M. Barbera1,2, D. Perera2,3, A. Matton2,3,4,5, F. Mangialasche3,4,6, A. Rosenberg4,7, L. Middleton2,8, T. Ngandu4,7, A. Solomon1,2,4, M. Kivipelto2,3,4,6,9
1. Department of Neurology, Institute of Clinical Medicine, University of Eastern Finland, Finland; 2. The Ageing Epidemiology Research Unit, School of Public Health, Imperial College London, United Kingdom; 3. FINGERS Brain Health Institute, Sweden; 4. Division of Clinical Geriatrics, Center for Alzheimer Research, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Sweden; 5. Division of Neurogeriatrics, Center for Alzheimer Research, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Sweden; 6. Theme Inflammation and Aging, Medical Unit Aging, Karolinska University Hospital, Sweden; 7. Population Health Unit, Finnish Institute for Health and Welfare, Finland; 8. Directorate of Public Health, Imperial College NHS Healthcare Trust Hospitals, United Kingdom; 9. Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Finland
Corresponding Author: Miia Kivipelto, Address: Division of Clinical Geriatrics, Center for Alzheimer Research, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Karolinska Vägen 37A, 171 64 Solna, Sweden, Email: miia.kivipelto@ki.se, Phone: +46 73-994-0922
J Prev Alz Dis 2023;4(10):718-728
Published online October 24, 2023, http://dx.doi.org/10.14283/jpad.2023.114
Abstract
At least 40% of all dementia has been linked to modifiable risk factors suggesting a clear potential for preventative approaches targeting these factors. Despite the recent promising findings from anti-amyloid monoclonal antibodies, a limited proportion of patients are expected to be eligible for these novel AD treatments. Given the heterogeneous nature of AD and the complex multi-level pathological processes leading to dementia (involving, e.g., shared risk factors, interaction of different pathology mechanisms, and their putative synergistic effects on cognition), targeting a single pathology may not be sufficient to halt or significantly impact disease progression. With exponentially increasing numbers of patients world-wide, in parallel to the unprecedented population ageing, new multimodal therapy approaches targeting several modifiable risk factors and disease mechanisms simultaneously are urgently required. Developing the next generation of combination therapies with lifestyle intervention and pharmacological treatments, implementing the right interventions for the right people at the right time, and defining accessible and sustainable strategies worldwide are crucial. Here, we summarize the state-of-the-art multimodal lifestyle-based approaches, especially findings and lessons learned from the FINGER trial, for prevention and risk reduction of cognitive impairment and dementia. We also discuss some emerging underlying biological mechanisms and the current development of precision prevention approaches. We present an example of a novel trial design combining healthy lifestyle changes with a repurposed putative disease-modifying drug and place this study in the context of the World-Wide FINGERS, the first interdisciplinary network of multimodal trials dedicated to the prevention and risk reduction of cognitive impairment and dementia.
Key words: Alzheimer’s disease, clinical trials, precision prevention, multimodal interventions.
Prevention potential of dementia and Alzheimer’s disease
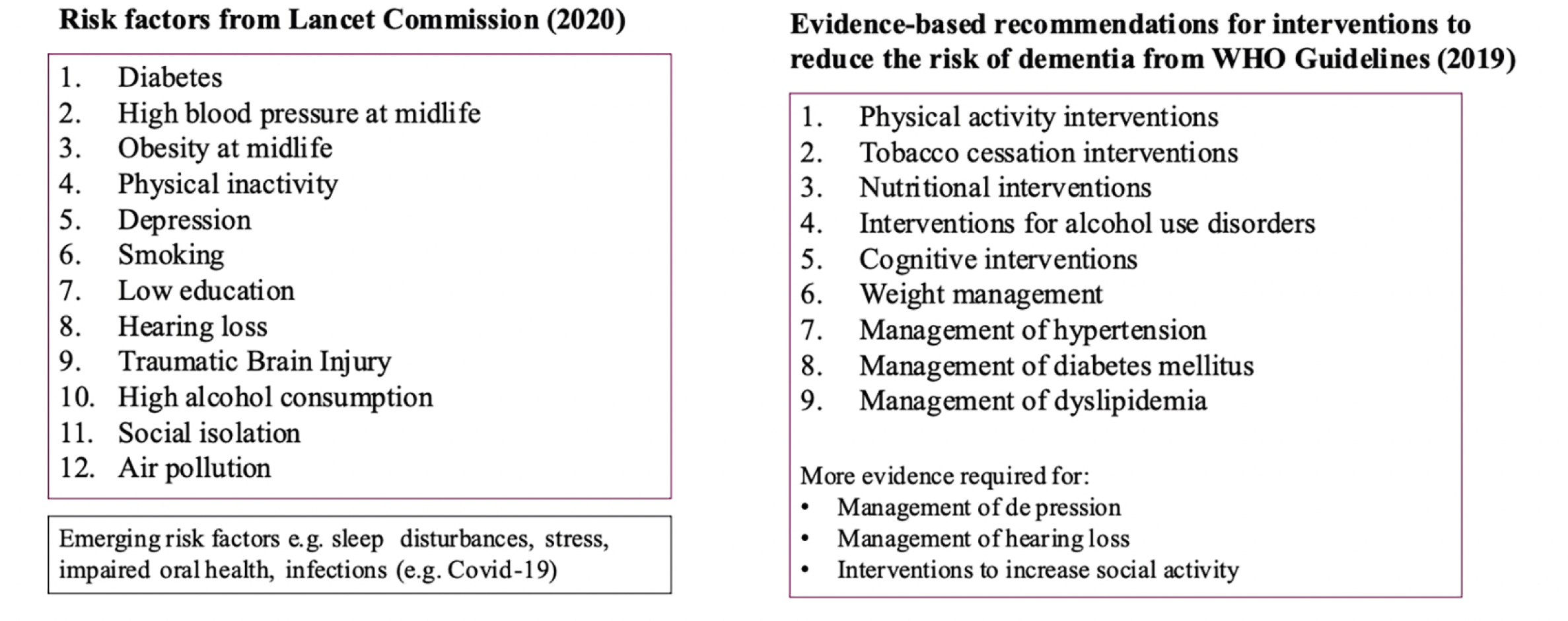
Preventing dementia and Alzheimer’s disease (AD) is a global priority. The long period of progressive accumulation of brain pathology during the asymptomatic stages to the onset of cognitive decline and dementia (1) creates a window of opportunity for both early detection and prevention. AD and related dementias (ADRDs) are complex and multifactorial, resulting from interactions between non-modifiable (e.g., age, sex, genetics) and modifiable risk factors (e.g., lifestyle, vascular, metabolic). According to the latest estimates, at least 40% of all dementia is attributable to modifiable lifestyle and environmental factors (2) and could, thus, be prevented or delayed (Figure 1). This prevention potential can be even higher in low-middle income countries (LMICs) where dementia burden is increasing very rapidly (3).
Given the multifactorial etiology of ADRD, a multimodal approach simultaneously targeting several risk factors and mechanisms may be needed for an optimal preventive effect. At the same time, such interventions need to adopt the ‘one size does not fit all’ paradigm and be adaptable to different populations and individuals across the globe (4). Furthermore, increasing our understanding of the biological mechanisms of multimodal interventions, of their optimal time windows, and target populations is crucial for precision prevention of ADRD.
Recent successes in trials of three Aβ-targeted monoclonal antibodies (donanemab, lecanemab, and aducanemab) suggest that the first disease modifying therapies (DMTs) may soon be available, at least in the early symptomatic disease stages (5). However, due to the complex nature of ADRD, targeting a single proteinopathy may not be sufficient to prevent dementia (6). It is well established that only 10-30% of late onset AD cases have pure AD pathology, with vascular pathology as most common comorbidity (7). Various AD profiles and subtypes have been identified (8) and recent advances in biomarkers (fluid biomarkers, neuroimaging) facilitate moving towards precision prevention and targeting the interventions for various at-risk and pathological profiles (9). Results from a real-world memory clinic setting at Karolinska University Hospital (Sweden) suggest that, depending on the applied cut-offs for amyloid positivity, only 13-27% of patients with Mild Cognitive Impairment (MCI) and 17-28% of patients with early dementia would be eligible for anti-amyloid treatments (10). Additional DMT interventions targeting other mechanisms and multimodal preventive therapies combining pharmacological and non-pharmacological approaches are currently progressing.
