L. Bransby1, E. Rosenich1, P. Maruff1,2,3, Y.Y. Lim1
1. Turner Institute for Brain and Mental Health, School of Psychological Sciences, Monash University, Melbourne, Victoria, Australia; 2. Florey Institute of Neuroscience and Mental Health, Parkville, Victoria, Australia; 3. Cogstate Ltd., Melbourne, Victoria, Australia
Corresponding Author: Lisa Bransby, Turner Institute for Brain and Mental Health, 18 Innovation Walk, Clayton, VIC 3800, Australia; Email: lisa.bransby@monash.edu
J Prev Alz Dis 2024;1(11):22-37
Published online October 4, 2023, http://dx.doi.org/10.14283/jpad.2023.119
Abstract
Many risk factors for dementia, identified from observational studies, are potentially modifiable. This raises the possibility that targeting key modifiable dementia risk factors may reduce the prevalence of dementia, which has led to the development of dementia risk reduction and prevention strategies, such as intervention trials or dementia prevention guidelines. However, what has rarely been considered in the studies that inform these strategies is the extent to which modifiable dementia risk factors can (1) be identified by individuals, and (2) be readily modified by individuals. Characteristics of modifiable dementia risk factors such as readiness of identification and targeting, as well as when they should be targeted, can influence the design, or success of strategies for reducing dementia risk. This review aims to develop a framework for classifying the degree of modifiability of dementia risk factors for research studies. The extent to which these modifiable dementia risk factors could be modified by an individual seeking to reduce their dementia risk is determined, as well as the resources that might be needed for both risk factor identification and modification, and whether modification may be optimal in early-life (aged <45 years), midlife (aged 45-65 years) or late-life (aged >65 years). Finally, barriers that could influence the ability of an individual to engage in risk factor modification and, ultimately, dementia risk reduction are discussed.
Key words: Dementia, risk factors, modifiable, prevention, risk reduction, Alzheimer’s disease.
Introduction
Clinicopathological and epidemiological studies have identified a wide range of dementia risk factors on the basis of their association with cognitive decline as well as increased risk for cognitive impairment and dementia onset (1–7). The most unequivocal risk factors for dementia are biologically determined, such as older age, with 1 in 10 people over 65 but 3 in 10 people over 85 living with dementia (1). The Apolipoprotein E (APOE) ε4 allele is the strongest genetic risk factor for dementia caused by sporadic Alzheimer’s disease (AD), and is present in 9-23% of various ethnic populations (8). The risk of developing AD by 85 years of age is 18.4% for APOE ε4 heterozygotes and 48.3% for APOE ε4 homozygotes (9). Sex has also been identified as a risk factor, with more women developing AD dementia than men (1). While these risk factors cannot be modified to reduce dementia risk, there is now consensus that many dementia risk factors can be modified. For example, based on a large-scale review of observational studies, the Lancet Commission of Dementia Prevention, Intervention and Care estimated that approximately 40% of cases of clinically classified dementia could be attributed to twelve key modifiable dementia risk factors (MDRFs) (10). This supports that dementia has a multifactorial etiology and raises the possibility that many cases of dementia could be prevented by strategies that act to reduce MDRFs (11, 12).
MDRFs are increasingly the targets of dementia risk reduction and prevention strategies, many of which are now underway. For example, several randomized controlled trials (RCT) have been conducted to determine whether single- or multi-domain interventions that target subsets of MDRFs may delay or even prevent cognitive decline or onset of dementia (13), such as the global multi-domain Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial (14). In addition, dementia prevention guidelines have been published by the World Health Organisation (WHO) that outline recommendations of strategies for reducing dementia (15). Recently, a brain health service model to facilitate dementia risk detection and interventions in at-risk but cognitively unimpaired individuals has been introduced and proposed as a potential method for implementation of dementia risk reduction strategies into clinical practice (16).
