinal Fluid Biomarker Changes in Preclinical Alzheimer Disease During Middle Age. JAMA Neurol, 2015. 72(9): p. 1029-42.
64. Suarez-Calvet, M., et al., sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol Med, 2016. 8(5): p. 466-76.
65. Hu, W.T., et al., Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease. Neurology, 2012. 79(9): p. 897-905.
66. Ray, S., et al., Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat Med, 2007. 13(11): p. 1359-62.
67. Soares, H.D., et al., Plasma biomarkers associated with the apolipoprotein E genotype and Alzheimer disease. Arch Neurol, 2012. 69(10): p. 1310-7.
68. Britschgi, M., et al., Modeling of pathological traits in Alzheimer’s disease based on systemic extracellular signaling proteome. Mol Cell Proteomics, 2011. 10(10): p. M111 008862.
69. Johnstone, D., et al., Multivariate protein signatures of preclinical Alzheimer’s disease in the Alzheimer’s disease neuroimaging initiative (ADNI) plasma proteome dataset. PLoS One, 2012. 7(4): p. e34341.
70. Duering, M., et al., Free water determines diffusion alterations and clinical status in cerebral small vessel disease. Alzheimers Dement, 2018. 14(6): p. 764-774.
71. Dumont, M., et al., Free Water in White Matter Differentiates MCI and AD From Control Subjects. Front Aging Neurosci, 2019. 11: p. 270.
72. Edde, M., et al., Free water: A marker of age-related modifications of the cingulum white matter and its association with cognitive decline. PLoS One, 2020. 15(11): p. e0242696.
73. Nakaya, M., et al., Free water derived by multi-shell diffusion MRI reflects tau/neuroinflammatory pathology in Alzheimer’s disease. Alzheimers Dement (N Y), 2022. 8(1): p. e12356.
74. Lista, S., F. Faltraco, and H. Hampel, Biological and methodical challenges of blood-based proteomics in the field of neurological research. Prog Neurobiol, 2013. 101-102: p. 18-34.
75. Swardfager, W., et al., A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry, 2010. 68(10): p. 930-41.
76. Green, M.J., et al., Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry, 2011. 16(9): p. 960-72.
77. Kurita, M., et al., Plasma brain-derived neurotrophic factor levels predict the clinical outcome of depression treatment in a naturalistic study. PLoS One, 2012. 7(6): p. e39212.
78. Lee, B.H., et al., Decreased plasma BDNF level in depressive patients. J Affect Disord, 2007. 101(1-3): p. 239-44.
79. Martinotti, G., et al., Nerve growth factor and brain-derived neurotrophic factor concentrations in schizophrenia: a review. J Biol Regul Homeost Agents, 2012. 26(3): p. 347-56.
80. Ishii, T. and S. Haga, Identification of components of immunoglobulins in senile plaques by means of fluorescent antibody technique. Acta Neuropathol, 1975. 32(2): p. 157-62.
81. Eikelenboom, P. and F.C. Stam, Immunoglobulins and complement factors in senile plaques. An immunoperoxidase study. Acta Neuropathol, 1982. 57(2-3): p. 239-42.
82. Zhou, R., et al., PET Imaging of Neuroinflammation in Alzheimer’s Disease. Front Immunol, 2021. 12: p. 739130.
83. Fan, Z., et al., An early and late peak in microglial activation in Alzheimer’s disease trajectory. Brain, 2017. 140(3): p. 792-803.
84. Metzler-Baddeley, C., et al., Sex-specific effects of central adiposity and inflammatory markers on limbic microstructure. Neuroimage, 2019. 189: p. 793-803.
85. Queiroz, S.A.L., et al., The Gut Microbiota-Brain Axis: A New Frontier on Neuropsychiatric Disorders. Front Psychiatry, 2022. 13: p. 872594.
86. Zhang, C., et al., Cromolyn Reduces Levels of the Alzheimer’s Disease-Associated Amyloid beta-Protein by Promoting Microglial Phagocytosis. Sci Rep, 2018. 8(1): p. 1144.
87. Wang, Y.J., et al., Cromolyn inhibits the secretion of inflammatory cytokines by human microglia (HMC3). Sci Rep, 2021. 11(1): p. 8054.