Clinical trial evidence
Until recently, translating the epidemiological and experimental evidence of risk factors and therapeutic targets to successful clinical trials has been challenging, in the AD field. Important lessons have been learned from recent randomized controlled trials (RCTs), in terms of study designs, target populations, interventions, and outcome measures. On the other hand, single-domain lifestyle interventions, e.g., focusing on diet, have mainly yielded negative or modest effects, further highlighting the importance of multimodal interventions. One recent example is the MIND-Diet trial testing the MIND-Diet compared to control among ‘at-risk’ population (family history of dementia, Body Mass Index (BMI) >25, and a suboptimal diet, n=1929). Both groups improved in global cognition, but there were no significant differences in cognition or brain structure (Magnetic Resonance Imaging, MRI) measures after 3 years (11, 12). Encouraging results have been reported from interventions based on physical activity, such as the Exercise in Adults With Mild Memory Problems (EXERT) RCT (13). In this study, sedentary older adults (age 65-89 years) with mild memory problems, experienced a stability in cognition after 12 months of moderate intensity aerobic exercise, or stretching and balance training, suggesting that regular physical activity can be beneficial in sedentary people, independently of its intensity (13, 14). Another large, single-domain clinical trial, the SPRINT-MIND study, included older individuals with increased risk for cardiovascular disease, and reported a reduced occurrence of cognitive decline, defined as MCI or combined MCI plus dementia, through a stricter control of systolic blood pressure (blood pressure goal less than 120 mmHg) compared to standard treatment (blood pressure goal less than 140 mmHg) (15).
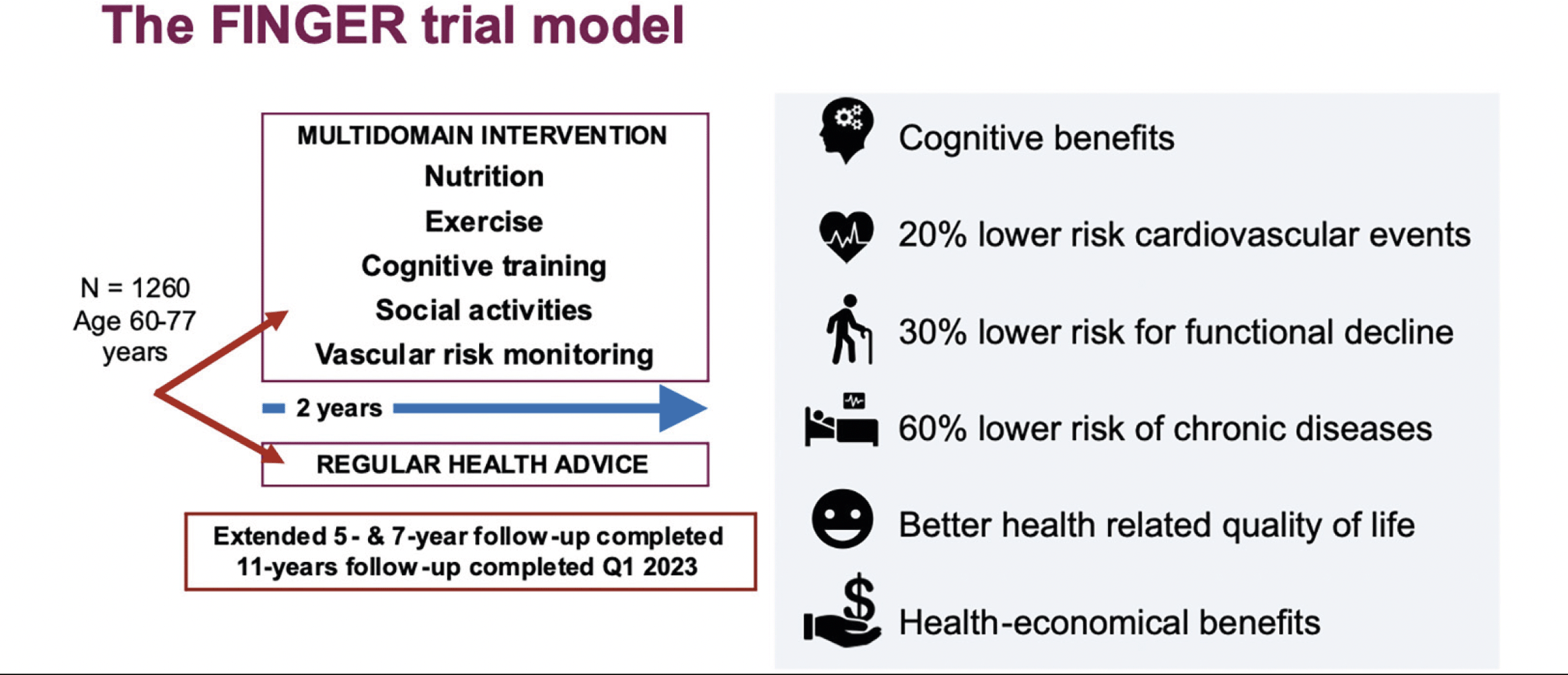
On the front of multimodal studies, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) was the first larger (n=1260) multimodal lifestyle-based RCT to show significant benefits on cognition in at-risk older individuals from general population (16). The FINGER trial combined five different intervention domains: 1) healthy balanced nutrition, 2) physical exercise, 3) cognitive training, 4) social activities, and 5) vascular/metabolic risk management, compared to the control arm receiving regular health advice. The beneficial effects of the 2-year trial were seen on global cognition (primary outcome; 25% greater improvement in the multimodal intervention, compared to the control group), as well as for various cognitive sub-domains; executive function, processing speed, and complex memory. The control group had 30% increased risk for cognitive decline after 2 years. Of note, participants carrying the APOE4 risk allele had marked beneficial effects from the intervention (17), pointing to potential environmental-genetic interactions which can be leveraged on to optimize dementia risk reduction.
Long-term adherence and sustained pattern of lifestyle modification is one critical factor that may impact the effect of the intervention. In the FINGER trial, active participation was associated with better cognition, and a dose-response was reported indicating that those individuals attending at least 50% of the various intervention domains got significant benefits on cognition (18). Thus, supporting long-term adherence is important in future interventions and implementation.
The FINGER 2-year intervention also had several longer-term beneficial effects beyond cognition (Figure 2). The risk of functional decline, i.e., development of disability and other chronic diseases, especially multimorbidity (60% lower risk) were reduced (19–21). The risk of cardiovascular events was reduced by 20% after extended follow-up (22). The FINGER intervention was also found to be cost-effective, with potentially substantial societal benefits (23). Follow-up studies of the FINGER participants after five, seven, and eleven years have been completed and will provide further valuable insights concerning long-term effects and underlying mechanisms of the multimodal intervention.
Overall, the design of dementia prevention/risk reduction RCTs has evolved considerably in the last two decades, from single-domain and -component studies towards more integrated multimodal approaches that are more in line with the heterogeneous and complex nature of ADRDs (4). Indeed, a new generation of multimodal RCTs targeting simultaneously multiple risk factors with multicomponent interventions have recently emerged with trials such as the Multidomain Alzheimer Preventive Trial (MAPT) and the Prevention of Dementia by Intensive Vascular care (preDIVA) and other smaller-size studies (4). Although both these trials reported no intervention effect on their primary outcomes, positive results were found in sub-group analyses. For example, beneficial intervention effects were reported in preDIVA participants with elevated and untreated blood pressure at baseline who initiated antihypertensive treatment as part of the intervention (24), and in MAPT participants with an increased risk of dementia such as high Cardiovascular Risk Factors, Aging and Dementia (CAIDE) Risk Score (of 6 or more), or having evidence of abnormal brain amyloid load (25).
Based on the review of the evidence of the first World Health Organization (WHO) Guidelines for Dementia Risk Reduction (4), the main lessons learned so far indicate that effective interventions should: a. target at-risk individuals who are most likely to benefit from the intervention (e.g. defined with high CAIDE or other validated risk score, presence of amyloid, genetic, or modifiable risk factors; the recently emerging blood-based biomarkers, showing high sensitivity and specificity for the AD biological signature of increased brain load of Amyloid and Tau along with evidence of Neurodegeneration (AT[N]) could guide both selection of target populations and matching them with suitable interventions); b. start early enough, preferably before substantial brain pathology; and c. be intensive enough and include sufficient guidance and follow-up (do the right things and do enough of them).