While there is growing consensus regarding which dementia risk factors are modifiable, little consideration has been given to the extent to which individuals could identify whether they have one or more MDRFs, and if they do, how easy such factors are to modify. For example, for an individual seeking to reduce their dementia risk, an MDRF such as physical inactivity may be easier to identify and modify compared to air pollution. Relatedly, the time required for any modification of an MDRF to influence and then maintain any meaningful reduction in dementia risk is also likely to differ across MDRFs. Thus, characteristics of MDRFs such as ease of identification and targeting, and the period of modification required for reduction of dementia risk will each influence the extent to which strategies designed to minimize MDRFs could be implemented. This also becomes important for planning RCTs, where time intervals are often fixed and limited to less than five years (11, 17, 18). To date, factors that influence the feasibility of implementing behavior change strategies for dementia risk reduction or prevention have not been considered formally or conceptualized in the observational studies that have informed the design of dementia risk reduction strategies. Thus, despite widespread agreement that targeting MDRFs is important for dementia prevention, the guidelines and policies for brain health that are based on these studies do not contain information on the feasibility, or time interval necessary, for MDRF modification approaches. For example, in the Lancet Commission Report, a range of recommended strategies were simply listed for targeting MDRFs at an individual or population-based level (10). Further, in a recent report that considered the societal and equity challenges of a proposed brain health service model, it was acknowledged that for some individuals, accessing a brain health service may not be feasible due to disadvantage and that population-based approaches may be more appropriate for equitable dementia prevention (19). Whilst these reports introduced the premise of interventions that target MDRFs needing to occur at different levels, no definition or characterization of these different levels were provided which inhibits understanding of the feasibility of how these recommended strategies for dementia risk reduction can be implemented. As a foundational step to strengthen these and future recommendations for dementia risk reduction from research studies, the degree of modifiability of key MDRFs requires more formal conceptualization, which will ultimately increase the feasibility of achieving dementia risk reduction and prevention.
The primary aim of this review is to develop a framework for classifying the degree of modifiability of MDRFs for research studies. This framework interprets the modifiability of MDRFs from the perspective of an individual seeking to reduce their dementia risk and considers non-modifiable dementia risk factors, such as older age, the APOE ε4 allele and sex, as a basis for comparison or contextualization of MDRFs. Each dementia risk factor is classified as one of the following: (i) non-modifiable; (ii) modifiable with intervention at societal or community level; or (iii) modifiable with intervention at the individual level. This framework is applied to a review of well-established MDRFs, such as those identified in the Lancet Commission Report (10), as well as other MDRFs for which there is accumulating evidence for their association with increased risk for cognitive impairment, cognitive decline and dementia onset. The extent to which these MDRFs could be modified by an individual seeking to reduce dementia risk is determined, as well as the resources that might be needed for both MDRF identification and modification, and whether modification would be ideal in early-life (aged <45 years), midlife (aged 45-65 years) and late-life (aged >65 years) (10). Finally, this review considers some of the barriers and enablers that influence the feasibility of modifying MDRFs.
Framework of modifiability for dementia risk factors
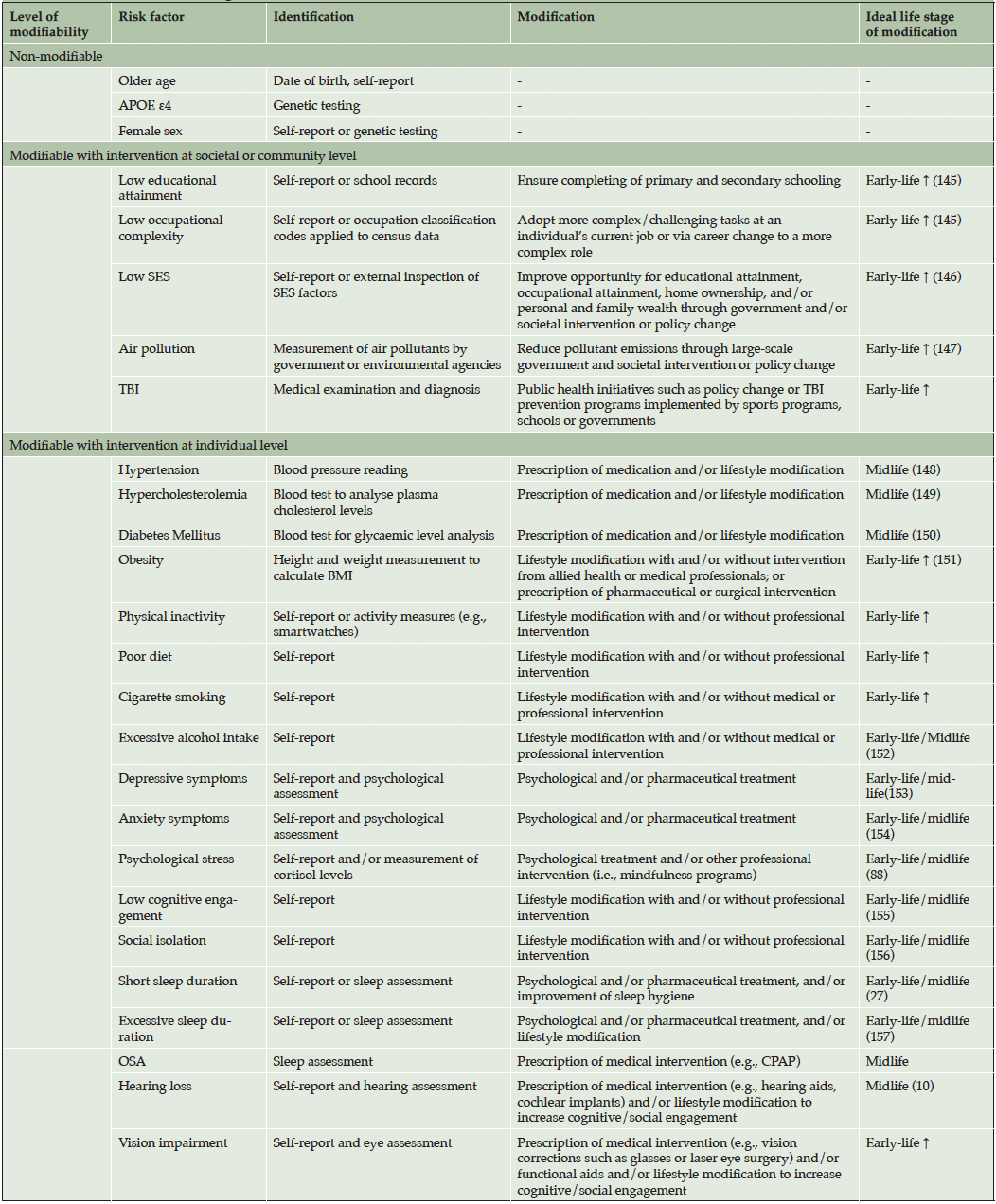
The framework for the degree of modifiability of dementia risk factors is summarized in Table 1. In this framework, each MDRF is classified according to its potential for modification by an individual seeking to reduce their dementia risk. This can occur in formal intervention trials or in everyday life. Three levels were developed for classifying the modifiability of each dementia risk factor which include:
(i) Non-modifiable – dementia risk factors that cannot be modified to reduce dementia risk.
(ii) Modifiable with intervention at the societal or community level – dementia risk factors that could potentially be modified but which require interventions from agencies, governments, or communities (e.g., government policy, societal change).
(iii) Modifiable with intervention at the individual level – dementia risk factors that can be modified by the individual with and/or without professional/medical interventions.
Note. References in the ideal life stage of modification column represent empirical evidence supporting the proposed life stage for the MDRF. If there is no reference, then the proposed life stage is hypothetical. APOE ε4=Apolipoprotein E ε4 allele; SES=socioeconomic status; TBI=traumatic brain injury; OSA=obstructive sleep apnea; BMI=body mass index; CPAP= continuous positive airway pressure; Early life ↑=ideal to modify early life and onwards.
For each MDRF, the classification was made by characterizing information on how an MDRF can be modified, the resources required for modification, and the potential for modification by the individual. Whilst this framework does not offer quantification for the levels of modifiability, it introduces the concept and forms the basis for this to be developed in future studies. The following section reviews each MDRF and provides the theoretical basis for its classification within the modifiability framework.
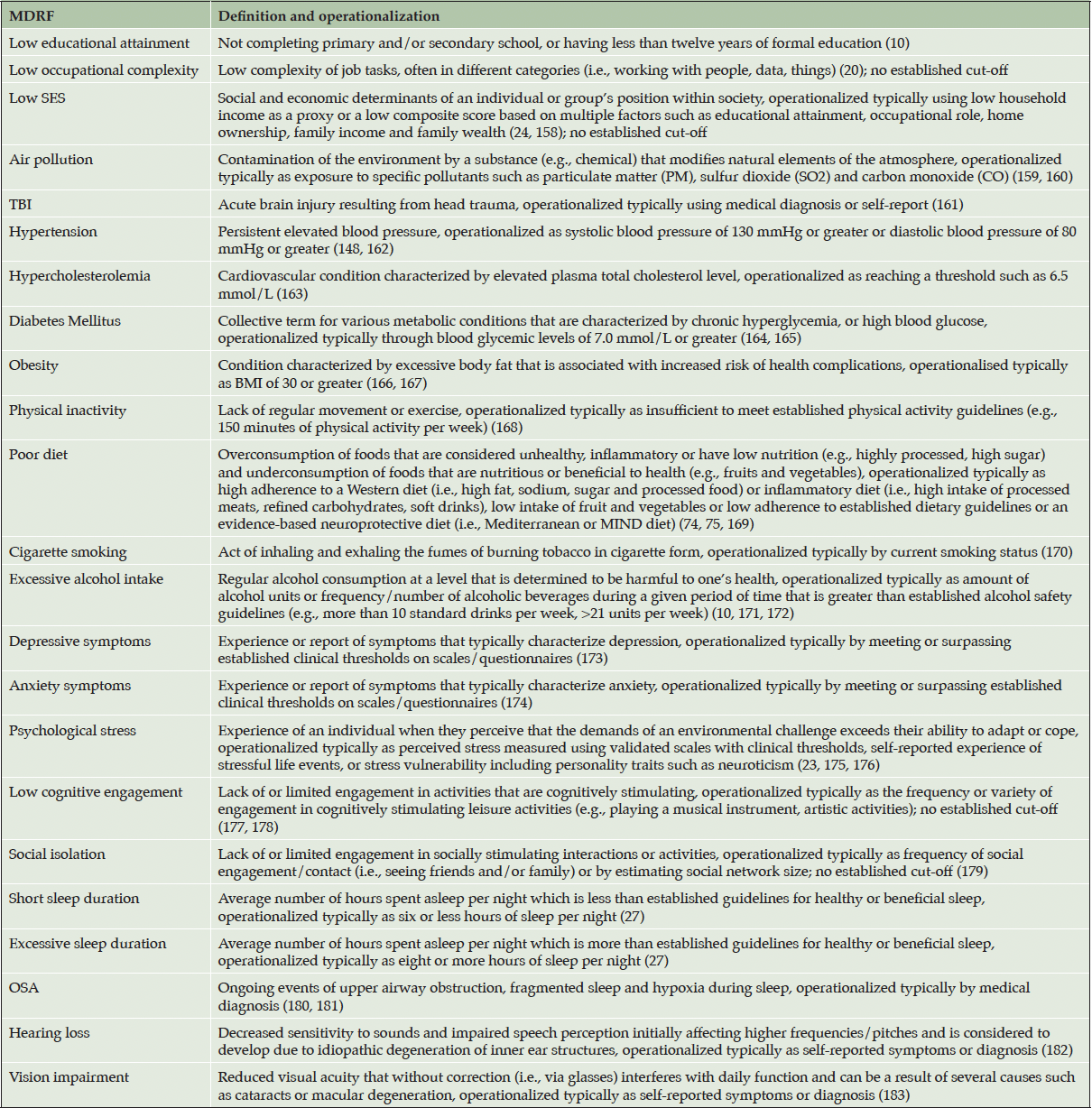