88. Lozupone, M., et al., ALZT-OP1: an experimental combination regimen for the treatment of Alzheimer’s disease. Expert Opin Investig Drugs, 2022. 31(8): p. 759-771.
89. Sandhu JK, K.M., Decoding mast cell-microglia communication in neurodegenerative diseases. Int. J. Mol. Sci. , 2021: p. https://doi.org/10.3390/ijms22031093.
90. Piette, F., et al., Masitinib as an adjunct therapy for mild-to-moderate Alzheimer’s disease: a randomised, placebo-controlled phase 2 trial. Alzheimers Res Ther, 2011. 3(2): p. 16.
91. Li, T., et al., Effects of Chronic Masitinib Treatment in APPswe/PSEN1dE9 Transgenic Mice Modeling Alzheimer’s Disease. J Alzheimers Dis, 2020. 76(4): p. 1339-1345.
92. Burns, L.H. and H.Y. Wang, Altered filamin A enables amyloid beta-induced tau hyperphosphorylation and neuroinflammation in Alzheimer’s disease. Neuroimmunol Neuroinflamm, 2017. 4(12): p. 263-271.
93. Wang, H.Y., et al., PTI-125 Reduces Biomarkers of Alzheimer’s Disease in Patients. J Prev Alzheimers Dis, 2020. 7(4): p. 256-264.
94. van Lengerich, B., et al., A TREM2-activating antibody with a blood-brain barrier transport vehicle enhances microglial metabolism in Alzheimer’s disease models. Nat Neurosci, 2023. 26(3): p. 416-429.
95. Vissers, M., et al., Safety, pharmacokinetics and target engagement of novel RIPK1 inhibitor SAR443060 (DNL747) for neurodegenerative disorders: Randomized, placebo-controlled, double-blind phase I/Ib studies in healthy subjects and patients. Clin Transl Sci, 2022. 15(8): p. 2010-2023.
96. Figley MD, L.K., Renous A, Tchessalova D, Gergits FW, Pandya B, Houze J, Thackaberry EA, Colonna M, Gray D, Mirescu C, Dejanovic B. Unique mechanism of action of highly potent, orally bioavailable and brain penetrant small molecule TREM2 agonists for the potential treatment of Alzheimer’s disease in Keystone Symposium on Molecular and Cellular Biology. . 2023.
97. Sekar, K. and A. Pack, Epidiolex as adjunct therapy for treatment of refractory epilepsy: a comprehensive review with a focus on adverse effects. F1000Res, 2019. 8.
98. Matsushita, T., et al., Inhibitory effect of baricitinib on microglia and STAT3 in a region with a weak blood-brain barrier in a mouse model of rheumatoid arthritis. Rheumatology (Oxford), 2023.
99. Decourt, B., et al., MCLENA-1: A Phase II Clinical Trial for the Assessment of Safety, Tolerability, and Efficacy of Lenalidomide in Patients with Mild Cognitive Impairment Due to Alzheimer’s Disease. Open Access J Clin Trials, 2020. 12: p. 1-13.
100. Xiong, L.Y., et al., Leukotriene receptor antagonist use and cognitive decline in normal cognition, mild cognitive impairment, and Alzheimer’s dementia. Alzheimers Res Ther, 2021. 13(1): p. 147.
101. Potter, H., et al., Safety and efficacy of sargramostim (GM-CSF) in the treatment of Alzheimer’s disease. Alzheimers Dement (N Y), 2021. 7(1): p. e12158.
102. Shi, Q., et al., Microglial mTOR Activation Upregulates Trem2 and Enhances beta-Amyloid Plaque Clearance in the 5XFAD Alzheimer’s Disease Model. J Neurosci, 2022. 42(27): p. 5294-5313.
103. Callizot, N., et al., AZP2006, a new promising treatment for Alzheimer’s and related diseases. Sci Rep, 2021. 11(1): p. 16806.
104. Imbimbo, B.P., et al., CHF5074, a novel gamma-secretase modulator, attenuates brain beta-amyloid pathology and learning deficit in a mouse model of Alzheimer’s disease. Br J Pharmacol, 2009. 156(6): p. 982-93.