Emerging biological mechanisms underlying multimodal interventions
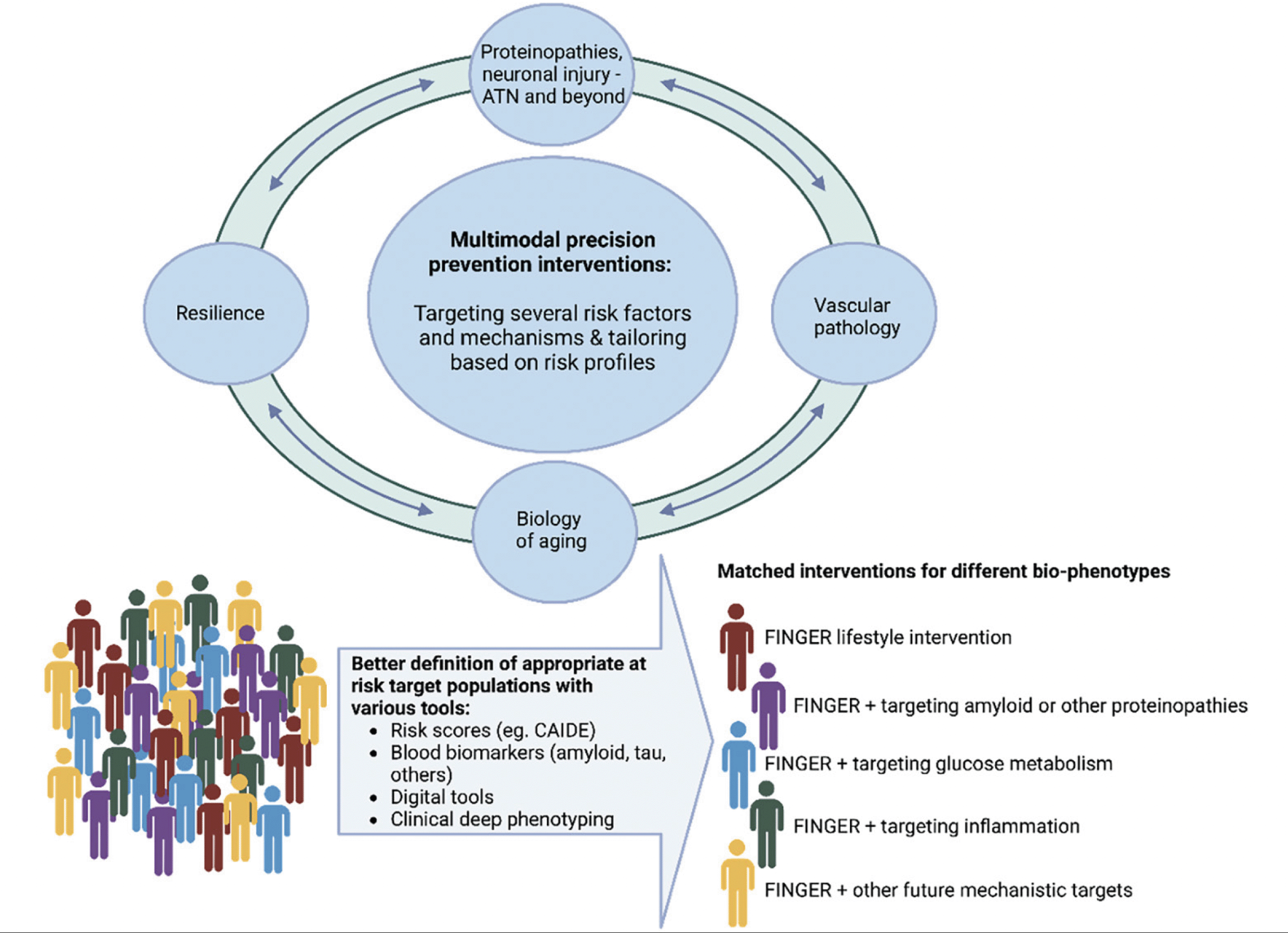
There is an increasing interest in exploring the biological mechanisms underlying multimodal interventions and related cognitive gains. Deeper understanding of these mechanisms can help to further tailor the interventions for various risk profiles and reveal novel targets for interventions (pharmacological and lifestyle). Knowledge about the precise biological mechanisms is still limited, largely because evidence from RCTs is new and still emerging, and novel biomarkers reflecting the key pathological features (e.g., AD blood-based biomarkers) have only recently become more widely available for use in large-scale prevention trials. The evidence so far suggests that multimodal interventions may exert their effects on cognition through different mechanisms, and the hypothesis is that there may be also mechanistic synergies. Mechanisms such as vascular-related pathways, synaptic plasticity, inflammatory-immune mediated responses, glucose/lipid homeostasis, insulin signaling and bioenergetic metabolism, mechanisms related to brain biological ageing (such as senescence cells, altered DNA damage/repair, loss of neurotrophic factors, and oxidative stress, among others) warrant further research. It is also likely that the multimodal interventions or some of their domains increase cognitive reserve and resilience to brain pathologies, mitigating the impacts of age- or disease-related brain changes on cognition (26). Recent observational studies suggest that lifestyle factors may indeed protect against cognitive decline by increasing resilience to a higher burden of AD pathology (27, 28), but evidence from RCTs is still limited.
Genetic-lifestyle interactions may also play an important role. For instance, APOE4 carriers have been reported to be more vulnerable to unhealthy lifestyle and other risk factors. On the other hand, epidemiological studies have indicated that protective factors e.g., education, occupational complexity, physical activity, unsaturated fatty acids, and non-smoking can reduce the risk of dementia among APOE4 carriers to the level of non-carriers (29–31). Indeed, the FINGER trial showed evidence of a clear beneficial effect in APOE4 carriers, suggesting that persons with genetical vulnerability may be an important target group for the interventions (17). Similar results have also been reported in some other multimodal interventions (32) and will be further studied within World-Wide FINGERS and other initiatives. Other life-course exposures may also have an effect on the individual prevention potential. For instance, previous engagement in mentally stimulating occupations have been recently linked to an increased beneficial response to the FINGER intervention (33).
Vascular factors and mechanisms have been linked to cognition and dementia in several epidemiological, clinical, and experimental studies. Evidence from the FINGER trial also points to the importance of vascular pathways and showed that the incidence of cardiovascular events was significantly reduced during the post-trial extended follow-up (20% risk reduction) (22). When stratified by pre-existing cardiovascular disease (CVD) history, the risk reduction was even higher, up to 50%, highlighting the importance of secondary prevention (22). The intervention also seems to affect white matter microstructure, and further studies within FINGER and World-Wide FINGERS network are ongoing to further clarify the impact on various vascular lesions, which commonly increases with aging (34). Additionally, there is a need to further develop and validate blood-based biomarkers reflecting the vascular changes that may be relevant for cognition.
The brain is highly rich in lipids, key energy supplier and main component of cell membranes maintaining lipid homeostasis, both fundamental for optimal brain function. The therapeutic potential of modifying lipids in neurodegenerative disease was highlighted in the 3-year LipiDiDiet RCT, where patients with prodromal AD receiving the medical food Fortasyn Connect (Souvenaid™; specific omega-3 based multi-nutrient combination) versus placebo had significant benefits on the rate of cognitive- functional decline and brain volumes (35). In the FINGER RCT, reducing the blood-brain-permeable oxysterol 27-hydroxycholesterol (27-OH) was associated with more cognitive gains (36), suggesting a role for cholesterol metabolism in lifestyle modification on brain function.
Neurotrophins (e.g., brain-derived neurotrophic factor (BDNF)) are crucial mediators of neuroplasticity and memory formation (37) and thus interesting candidates to study in relation to cognitive gains. In the SoUth Korean study to PrEvent cognitive impaiRment and protect BRAIN health through lifestyle intervention in at-risk elderly people (SUPERBRAIN), the multimodal lifestyle intervention significantly increased serum BDNF levels (38, 39) whereas other trials, such as the Movimente trial did not detect any BDNF changes (40). Further, levels of proBDNF, the precursor form of BDNF, was shown to be associated with memory gains in the FINGER RCT (41), implying that lifestyle modification may be more efficient in genetically or environmentally predisposed individuals where proBDNF could be one factor. Identifying such markers of high-responders will be important tools for future precision prevention. As an important aspect in the biology of aging, telomere length was studied in the FINGER RCT where it was shown that the lifestyle intervention facilitated leucocyte telomere length maintenance, especially amongst APOE4 carriers (42). Given that age is the main risk factor for dementia and many of the mechanisms discussed above are age-related, further studies investigating the biology of aging, with respect to dementia risk are warranted and could also provide novel targets for intervention strategies.
Evidence linking lifestyle changes directly to AD imaging or the new AD blood-based biomarkers is currently limited and no data from larger RCTs are available. In relation to clinical AD biomarkers, studies of monozygotic twins have shown that the level of tau pathology may be modifiable by lifestyle factors (43, 44). Ongoing and planned studies in the FINGER RCT include new AD blood biomarkers (several ptau species, Aβ42, Aβ40, NfL and GFAP), as well as -omics where an exceptionally long follow-up time of 11 years will be included in some of the analyses. Furthermore, the FINGER model is currently applied in various mice models, where multimodal intervention brain mechanisms of action will be studied in detail (45).