Substantial evidence from observational studies, synthesized in the Lancet Commission Report,10 has identified low education, hearing loss, traumatic brain injury (TBI), hypertension, excessive alcohol intake, obesity, smoking, depression, social isolation, physical inactivity, air pollution, and diabetes as well-established MDRFs (10). Other MDRFs, that were not highlighted by the Lancet Commission Report but for which there is a growing evidence-base from epidemiological and clinicopathological studies include low occupational complexity (20), hypercholesterolemia (21), anxiety (22), psychological stress (23), low socioeconomic status (24), poor diet (25), low cognitive engagement (26), short sleep duration (27, 28), excessive sleep duration (29), obstructive sleep apnea (OSA) (30) and vision impairment (31). These MDRFs are therefore also included in the framework to ensure it is as comprehensive as possible. Definitions and operationalization for each MDRF are summarized in Table 2.
Note. SES=socioeconomic status; TBI=traumatic brain injury; BMI=body mass index; MIND= Mediterranean-DASH Intervention for Neurodegenerative Delay; OSA=obstructive sleep apnea.
Search criteria
PubMed and Google Scholar databases were searched for articles published in English with no date or time restrictions. There were several steps to the literature search process for the current review. First, to obtain evidence of an association between a given MDRF with cognitive decline and risk of cognitive impairment and/or dementia, a combination of search terms was included such as “modifiable dementia risk factor”, or the specific MDRF (e.g., “hypertension”) and “cognitive decline”, “cognitive impairment”, or “dementia risk”. To determine from previous literature the methods for identification of MDRFs, a term for the specific MDRF was searched for in combination with terms relating to identification such as “detection”, “measurement”, “identification”. To determine from previous literature strategies for MDRF modification or intervention, combinations of search terms then included the specific MDRF and terms for modification such as “intervention”, “modification”, “treatment”. In some cases, this process was guided by intuition and prior knowledge as for some MDRFs such specific details have not been explicitly defined in previous studies. To propose ideal life stages of modification for MDRFs, search terms included the specific MDRF, “dementia risk” and “age”, or “early life”, “midlife”, or “late life”, or “middle-aged adults”, or “older adults”. Finally, to search previous literature for barriers and enablers to dementia risk reduction, search terms included “dementia risk reduction”, “dementia prevention”, or “dementia risk”, and “barrier” or “enablers” and “engagement”, “intervention”, or “behavior”. Terms such as “external”, “population-based”, “internal” or “individual” were also subsequently added to the literature search for dementia risk reduction barriers.
Dementia risk factors that are modifiable with intervention at societal or community level
Table 1 shows several MDRFs that are classified as modifiable with societal or community level intervention. The modification of these MDRFs is difficult or not feasible for individuals to achieve themselves. Rather, for modification of these MDRFs, interventions from agencies, governments, or communities (e.g., government policy, societal change) may be required.