105. Correia, S.S., et al., The CNS-Penetrant Soluble Guanylate Cyclase Stimulator CY6463 Reveals its Therapeutic Potential in Neurodegenerative Diseases. Front Pharmacol, 2021. 12: p. 656561.
106. Mancuso, R., et al., CSF1R inhibitor JNJ-40346527 attenuates microglial proliferation and neurodegeneration in P301S mice. Brain, 2019. 142(10): p. 3243-3264.
107. Xiao, S., et al., A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer’s dementia. Alzheimers Res Ther, 2021. 13(1): p. 62.
108. Rutigliano, G., et al., An isoform-selective p38alpha mitogen-activated protein kinase inhibitor rescues early entorhinal cortex dysfunctions in a mouse model of Alzheimer’s disease. Neurobiol Aging, 2018. 70: p. 86-91.
109. Reading, C.L., C.N. Ahlem, and M.F. Murphy, NM101 Phase III study of NE3107 in Alzheimer’s disease: rationale, design and therapeutic modulation of neuroinflammation and insulin resistance. Neurodegener Dis Manag, 2021. 11(4): p. 289-298.
110. Prins, N.D., et al., A phase 2 double-blind placebo-controlled 24-week treatment clinical study of the p38 alpha kinase inhibitor neflamapimod in mild Alzheimer’s disease. Alzheimers Res Ther, 2021. 13(1): p. 106.
111. Jin, L.W., et al., Repurposing the KCa3.1 inhibitor senicapoc for Alzheimer’s disease. Ann Clin Transl Neurol, 2019. 6(4): p. 723-738.
112. Vijverberg, E.G.B., et al., Rationale and study design of a randomized, placebo-controlled, double-blind phase 2b trial to evaluate efficacy, safety, and tolerability of an oral glutaminyl cyclase inhibitor varoglutamstat (PQ912) in study participants with MCI and mild AD-VIVIAD. Alzheimers Res Ther, 2021. 13(1): p. 142.
113. Li, Y., et al., Etanercept Reduces Neuron Injury and Neuroinflammation via Inactivating c-Jun N-terminal Kinase and Nuclear Factor-kappaB Pathways in Alzheimer’s Disease: An In Vitro and In Vivo Investigation. Neuroscience, 2022. 484: p. 140-150.
114. Hannestad, J., et al., Safety and Tolerability of GRF6019 Infusions in Severe Alzheimer’s Disease: A Phase II Double-Blind Placebo-Controlled Trial. J Alzheimers Dis, 2021. 81(4): p. 1649-1662.
115. Koh, S.H., et al., Efficacy and safety of GV1001 in patients with moderate-to-severe Alzheimer’s disease already receiving donepezil: a phase 2 randomized, double-blind, placebo-controlled, multicenter clinical trial. Alzheimers Res Ther, 2021. 13(1): p. 66.
116. Brody, M., et al., Results and insights from a phase I clinical trial of Lomecel-B for Alzheimer’s disease. Alzheimers Dement, 2023. 19(1): p. 261-273.
117. Zauderer, M. and E.E. Evans, Conclusions of the SIGNAL study in Huntington and implications for treatment of other slowly progressive neurodegenerative diseases. Clin Transl Med, 2023. 13(2): p. e1169.
118. MacPherson, K.P., et al., Peripheral administration of the soluble TNF inhibitor XPro1595 modifies brain immune cell profiles, decreases beta-amyloid plaque load, and rescues impaired long-term potentiation in 5xFAD mice. Neurobiol Dis, 2017. 102: p. 81-95.
119. Gibson, G.E., et al., Benfotiamine and Cognitive Decline in Alzheimer’s Disease: Results of a Randomized Placebo-Controlled Phase IIa Clinical Trial. J Alzheimers Dis, 2020. 78(3): p. 989-1010.
120. Payne, A., et al., Epigallocatechin-3-Gallate (EGCG): New Therapeutic Perspectives for Neuroprotection, Aging, and Neuroinflammation for the Modern Age. Biomolecules, 2022. 12(3).
© The Authors 2023