From FINGER to FINGER 2.0: The first lifestyle + pharmacological combination therapies
Approaches combining multimodal lifestyle intervention with disease-modifying drugs (novel or repurposed) could provide effective strategies for precision prevention or treatment of ADRDs (46). The value of such combination therapies of multimodal lifestyle interventions and pharmacological treatments has been demonstrated in at-risk individuals for the prevention of other conditions, such as CVD (47) and type-2 diabetes (T2D) (48, 49), which share several modifiable risk factors (2) and potential underlying mechanisms with ADRD (50).
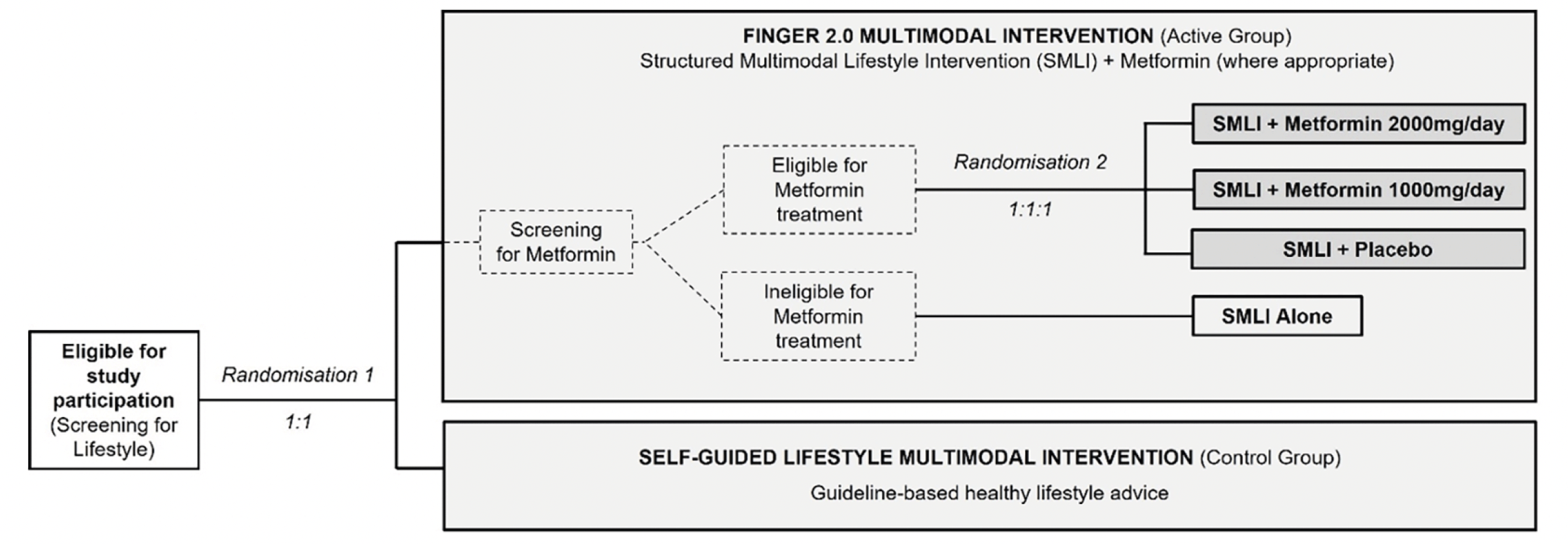
The “Multimodal METformin and FINGER lifestyle intervention to prevent cognitive impairment and disability in older adults at risk for dementia: a phase IIb multi-national randomized, controlled trial” (MET-FINGER) study (ClinicalTrials.gov, NCT05109169) aims to test a FINGER 2.0 multimodal intervention, combining a Structured Multimodal Lifestyle Intervention (SMLI, based on the original FINGER model) with metformin (where appropriate) in an APOE ε4-enriched population of older adults at-risk for dementia, compared to a Self-Guided Multimodal Intervention (general health advice).
The study’s main objectives are to test the effect of the FINGER 2.0 intervention versus a Self-Guided Multimodal Intervention on change in global cognition (primary objective) and in individual cognitive domains, functional status, and lifestyle, vascular, metabolic, and other dementia-related risk factors (secondary objectives). Exploratory objectives will investigate, within the SMLI-metformin combination groups (i) potential interactions between metformin and lifestyle changes; (ii) potential disease-modifying effects of the lifestyle-metformin combination; and (iii) feasibility of the metformin + lifestyle combination; in the context of prevention of cognitive impairment.
Metformin, the recommended first-line treatment in adults with type-2 diabetes (T2D), was selected as a promising candidate for a disease-modifying drug repurposing, and lifestyle intervention-pharmacological treatment combination to prevent or delay cognitive impairment. The rationale for this choice was firstly based on the well-established link between T2D and AD (51–53). In particular, unhealthy lifestyle habits, T2D, insulin resistance, and increased adiposity (overweight/obesity) have been consistently linked to the risk of dementia/AD through several mechanisms which could be targeted by metformin (51–54). Recently, a large (n=210,000+) cohort study also reported that, in patients with T2D, metformin treatment was significantly associated with lower dementia risk compared with no treatment (55) Additionally, metformin has shown potential neuro-protective effects (e.g., vascular, metabolic, anti-senescence via genetic modulation) (56–59). Increasing evidence also supports a potential role of metformin in counteracting biological mechanisms of aging, which may also play an important role in neurodegenerative disorders (56). This suggests that pharmacologic strategies for decreasing insulin resistance and preventing T2D may also help reduce the risk of cognitive impairment.
MET-FINGER is a 2-year randomized, controlled, parallel-group, multicenter phase-IIb proof-of-concept clinical trial (Figure 3). Participants will be first randomized 1:1 to the FINGER 2.0 intervention (SMLI for all participants + metformin if eligible) or the Self-Guided Multimodal Intervention (evidence-based generic health advice). Participants allocated to the FINGER 2.0 intervention, and eligible for metformin treatment, will be randomized 1:1:1 to receive metformin 2000mg/day, metformin 1000mg/day, or placebo, together with the SMLI. Eligibility to metformin is based on presence of indicators of increased risk for T2D, due to elevated adiposity or mildly impaired fasting glucose, in the absence of T2D diagnosis or intolerance/contraindications to metformin. Participants allocated to the FINGER 2.0 intervention but not eligible for metformin treatment will receive the SMLI alone. The metformin/placebo allocation and treatment will be double-blinded. 600 participants will be included across three sites (in the UK, Finland, and Sweden) with the aim to recruit an APOE4 ε4-enriched population (≥ 50%) (Figure 3). Metformin will be administered as Glucophage®XR 500 (“SR” in the UK), in form of 500mg oral tablets, manufactured in bulk by Merck KGaA (Darmstadt, Germany).
MET-FINGER will test, for the first time, a combination therapy of a multimodal lifestyle intervention with a repurposed putative disease-modifying drug. This trial bridges the gap between pharmacological and non-pharmacological interventions for dementia prevention and proposes a new precision prevention approach which addresses multiple risk factors and disease mechanisms simultaneously, while accounting that their contributions to the overall dementia risk may have a different weight in different people. The study includes metformin/placebo treatment in a trial-within-trial design, and may, thus, provide exploratory rather than efficacy data on the added benefits of metformin compared with multimodal lifestyle intervention alone. However, its findings will inform the design of future phase III precision prevention trials, including selection criteria, metformin dose for combination with lifestyle intervention; and overall trial design, including target at-risk population and evidence-based estimates for power calculations, e.g. 3- or 4-arm design (60).
This lifestyle intervention-pharmacological treatment combination will include adjustments tailored to each person, and metformin will be given only to people who are most likely to benefit from it, in a pragmatic approach close to a real-life scenario where disease-modifying drugs can achieve optimal effects only in specific at-risk individuals. Findings from this trial may provide invaluable evidence for novel lines of dementia prevention strategies based on implementing the most effective solution, targeted to individuals who may benefit most, at the most appropriate time. In the future, this approach, potentially as part of RCTs adopting the adaptive trial design model, may be employed in novel lifestyle intervention-pharmacological treatment combinations (Figure 4) including those where newly developed AD drugs could be tested.