Low educational attainment
Low educational attainment, or less education, can be identified in research studies or in the general population by self-report or via inspection of school records. Low educational attainment may be modified in early-life by ensuring completion of at least primary and secondary schooling (10), however, the evidence is equivocal on whether additional education in later life stages is associated with reduced dementia risk (32). Importantly, low educational attainment is related to low socioeconomic status (SES) (33), which can create barriers to health literacy, healthcare access and opportunity to engage in health-promoting behaviors. Thus, increasing educational attainment in early life is facilitated by factors external to the individual such as parents or guardians (e.g., ensuring completion of secondary school), or at a government or societal level (e.g., free, or subsidized education, education promotion campaigns, universal access to education).
Low occupational complexity
Low occupational complexity can be identified by self-report or, in some research studies, by occupational classification codes applied to census data (34). Low occupational complexity may be modified by adopting more complex or challenging tasks at an individual’s current job or via career change to a more complex role or industry. However, the ability to make such modifications may differ according to occupation type and industry, and in many cases, may rely on opportunities for further education or upskilling. Importantly, this may not be possible for individuals seeking to reduce their dementia risk due to challenges such as financial responsibilities and caregiving. Thus, modification of occupational complexity may need to occur in early adulthood, the typical time of occupational training and career/work initiation. However, opportunity to increase occupational complexity may also be related to SES (35) and may require intervention from factors external to the individual (e.g., workplace, government/policy, and even parents or guardians, given its relation to early-life educational attainment). Further, opportunity to increase occupational complexity can also depend on whether free or subsidized tertiary education is available where the individual lives.
Low SES
Low SES can be identified via self-report or external inspection of socioeconomic factors including educational attainment, occupational role, home ownership, family income and family wealth. Modification of low SES may not be in the control of the individual. Low SES likely relates to multiple converging factors and social determinants of health such as barriers to health literacy (36), limited access to healthcare and community resources that promote healthy behaviours (e.g., green spaces, affordable fresh food) (37), as well as structural factors, such as social exclusion, discrimination, and racism (38, 39). These factors may affect individuals throughout the lifespan, and may work independently or in synergy to influence engagement in risky lifestyle behaviors that often promote morbidity such as cardiovascular conditions and dementia (40). SES may therefore be difficult and complex to modify at the individual level and likely requires larger scale government and societal intervention or policy change.
Air pollution
Air pollution can be identified by the measurement of air pollutants such as particulate matter (PM), sulfur dioxide (SO2) and carbon monoxide (CO) by government bodies or environmental agencies (41, 42). Air pollution may possibly be modified by targeting known contributors (41, 43) at the individual-level (e.g., smoking cessation, the adoption of electric cars and air purifiers). However, this can require significant financial and personal resources, and it remains unclear whether this will result in positive short-term health changes for the individual. Further, it may not be feasible for individuals to relocate from an area or city with high air pollution to another with lower pollution to reduce their dementia risk. Given this, strategies to reduce dementia risk from air pollution are more likely to occur through modification at a governmental and/or large-scale societal level (44). The Lancet Commission Report identified air pollution as a late-life MDRF (10), however, it is unclear whether this reflects lifetime exposure to air pollution or whether air pollutants exacerbate neurodegenerative changes already occurring in late life (45).
Traumatic brain injury
TBI can be identified by medical examination and diagnosis or by self-report diagnosis by the individual after the event. If an individual has already experienced TBI, some treatments can be administered to individuals by medical practitioners to reduce the severity of damage to the brain (46), although it is unclear whether these treatments will then, in turn, reduce dementia risk. Thus, once an individual has already experienced TBI, the risk for dementia posed by TBI may not be possible to modify or reverse at the individual level. Contact sports and motor vehicle accidents both have high incidence rates of TBI (47, 48). Thus, it may be more practical to modify TBI associated risk for dementia through public health initiatives such as targeted preventative educational programs and policy changes that are external to the individual and focused on protecting the brain from injury (i.e., mandatory helmets for football players, safer roads/cars). This may occur either at the government level, by schools and sport clubs, or by raising public awareness of the guidelines and recommendations around TBI risk. The Lancet Commission Report identified TBI as a midlife MDRF likely due to the amount and quality of evidence that exists for this life stage (10). It is unclear whether experiencing TBI in childhood or adolescence (e.g., though playing sport) is also associated with dementia risk in later life, although there is some evidence that TBI-related dementia risk is attenuated with time (49).