From FINGER to WW-FINGERS: the value of harmonized big data
The World-Wide FINGERS (WW-FINGERS) Network was established with the primary objective of sharing knowledge and experiences, as well as harmonizing trials methodologies investigating the effect of multimodal dementia prevention/risk reduction interventions in different populations, with the aim of generating robust evidence globally to advance the implementation of dementia prevention strategies. The network builds on the experiences and expands the work of the original FINGER trial, by adapting the FINGER model to reflect cultural, regional, and economic needs of different populations around the globe, and testing and future developing it in different settings. Target populations cover the whole spectrum from at-risk persons from general population to prodromal AD in clinic settings (pre-dementia stage).
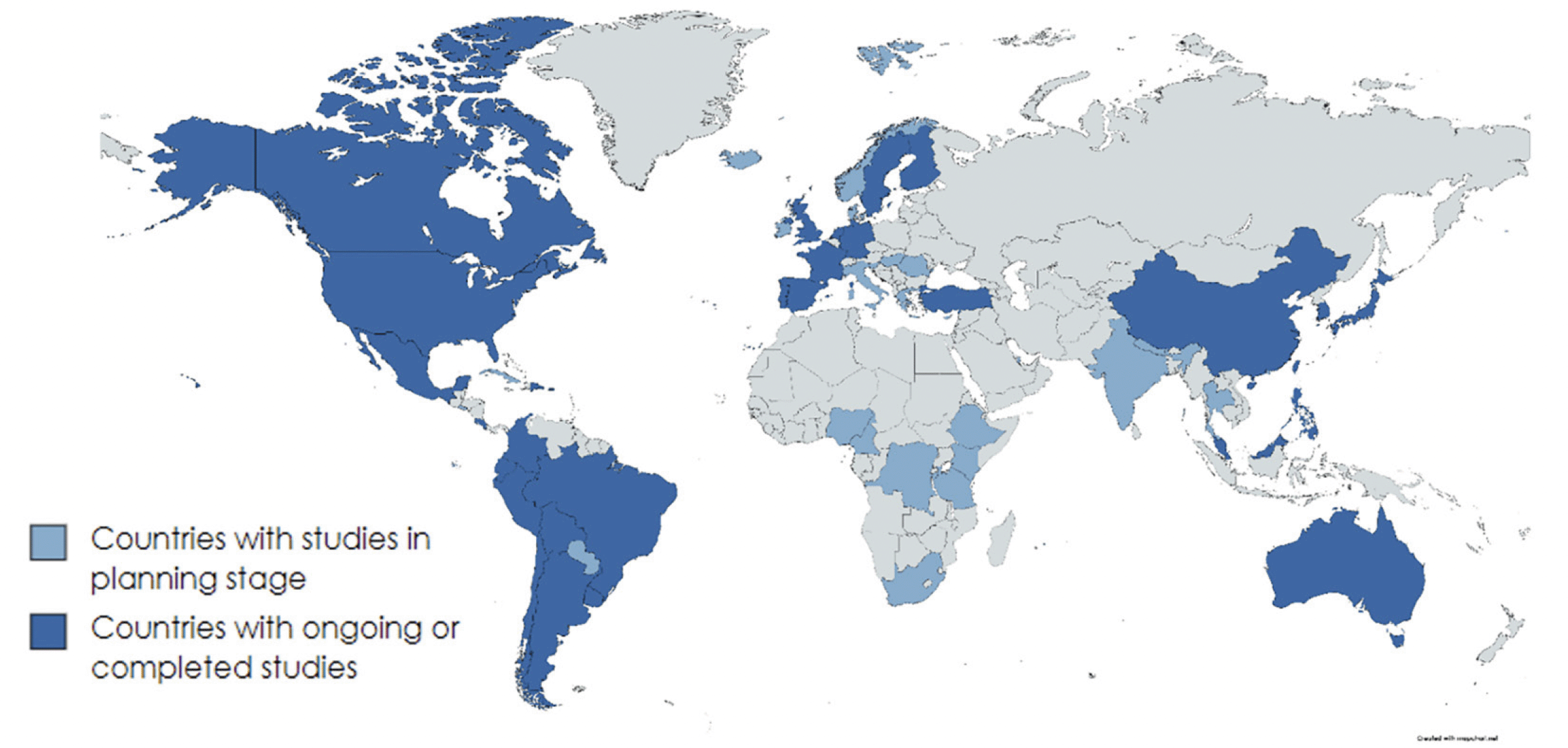
Starting, in 2017, the Network has grown to include 61 different countries from across the globe, including several LMICs (as of 22 Aug 2023 – Figure 5).
The FINGER methodology is the core to all WW-FINGERS trials: a multimodal lifestyle-based intervention aimed at mitigating various dementia risk factors; intervention delivery through individual and group sessions, aimed at optimizing the intervention at an individual level, whilst also enhancing social stimulation and peer support. Other key methodological pillars include randomization in the trial design, and prospective harmonization of cognitive and other outcomes to allow for joint analysis across different cohorts (61). Digitally enhanced interventions are also being tested (e-FINGERS). The strength of the Network lies in the fact that the more trials are included and harmonized with each other, the greater its statistical power is. By conducting joint analyses across multiple world-wide cohorts, the Network will be able to generate more robust and impactful results. Data sharing is supported by using federated database models, e.g., through the AD Workbench (https://www.alzheimersdata.org/ad-workbench).
New members to the Network can join at any stage of activity, from new researchers interested in the FINGER model, to existing research groups interested in harmonizing their trials to the FINGER methodology. The WW-FINGERS Global Scientific Coordinating Center, hosted at the FINGERS Brain Health Institute (FBHI, https://fbhi.se/), brings together experts on the FINGER model from multiple institutions, to provide a range of scientific support, from guidance on trial designs and outcome measures, to support the preparation of grant applications. The Network meets regularly to share knowledge, both virtually and annually in-person. International Working Groups (IWG) within the Network are also focusing on harmonizing activities on a larger scale, for example the Biomarkers and Biorepositories IWG are investigating potential solutions for harmonizing biomarker and bio-sample analysis and sharing platforms across the Network.
With its adaptive framework applied in diverse contexts, the Network is at the forefront of precision prevention medicine, testing different combinations of multimodal interventions to identify appropriately and precisely tailored approach targeted to reduce individual risk of cognitive decline, but with the potential for large-scale dementia prevention effects in wider populations. Furthermore, the Network is leading the next generation of trials, combining precision lifestyle interventions with pharmacological treatments to further investigate potential benefits in at-risk individuals. The MIND-AD trial targeted patients with prodromal AD with lifestyle and vascular risk factors and combined the multimodal lifestyle- based intervention with medical food Fortasyn Connect (Souvenaid™) in one arm to test the feasibility and possible synergistic effects (62). In the context of dementia prevention/pre-symptomatic risk reduction, MET-FINGER is the first trial of this kind, and is laying the foundation for future trials combining multimodal interventions with either novel or repurposed medications, whilst also testing adaptive platform design methodologies. For instance, sodium-glucose cotransporter-2 inhibitors (SGLT2i) are a newer class of oral glucose-lowering medication with neuroprotective properties which can be relevant to AD prevention (63). Interestingly, repurposed agents represent 28% of clinical trials in the current AD pipeline (64). Through the WW-FINGERS Network, the so-defined FINGER 2.0 model will be expanded to create even more personalized, and therefore effective, preventative treatments.
From research to implementation
Prevention is highlighted as the key element in managing the dementia epidemic and it is imperative to develop and implement large-scale global strategies for prevention and risk reduction. The WHO Guidelines on risk reduction of cognitive decline and dementia (65) provide support for countries in designing public health interventions and implementation activities. The guidelines and the WHO Blueprint for dementia research (66) highlights multimodal approaches targeting multiple risk factors and tailored at individual and population level. However, there are still significant evidence gaps and need for new high-quality RCT evidence (when feasible) to refine the evidence, studies from LMICs and increased diversity and representativeness, harmonization in trial design and data, and testing new populations and combination therapies that target many pathways involved.
While the absolute numbers of individuals living with dementia are exponentially increasing, observational population-based studies have indicated that the age-specific incidence and prevalence of dementia is decreasing in recent decades, at least in some high-income countries (67). This suggests that dementia prevention through lifestyle modification is not only a future possibility but may already be under way, possibly related to increased educational attainment and better management of several modifiable risk factors, including CVD and metabolic diseases, and more active lifestyle in late-life. At the same time, opposite trends have been observed in other countries and the incidence of modifiable risk factors such as diabetes and obesity seem to be increasing globally. Novel risk factors are currently being investigated and may emerge as important targets for risk reduction (many of them are common and increasing, e.g., sleep disturbances, stress, social isolation, impaired oral health, infections) (12). Furthermore, the COVID-19 pandemic has negatively influenced many of the modifiable risk factors (68). It is important to adopt a life-course perspective, including early life experiences and exposures. The importance of risk factors may vary during the life course and among population groups. Consistently with this, regional or national estimates on risk factors prevalence are crucial to inform the prioritization of public health efforts and resource allocation. For instance, two European studies recently replicated the Lancet Commission work for estimates of dementia prevention (69, 70). In Germany, it was reported that 38% of the cases of dementia could potentially be prevented as associated with eleven modifiable risk-factors, the main being hearing loss, hypertension, depression, obesity, and smoking (69). The Danish estimates reported that approximately 35% of national dementia cases were due to modifiable risk factors, with physical inactivity, hearing loss, hypertension, and obesity being the main contributors, and, thus, more relevant for dementia prevention in Denmark (70). Similarly, another study including LMICs reported that five risk factors, including low education, smoking, hypertension, obesity and diabetes, had a higher population attributable fraction for dementia in China, India, ad most of Latin America, compared to the Lancet Commission global estimates, indicating a far larger prevention potential in these regions (71).
It is also relevant to consider that multimodal preventive interventions can be implemented on many levels in societies. The concept Brain Health Services (in addition to traditional memory clinics) has been recently highlighted aiming to implement dementia and cognitive decline prevention programs in an evidence-based way (72). Implementation programs are also currently being tested in primary care and municipality level in many countries.
Finally, both individual and population-based approaches are needed for effective and feasible dementia risk reduction. Sustainable public health prevention policies will indeed require synergy of individual-based approaches focusing on at-risk people, such as the FINGER model, and whole population-based approaches, which promote healthy lifestyle in whole populations by large-scale interventions, and require contribution of several stakeholders, such as national and local governments, industry, the education sector, town planners, and workplaces (73). Examples of tangible steps towards this can be found in the North of Europe, as in Sweden the updated national strategy for dementia has included prevention in the list of forthcoming activities (74), while in Finland the public health authority is leading a project on implementation which gathers multiple stakeholders across the society (75).
Conclusion
Evidence is increasing that longer-term multimodal lifestyle changes are both feasible and effective strategies for reducing an individual’s risk of cognitive decline. Although the age-specific prevention potential for older adults might not yet be fully clear, findings from FINGER and other trials clearly show that it is never too late for effective dementia risk reduction. However, to achieve the greatest benefit, prevention strategies should be further tailored, optimized and adapted for different target populations and their associated settings. The FINGER 2.0 model combines an updated, personalized lifestyle intervention with a pharmacological treatment and could become a blueprint for new combination therapies and for the next generation of clinical trials in the ADRD field. Increasing understanding of the underpinning biological mechanisms will facilitate the development towards precision prevention. Furthermore, it is important to continue advancing the drug pipeline to develop treatments targeting several mechanisms in addition to amyloid, also including the biology of brain ageing. Finally, a strong foundation of public and patient involvement and global multi-stakeholder collaborations are key factors to successful prevention trials and implementation and to drive the message that ‘it’s never too early or too late’ to make a change for better brain health.
Acknowledgements: For the MET-FINGER Trial, metformin (Glucophage® XR/SR 500mg) has been provided by Merck Healthcare KGaA (Darmstadt, Germany). Danone Nutricia Research provided Fortasyn Connect (Souvenaid™) for the LipiDiDiet and MIND-ADmini trials.
Funding: Alzheimer’s Association – Part the Cloud Gates Partnership 2020 (PTC); Alzheimer’s Drug Discovery Foundation (ADDF); Alzheimerfonden (Sweden); Region Stockholm (ALF grant); European Research Council (ERC, 804371); EU Joint Programme—Neurodegenerative Disease Research (JPND) EURO-FINGERS and Multi-MeMo grants; ERA-NET Cofund Scheme – Horizon 2020 Research and Innovation Framework Programme of the European Commission Research Directorate-General (ERA PerMed grant); NordForsk NJ-FINGERS grant; Swedish Research Council; Center for Innovative Medicine (CIMED) at Region Stockholm (Sweden); Stiftelsen Stockholms Sjukhem (Sweden); Swedish Research Council for Health, Working Life and Welfare; Hjärnfonden (Sweden); Academy of Finland. Open access funding provided by Karolinska Institute.
Conflict of interest: MK is member of the Combinostics, Biogen, BioArtic, Eli Lilly, and Nestle Advisory Boards; and Speaker for Biogen, Roche, Nestle, and Nutricia. On behalf of the other authors, the corresponding author declare no conflict of interest.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614–29. doi: 10.1016/S1474-4422(14)70090-0.
2. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46. doi: 10.1016/S0140-6736(20)30367-6.
3. Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer report 2015: The global impact of dementia. Alzheimer’s disease International; 2015. https://www.alzint.org/u/WorldAlzheimerReport2015.pdf
4. Stephen R, Barbera M, Peters R, Ee N, Zheng L, Lehtisalo J, et al. Development of the First WHO Guidelines for Risk Reduction of Cognitive Decline and Dementia: Lessons Learned and Future Directions. FrontNeurol. 2021;12(Journal Article):763573. doi: 10.3389/fneur.2021.763573
5. Cummings J. Lessons Learned from Alzheimer Disease: Clinical Trials with Negative Outcomes. ClinTranslSci. 2018;11(2):147–52. doi: 10.1111/cts.12491.
6. Ackley SF, Zimmerman SC, Brenowitz WD, Tchetgen Tchetgen EJ, Gold AL, Manly JJ, et al. Effect of reductions in amyloid levels on cognitive change in randomized trials: instrumental variable meta-analysis. BMJ. 2021 Feb 25;372:n156. doi: 10.1136/bmj.n156.
7. Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007 Dec 11;69(24):2197–204. doi: 10.1212/01.wnl.0000271090.28148.24.
8. Ferreira D, Nordberg A, Westman E. Biological subtypes of Alzheimer disease: A systematic review and meta-analysis. Neurology. 2020 Mar 10;94(10):436–48. doi: 10.1212/WNL.0000000000009058.
9. Mohanty R, Ferreira D, Nordberg A, Westman E, Alzheimer’s Disease Neuroimaging Initiative. Associations between different tau-PET patterns and longitudinal atrophy in the Alzheimer’s disease continuum: biological and methodological perspectives from disease heterogeneity. Alzheimers Res Ther. 2023 Feb 22;15(1):37. doi: 10.1186/s13195-023-01173-1.
10. Rosenberg A, Öhlund-Wistbacka U, Hall A, Bonnard A, Hagman G, Rydén M, et al. β-Amyloid, Tau, Neurodegeneration Classification and Eligibility for Anti-amyloid Treatment in a Memory Clinic Population. Neurology. 2022 Nov 8;99(19):e2102–13. doi: 10.1212/WNL.0000000000201043.
11. Barnes LL, Dhana K, Liu X, Carey VJ, Ventrelle J, Johnson K, et al. Trial of the MIND Diet for Prevention of Cognitive Decline in Older Persons. N Engl J Med. 2023 Jul 18; doi: 10.1056/NEJMoa2302368.
12. Lisko I, Kulmala J, Annetorp M, Ngandu T, Mangialasche F, Kivipelto M. How can dementia and disability be prevented in older adults: where are we today and where are we going? J Intern Med. 2021 Jun;289(6):807–30. doi: 10.1111/joim.13227.
13. Baker LD, Cotman CW, Thomas R, Jin S, Shadyab AH, Pa J, et al. Topline Results of EXERT: Can Exercise Slow Cognitive Decline in MCI? Alzheimer’s & Dementia. 2022 Dec;18(S11):e069700.
14. Shadyab AH, LaCroix AZ, Feldman HH, van Dyck CH, Okonkwo OC, Tam SP, et al. Recruitment of a multi-site randomized controlled trial of aerobic exercise for older adults with amnestic mild cognitive impairment: The EXERT trial. Alzheimers Dement. 2021 Nov;17(11):1808–17. doi: 10.1002/alz.12401.
15. SPRINT MIND Investigators for the SPRINT Research Group, Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA. Feb 12;321(6):553–61. doi: 10.1001/jama.2018.21442.
16. Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–63. doi: 10.1016/S0140-6736(15)60461-5.
17. Solomon A, Turunen H, Ngandu T, Peltonen M, Levalahti E, Helisalmi S, et al. Effect of the Apolipoprotein E Genotype on Cognitive Change During a Multidomain Lifestyle Intervention: A Subgroup Analysis of a Randomized Clinical Trial. JAMA Neurol. 2018;75(4):462–70. doi: 10.1001/jamaneurol.2017.4365.