Summary
The MDRFs considered so far have been classified as modifiable with intervention at societal or community level, such as government policy or even legislative change. Some interventions required to modify these MDRFs are preventative, such as educational programs and policy changes for preventing TBI, whilst others are likely ameliorative such as government policy to reduce emission of air pollutants. Many large-scale societal and community level interventions that require policy change take time, effort and resources and therefore may not be entirely feasible for meaningful dementia risk reduction. Further, the efficacy of these population-level approaches to dementia risk reduction is currently unclear as they have been severely under-researched (50). Additionally, most of these MDRFs require intervention in early life. Whilst some interventions may already occur early in life such as completion of schooling or educational programs to prevent TBI (i.e., in sports programs) for reasons other than dementia prevention (i.e., general child wellbeing or health), it is unclear the extent to which these interventions directly or indirectly influence dementia risk and whether public health initiatives specific for dementia prevention in children, adolescents or young adults are plausible.
Dementia risk factors that are modifiable with intervention at the individual level
Table 1 summarizes MDRFs classified as being modifiable with intervention by the individual. Depending on the individual, MDRF and specific circumstances, this may occur with or without the individual accessing medical or other professional intervention.
Hypertension and hypercholesterolemia
Hypertension can be identified through blood pressure monitoring which is a simple and non-invasive assessment that allied health professionals and medical practitioners can administer (51). Similarly, hypercholesterolemia can be identified by simple blood tests to monitor plasma cholesterol which can increase opportunity for early detection (52). Hypertension and hypercholesterolemia can both be modified at the individual level through the prescription of pharmaceutical treatment (e.g., benazepril, statins) (53, 54) and/or lifestyle modification (e.g., diet or exercise programs) (55, 56), by medical practitioners. The relationship between hypertension and hypercholesterolemia with increased dementia risk is strongest when these conditions present in midlife (57, 58), with some positing that chronicity (i.e., the condition is present for a longer timespan compared to if present from late-life only) may underly this observation (59). Accordingly, midlife is considered the ideal age for modification of these MDRFs (10, 58).
Diabetes Mellitus
Diabetes mellitus (DM) can be identified by blood tests for glycemic level and medical diagnosis. Blood tests are recommended for people at greater risk, including middle-aged adults, those with obesity, or a those with a family history of DM, which can facilitate early detection and treatment (60). DM can be modified by the individual through medications (e.g., metformin) and lifestyle modification such as limiting sugar intake and increased physical activity (61) prescribed by medical practitioners. Whilst DM has generally been considered a late-life MDRF, however, DM management is recommended to occur as early as possible (10, 62).
Obesity
Obesity can be identified via simple height and weight measurement to calculate BMI which can be done by individuals themselves or administered by allied health professionals/medical practitioners. Obesity can be addressed by individuals through lifestyle modification (e.g., increase in exercise, diet modification) (63, 64), which can be supported by allied health professionals (e.g., personal trainer, dietician, exercise physiologist), or pharmacological/surgical interventions (e.g., appetite suppressors, bariatric surgeries) (65). Obesity is most consistently associated with dementia risk outcomes when present in midlife (66), suggesting that midlife is an ideal timeframe for modification. However, given the strong link between obesity and other morbidities such as cardiovascular disease (67), it would be prudent for obesity to be addressed as early as possible.
Physical inactivity
Physical inactivity, or sedentary behavior, can be identified by self-report by the individual or through wearable measures of activity (e.g., smartwatches). Physical inactivity can be modified by individuals through lifestyle modification to increase physical activity, and can be supported by professional resources such as personal training, gym membership, and council-run community fitness programs (68). Community resources such as access to parks, walking trails, and bicycle paths can also promote engagement in physical activity (69). Whilst the Lancet Commission Report identified physical inactivity as a late-life MDRF (10), the relationship between physical inactivity and dementia risk in older adults is complex and may also contain reverse causation, i.e., sedentary behavior or physical inactivity in older adults may be indicative of underlying neurodegeneration, frailty, and incipient dementia as opposed to physical inactivity acting as a risk factor for dementia. Given its relationship with obesity, and the substantial benefits that physical activity has on improving cardiovascular health (70), it is therefore prudent for physical inactivity to be addressed at earlier life stages.
Cigarette smoking
Cigarette smoking is identified via self-report by the individual. Cigarette smoking can be reduced or ceased by individuals through lifestyle modification, however, nicotine addiction that is commonly associated with long-term smoking is a barrier to smoking reduction/cessation for many individuals. Smoking reduction/cessation can be aided with medical support, pharmaceutical aids (i.e., nicotine patches, medication), support hotlines and counselling (71). The Lancet Commission Report identified cigarette smoking as a late-life MDRF (10). However, the effects of cigarette smoking on dementia risk are likely a result of chronic or lifetime exposure, and may be confounded by survival bias given the greater risk of premature death associated with smoking (72). Importantly, general medical advice recommends strongly that smoking reduction or cessation occurs as early as possible (73).