18. Ngandu T, Lehtisalo J, Korkki S, Solomon A, Coley N, Antikainen R, et al. The effect of adherence on cognition in a multidomain lifestyle intervention (FINGER). Alzheimers Dement. 2022 Jul;18(7):1325–34. doi: 10.1002/alz.12492.
19. Marengoni A, Rizzuto D, Fratiglioni L, Antikainen R, Laatikainen T, Lehtisalo J, et al. The Effect of a 2-Year Intervention Consisting of Diet, Physical Exercise, Cognitive Training, and Monitoring of Vascular Risk on Chronic Morbidity-the FINGER Randomized Controlled Trial. JAmMedDirAssoc. 2018;19(4):355-360.e1. doi: 10.1016/j.jamda.2017.09.020.
20. Kulmala J, Ngandu T, Havulinna S, Levalahti E, Lehtisalo J, Solomon A, et al. The Effect of Multidomain Lifestyle Intervention on Daily Functioning in Older People. JAmGeriatrSoc. 2019;67(6):1138–44. doi: 10.1111/jgs.15837.
21. Strandberg TE, Levälahti E, Ngandu T, Solomon A, Kivipelto M, Kivipelto M, et al. Health-related quality of life in a multidomain intervention trial to prevent cognitive decline (FINGER). European Geriatric Medicine. 4AD Jan;8(2):164–7. doi: 10.1007/s40520-022-02088-x.
22. Lehtisalo J, Rusanen M, Solomon A, Antikainen R, Laatikainen T, Peltonen M, et al. Effect of a multi-domain lifestyle intervention on cardiovascular risk in older people: the FINGER trial. EurHeart J. 2022;(Journal Article). doi: 10.1093/eurheartj/ehab922.
23. Wimo A, Handels R, Antikainen R, Eriksdotter M, Jönsson L, Knapp M, et al. Dementia prevention: The potential long-term cost-effectiveness of the FINGER prevention program. Alzheimers Dement. 2022 Jul 16; doi: 10.1002/alz.12698.
24. Moll van Charante EP, Richard E, Eurelings LS, van Dalen JW, Ligthart SA, van Bussel EF, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet. Aug 20;388(10046):797–805. doi: 10.1016/S0140-6736(16)30950-3.
25. Chhetri JK, de Souto Barreto P, Cantet C, Pothier K, Cesari M, Andrieu S, et al. Effects of a 3-Year Multi-Domain Intervention with or without Omega-3 Supplementation on Cognitive Functions in Older Subjects with Increased CAIDE Dementia Scores. J Alzheimers Dis. 2018;64(1):71–8. doi: 10.3233/JAD-180209.
26. Song S, Stern Y, Gu Y. Modifiable lifestyle factors and cognitive reserve: A systematic review of current evidence. Ageing Res Rev. 2022 Feb;74:101551. doi: 10.1016/j.arr.2021.101551.
27. Desai P, Evans D, Dhana K, Aggarwal NT, Wilson RS, McAninch E, et al. Longitudinal Association of Total Tau Concentrations and Physical Activity With Cognitive Decline in a Population Sample. JAMA Netw Open. 2021 Aug 2;4(8):e2120398. doi: 10.1001/jamanetworkopen.2021.20398
28. Ballarini T, Melo van Lent D, Brunner J, Schröder A, Wolfsgruber S, Altenstein S, et al. Mediterranean Diet, Alzheimer Disease Biomarkers and Brain Atrophy in Old Age. Neurology. 2021 May 5;96(24):e2920-2932. doi: 10.1212/WNL.0000000000012067.
29. Jia J, Zhao T, Liu Z, Liang Y, Li F, Li Y, et al. Association between healthy lifestyle and memory decline in older adults: 10 year, population based, prospective cohort study. BMJ. 2023 Jan 25;380:e072691. doi: 10.1136/bmj-2022-072691.
30. Kivipelto M, Rovio S, Ngandu T, Kåreholt I, Eskelinen M, Winblad B, et al. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. J Cell Mol Med. 2008 Dec;12(6B):2762–71. doi: 10.1111/j.1582-4934.2008.00296.x.
31. Strand BH, Rosness TA, Engedal K, Magnus P, Bergem ALM, Schirmer H, et al. Interaction of Apolipoprotein E Genotypes, Lifestyle Factors and Future Risk of Dementia-Related Mortality: The Cohort of Norway (CONOR). Dement Geriatr Cogn Disord. 2015;40(3–4):137–47. doi: 10.1159/000431218.
32. Chhetri JK, de Souto Barreto P, Cantet C, Cesari M, Coley N, Andrieu S, et al. Trajectory of the MAPT-PACC-Preclinical Alzheimer Cognitive Composite in the Placebo Group of a Randomized Control Trial: Results from the MAPT Study: Lessons for Further Trials. J Prev Alzheimers Dis. 2018;5(1):31–5. doi: 10.14283/jpad.2017.21.
33. Rydström A, Darin-Mattsson A, Kåreholt I, Ngandu T, Lehtisalo J, Solomon A, et al. Occupational complexity and cognition in the FINGER multidomain intervention trial. Alzheimers Dement. 2022 Dec;18(12):2438–47. doi: 10.1002/alz.12561.
34. Stephen R, Solomon A, Ngandu T, Levälahti E, Rinne JO, Kemppainen N, et al. White Matter Changes on Diffusion Tensor Imaging in the FINGER Randomized Controlled Trial. J Alzheimers Dis. 2020;78(1):75–86. doi: 10.3233/JAD-200423.
35. Soininen H, Solomon A, Visser PJ, Hendrix SB, Blennow K, Kivipelto M, et al. 36-month LipiDiDiet multinutrient clinical trial in prodromal Alzheimer’s disease. Alzheimers Dement. 2021 Jan;17(1):29–40. doi: 10.1002/alz.12172.
36. Sandebring-Matton A, Goikolea J, Björkhem I, Paternain L, Kemppainen N, Laatikainen T, et al. 27-Hydroxycholesterol, cognition, and brain imaging markers in the FINGER randomized controlled trial. Alzheimers Res Ther. 2021 Mar 6;13(1):56. doi: 10.1186/s13195-021-00790-y.
37. Colucci-D’Amato L, Speranza L, Volpicelli F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int J Mol Sci. 2020 Oct 21;21(20):7777. doi: 10.3390/ijms21207777.
38. Moon SY, Kim S, Choi SH, Hong CH, Park YK, Na HR, et al. Impact of Multidomain Lifestyle Intervention on Cerebral Cortical Thickness and Serum Brain-Derived Neurotrophic Factor: the SUPERBRAIN Exploratory Sub-study. Neurotherapeutics. 2022 Sep;19(5):1514–25. doi: 10.1007/s13311-022-01276-x.
39. Moon SY, Shin SA, Jeong JH, Hong CH, Park YK, Na HR, et al. Impact of a multidomain lifestyle intervention on regional spontaneous brain activity. Front Aging Neurosci. 2022;14:926077. doi: 10.3389/fnagi.2022.926077.
40. Castells-Sánchez A, Roig-Coll F, Dacosta-Aguayo R, Lamonja-Vicente N, Torán-Monserrat P, Pera G, et al. Molecular and Brain Volume Changes Following Aerobic Exercise, Cognitive and Combined Training in Physically Inactive Healthy Late-Middle-Aged Adults: The Projecte Moviment Randomized Controlled Trial. Front Hum Neurosci. 2022;16:854175. doi: 10.3389/fnhum.2022.854175.
41. Matton A, Håkansson K, Goicolea J, Daniilidou M, Ngandu T, Gerenu G, et Al. Serum proBDNF predicts memory gains after lifestyle changes in elderly persons – A subgroup analysis among adherent participants in the FINGER study. Clinical Trials on Alzheimer’s Disease conference, 2022; P063.
42. Sindi S, Solomon A, Kåreholt I, Hovatta I, Antikainen R, Hänninen T, et al. Telomere Length Change in a Multidomain Lifestyle Intervention to Prevent Cognitive Decline: A Randomized Clinical Trial. J Gerontol A Biol Sci Med Sci. 2021 Feb 25;76(3):491–8. doi: 10.1093/gerona/glaa279.
43. Coomans EM, Tomassen J, Ossenkoppele R, Golla SSV, den Hollander M, Collij LE, et al. Genetically identical twins show comparable tau PET load and spatial distribution. Brain. 2022 Oct 21;145(10):3571–81. doi: 10.1093/brain/awac004.
44. Konijnenberg E, Tomassen J, den Braber A, Ten Kate M, Yaqub M, Mulder SD, et al. Onset of Preclinical Alzheimer Disease in Monozygotic Twins. Ann Neurol. 2021 May;89(5):987–1000. doi: 10.1002/ana.26048.
45. Alanko V, Eroli F, Solomon A, Hartmann T, Kivipelto M, Nilsson P, et Al. Prevention of Alzheimer’s by a Multimodal Lifestyle Protocol – Working-mechanisms for Memory Gains: Mouse-PAW. Alzheimer’s Association International Conference, 2023; Poster P1-198.
46. Middleton LT, Touchon J, Vellas B. Editorial: What Is Reasonable and Necessary for Alzheimer Patients from Randomized Clinical Trials to Clinical Practice? J Prev Alzheimers Dis. 2023;10(3):331–2. doi: 10.14283/jpad.2023.69.
47. Cutler JA. Combinations of lifestyle modification and drug treatment in management of mild-moderate hypertension: a review of randomized clinical trials. Clin Exp Hypertens. 1993 Nov;15(6):1193–204. doi: 10.3109/10641969309037105.
48. Sheng Z, Cao JY, Pang YC, Xu HC, Chen JW, Yuan JH, et al. Effects of Lifestyle Modification and Anti-diabetic Medicine on Prediabetes Progress: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne). 2019;10:455. doi: 10.3389/fendo.2019.00455.
49. Guardado-Mendoza R, Salazar-López SS, Álvarez-Canales M, Farfán-Vázquez D, Martínez-López YE, Jiménez-Ceja LM, et al. The combination of linagliptin, metformin and lifestyle modification to prevent type 2 diabetes (PRELLIM). A randomized clinical trial. Metabolism. 2020 Mar;104:154054. doi: 10.1016/j.metabol.2019.154054.
50. Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, et al. Vascular dysfunction-The disregarded partner of Alzheimer’s disease. Alzheimers Dement. 2019;15(1):158–67. doi: 10.1016/j.jalz.2018.07.222.
51. Yarchoan M, Arnold SE. Repurposing diabetes drugs for brain insulin resistance in Alzheimer disease. Diabetes. 2014;63(7):2253–61. doi: 10.2337/db14-0287.
52. Markowicz-Piasecka M, Sikora J, Szydlowska A, Skupien A, Mikiciuk-Olasik E, Huttunen KM. Metformin – a Future Therapy for Neurodegenerative Diseases : Theme: Drug Discovery, Development and Delivery in Alzheimer’s Disease Guest Editor: Davide Brambilla. PharmRes. 2017;34(12):2614–27. doi: 10.1007/s11095-017-2199-y.
53. Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. NatRevNeurol. 2018;14(3):168–81. doi: 10.1038/nrneurol.2017.185.
54. Charpignon ML, Vakulenko-Lagun B, Zheng B, Magdamo C, Su B, Evans K, et al. Causal inference in medical records and complementary systems pharmacology for metformin drug repurposing towards dementia. Nat Commun. 2022 Dec 10;13(1):7652. doi: 10.1038/s41467-022-35157-w.
55. Zheng B, Su B, Ahmadi-Abhari S, Kapogiannis D, Tzoulaki I, Riboli E, et al. Dementia risk in patients with type 2 diabetes: Comparing metformin with no pharmacological treatment. Alzheimers Dement. 2023 Jul 3; doi: 10.1002/alz.13349.
56. Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019 Oct;15(10):565–81. doi: 10.1038/s41582-019-0244-7.
57. Hara Y, McKeehan N, Fillit HM. Translating the biology of aging into novel therapeutics for Alzheimer disease. Neurology. 2019 Jan 8;92(2):84–93. doi: 10.1212/WNL.0000000000006745.
58. Luo S, Wong ICK, Chui CSL, Zheng J, Huang Y, Schooling CM, et al. Effects of putative metformin targets on phenotypic age and leukocyte telomere length: a mendelian randomisation study using data from the UK Biobank. Lancet Healthy Longev. 2023 Jul;4(7):e337–44. doi: 10.1016/S2666-7568(23)00085-5.
59. Xenos D, Mecocci P, Boccardi V. A blast from the past: To tame time with metformin. Mech Ageing Dev. 2022 Dec;208:111743. doi: 10.1016/j.mad.2022.111743.
60. Friedman LG, McKeehan N, Hara Y, Cummings JL, Matthews DC, Zhu J, et al. Value-Generating Exploratory Trials in Neurodegenerative Dementias. Neurology. 2021 May 18;96(20):944–54. doi: 10.1212/WNL.0000000000011774.
61. Kivipelto M, Mangialasche F, Snyder HM, Allegri R, Andrieu S, Arai H, et al. World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement. Jul;16(7):1078–94. doi: 10.1002/alz.12123.
62. Sindi S, Thunborg C, Rosenberg A, Andersen P, Andrieu S, Broersen LM, et al. Multimodal Preventive Trial for Alzheimer’s Disease: MIND-ADmini Pilot Trial Study Design and Progress. J Prev Alzheimers Dis. 2022;9(1):30–9. doi: 10.14283/jpad.2022.4.
63. Mancinetti F, Xenos D, De Fano M, Mazzieri A, Porcellati F, Boccardi V, et al. Diabetes-Alzheimer’s connection in older age: SGLT2 inhibitors as promising modulators of disease pathways. Ageing Res Rev. 2023 Jul 20;90:102018. doi: 10.1016/j.arr.2023.102018.
64. Cummings J, Zhou Y, Lee G, Zhong K, Fonseca J, Cheng F. Alzheimer’s disease drug development pipeline: 2023. Alzheimers Dement (N Y). 2023;9(2):e12385. doi: 10.1002/trc2.12385.
65. Chowdhary N, Barbui C, Anstey KJ, Kivipelto M, Barbera M, Peters R, et al. Reducing the Risk of Cognitive Decline and Dementia: WHO Recommendations. FrontNeurol. 2022;12(Journal Article):765584. doi: 10.3389/fneur.2021.765584.
66. A blueprint for dementia research. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO.
67. Wolters FJ, Chibnik LB, Waziry R, Anderson R, Berr C, Beiser A, et al. Twenty-seven-year time trends in dementia incidence in Europe and the United States: The Alzheimer Cohorts Consortium. Neurology. 2020 Aug 4;95(5):e519–31. doi: 10.1212/WNL.0000000000010022.
68. Lehtisalo J, Palmer K, Mangialasche F, Solomon A, Kivipelto M, Ngandu T. Changes in Lifestyle, Behaviors, and Risk Factors for Cognitive Impairment in Older Persons During the First Wave of the Coronavirus Disease 2019 Pandemic in Finland: Results From the FINGER Study. Front Psychiatry. 2021;12:624125. doi: 10.3389/fpsyt.2021.624125
69. Blotenberg I, Hoffmann W, Thyrian JR. Dementia in Germany: Epidemiology and Prevention Potential. Dtsch Arztebl Int. 2023 Jun 30;(Forthcoming):arztebl.m2023.0100. doi: 10.3238/arztebl.m2023.0100.
70. Jørgensen K, Nielsen TR, Nielsen A, Waldemar G. Potential for prevention of dementia in Denmark. Alzheimers Dement. 2023 Mar 18; doi: 10.1002/alz.13030.
71. Mukadam N, Sommerlad A, Huntley J, Livingston G. Population attributable fractions for risk factors for dementia in low-income and middle-income countries: an analysis using cross-sectional survey data. Lancet Glob Health. 2019 May;7(5):e596–603. doi: 10.1016/S2214-109X(19)30074-9.
72. Solomon A, Stephen R, Altomare D, Carrera E, Frisoni GB, Kulmala J, et al. Multidomain interventions: state-of-the-art and future directions for protocols to implement precision dementia risk reduction. A user manual for Brain Health Services-part 4 of 6. Alzheimers Res Ther. 2021 Oct 11;13(1):171. doi: 10.1186/s13195-021-00875-8.
73. Walsh S, Govia I, Wallace L, Richard E, Peters R, Anstey KJ, et al. A whole-population approach is required for dementia risk reduction. Lancet Healthy Longev. 2022 Jan;3(1):e6–8. doi: 10.1016/S2666-7568(21)00301-9.
74. Regeringskansliet. (2023, May 19). Uppdrag att ta fram underlag till en utvecklad nationell demensstrategi. Regeringskansliet; Regeringen och Regeringskansliet. https://www.regeringen.se/regeringsuppdrag/2023/05/uppdrag-att-ta-fram-underlag-till-en-utvecklad-nationell-demensstrategi/ . Last Accessed, September 2023.
75. Valtioneuvosto. (2023, April 4). National Programme on Ageing to 2030. Valtioneuvosto. https://julkaisut.valtioneuvosto.fi/handle/10024/164823 . Last Accessed, September 2023.
© The Authors 2023