Poor diet
Poor diet is identified by self-report of dietary patterns by individuals. Diet can be modified by individuals through lifestyle modification to reduce intake of unhealthy or inflammatory foods (74) and increase adherence to dietary guidelines or neuroprotective diets (e.g., Mediterranean-DASH Intervention for Neurodegenerative Delay diet) (75). Individuals can also be supported through consultation with dieticians or nutritionists, or access to meal plans/recipes. Poor diet is related to increased inflammation (76) and health conditions such as cardiovascular conditions (e.g., hypercholesterolemia) (77) which are also associated with increased dementia risk. Thus, given the robust evidence for associations between neuroinflammation and midlife cardiovascular conditions with dementia risk, it may be prudent for poor diet to be targeted at midlife or an earlier life stage.
Excessive alcohol intake
Excessive alcohol intake can be identified by self-report of alcohol intake by individuals, which can be assessed as excessive by medical or health professionals using established alcohol safety guidelines (e.g., no more than 10 standard drinks per week), or via self-reported concerns by individuals themselves, their family and/or friends. Excessive alcohol intake can be reduced by individuals through lifestyle modification. However, alcohol addiction and dependence can act as significant barriers to reduction (78), and medical or psychological intervention may be required for individuals that experience alcohol addiction/dependence. Whilst midlife is generally considered to be the ideal stage to reduce alcohol intake (10), general medical practice recommends limiting excessive alcohol intake as early as possible (79).
Elevated depression and anxiety symptoms
Elevated depression and anxiety symptoms can be identified by self-report of symptoms by individuals which can then lead to psychological assessment. Depression and anxiety symptomatology can be reduced using psychological (e.g., cognitive behavioral therapy) (80) or pharmaceutical (e.g., anti-depressant medication) (81) interventions administered to individuals by psychiatrists, general practitioners, psychologists and counsellors. Other interventions can complement pharmaceutical or psychological therapy such as physical activity (82) and mindfulness practice (83) which individuals can engage in with assistance from professional programs, online resources, or digital application-based interventions. The Lancet Commission Report identified depression as a late-life MDRF (10), however, anxiety or depression in late-life are also symptoms of incipient dementia (84, 85). Therefore, risk for dementia that is contributed by depression and anxiety symptomatology may need to be targeted at earlier life stages.
Psychological stress
Psychological stress can be identified by self-report experience of individuals or measurement of cortisol levels in blood, saliva or hair samples (86). Psychological stress may be addressed by individuals via psychological treatment which can be administered by psychiatrists, psychologists, or counsellors. Complementary to psychological treatment, mindfulness practice has also been associated with improvements in psychological stress which individuals can access via professional programs and online resources (87). Several studies show that midlife is typically stressful due to career demands and caregiving responsibilities, and this heightened psychological stress is associated with increased dementia risk several decades later (88). Therefore, although reducing psychological stress is recommended at any life stage, midlife is an important time to implement strategies to cope with stress.
Low cognitive engagement and social isolation
Low cognitive engagement and social isolation can be identified by self-report of engagement in cognitively and socially stimulating activities or interactions by individuals. Attempts to increase cognitive and social engagement (e.g., engagement in creative and/or social activities) can be made by individuals, and can be facilitated by professional programs (e.g., community groups, piano lessons) in the community and/or online. Social isolation was identified as a late-life MDRF in the Lancet Commission Report (10), however, social isolation in late-life may also reflect withdrawal from usual activities associated with incipient dementia (89). It is possible that any neurological benefits of cognitive and social engagement (i.e., cognitive reserve) (90) occur as a lifelong process, suggesting that low cognitive engagement and social isolation should be addressed as early as possible.
Sleep-related characteristics and disorders
Short (≤6 hours per night) or excessive (≥8 hours per night) sleep duration can be identified by self-report and in some cases, by consumer grade devices such as smartwatches or polysomnography (i.e., in research studies, sleep clinic). Short sleep duration can be addressed and increased to the optimal seven hours with interventions such as pharmaceutical treatment (e.g., melatonin) (91), or improvement of sleep hygiene (92). Excessive sleep duration can also be addressed by pharmaceutical treatment (e.g., wakefulness promoting agents or stimulants) and lifestyle modification (e.g., alarm use, caffeine), however, is often associated with underlying conditions which themselves require treatment (93). Further, as both short and excessive sleep duration are related to depressive symptoms (29), psychological treatment or practices may be required for some individuals (94). These interventions can be administered to individuals by medical practitioners, sleep specialists, psychologists or can be accessed through community and online resources (e.g., sleep hygiene information, mindfulness classes, digital application-based interventions). While short sleep is very common in late-life and has also been proposed to be a symptom of incipient dementia (95), short sleep in midlife is also associated with increased dementia risk (27) indicating that intervention should occur in midlife or earlier life stages. While the relationship between excessive sleep duration and risk of dementia in midlife or earlier is unclear (27) and late life excessive sleep is also proposed as a symptom of incipient dementia (96) intervention should likely also occur as early as possible.
OSA can be identified by medical diagnosis following sleep analysis using polysomnography. OSA can be treated through prescription of interventions for OSA to individuals by medical practitioners, such as continuous positive airway pressure (CPAP) (97), which has shown promise in attenuating the negative consequences of OSA on cognition and the brain (98). OSA that presents at any time during adulthood, but particularly from midlife, has been shown to have deleterious effects on the brain (99) and thus requires modification as early as possible once diagnosed.
Sensory impairments
Hearing loss and vision impairment can be identified by self-reported symptoms by the individual and/or assessment by health professionals (i.e., audiologist, optometrist). Age-related hearing loss can be addressed by individuals through the prescription of interventions such as hearing aids or cochlear implants by medical practitioners and audiologists, with some studies showing improvements in cognition and diminished association with cognitive decline associated with these interventions (100). Vision impairment can be addressed by individuals through prescription of corrective interventions such as glasses, contact lenses, or laser eye surgery (101), or functional aids such as visual aid technologies and rehabilitation (102). For instance, one study showed that individuals with cataracts that had corrective surgery had a 50% reduced risk of dementia compared to those with cataracts who did not have surgery (31). One hypothesis for how hearing loss and vision impairment may increase risk for dementia is through diminished cognitive and social engagement and depressive symptoms, which themselves are risk factors for dementia (31, 103). For instance, reduction in social isolation, loneliness and depressed mood partially mediated the association between hearing aid use and risk of dementia (103). Age-related hearing loss begins to emerge in midlife (104), thus making midlife the ideal timeframe for intervention (10). Vision impairment can impact an individual at any age so is recommended to be addressed or corrected as early as possible.
Summary
Classifying MDRFs according to this framework of modifiability highlights the issue that showing associations between MDRFs and cognitive decline or dementia may not necessarily translate readily to simple and easy MDRF identification, modification and dementia risk reduction for individuals. Therefore, the characterization of the feasibility of identification and modification of MDRFs, and the resources needed, contributes to enhancing understanding of how meaningful dementia risk reduction can be achieved. Thus, implementing a framework such as this to current and future studies of MDRFs forms a basis for reducing heterogeneity in the field, increasing general understanding about dementia risk reduction, and optimizing risk reduction strategies that target MDRFs.
Ideal life-stage for mdrf modification
As is illustrated in Table 1, most MDRFs were classified as being modifiable with intervention at the individual level which indicates considerable opportunity and feasibility for dementia risk reduction. Table 1 also shows that the ideal life stage proposed for modification of all MDRFs is early-to-mid-life and onwards. This is in contrast to the Lancet Commission Report which indicated that six MDRFs were classified as late-life MDRFs (10). However, these six late life MDRFs, such as physical inactivity, social isolation and depression, might be indicators of withdrawal or symptoms attributable to incipient dementia and thus indicative of reverse causation. Importantly, underlying AD pathological processes can develop over decades before clinically recognizable symptoms are detectable, even in middle-aged adults (105, 106). Therefore, although the life-course model of dementia risk proposed by the Lancet Commission Report suggests that some MDRFs have the greatest influence on dementia risk in late life, it is possible that, for some individuals, this may be too late for dementia risk reduction to be achieved. Additionally, patterns of health and lifestyle behaviors likely develop from an early age (107), which may mean that the influence of some MDRFs accumulate across the lifespan and meaningful behavior change and dementia risk reduction in later life can be difficult. Further, many MDRFs (e.g., smoking, physical inactivity) are also risk factors for other diseases and negative health consequences, such as cardiovascular disease, which may emerge prior to dementia. The current framework thus proposes that if MDRFs are present earlier in life, it would be prudent for modification to occur as early as possible. However, it is important to acknowledge that several factors, internal and external to the individual, may contribute to the extent to which an individual can engage in dementia risk reduction behavior even if their MDRFs are readily modifiable. The following sections discuss barriers and enablers to dementia risk reduction behavior.
Barriers and enablers to dementia risk reduction
Dementia risk factors that are modifiable by the individual have a higher level of modifiability as they can be targeted on an individual basis. Individuals can make major contributions to their own health and wellbeing in their everyday lives through adopting health-promoting behaviors (e.g., physical activity) and avoiding health-compromising behaviors (e.g., smoking), with or without medical or professional intervention (108). Further, by seeking assistance from medical or other professional interventions, individuals can take steps to improve health conditions (e.g., hypertension, OSA). However, even if some MDRFs are more feasible to target on an individual level, modification of these MDRFs may not necessarily occur or be easy to achieve. Both internal and external barriers can negatively influence engagement and maintenance of health behavior change relating to dementia risk. Alternatively, there are also internal and external enablers that can promote or facilitate engagement in dementia risk reduction behavior. These are summarized below and synthesized in Figure 1.
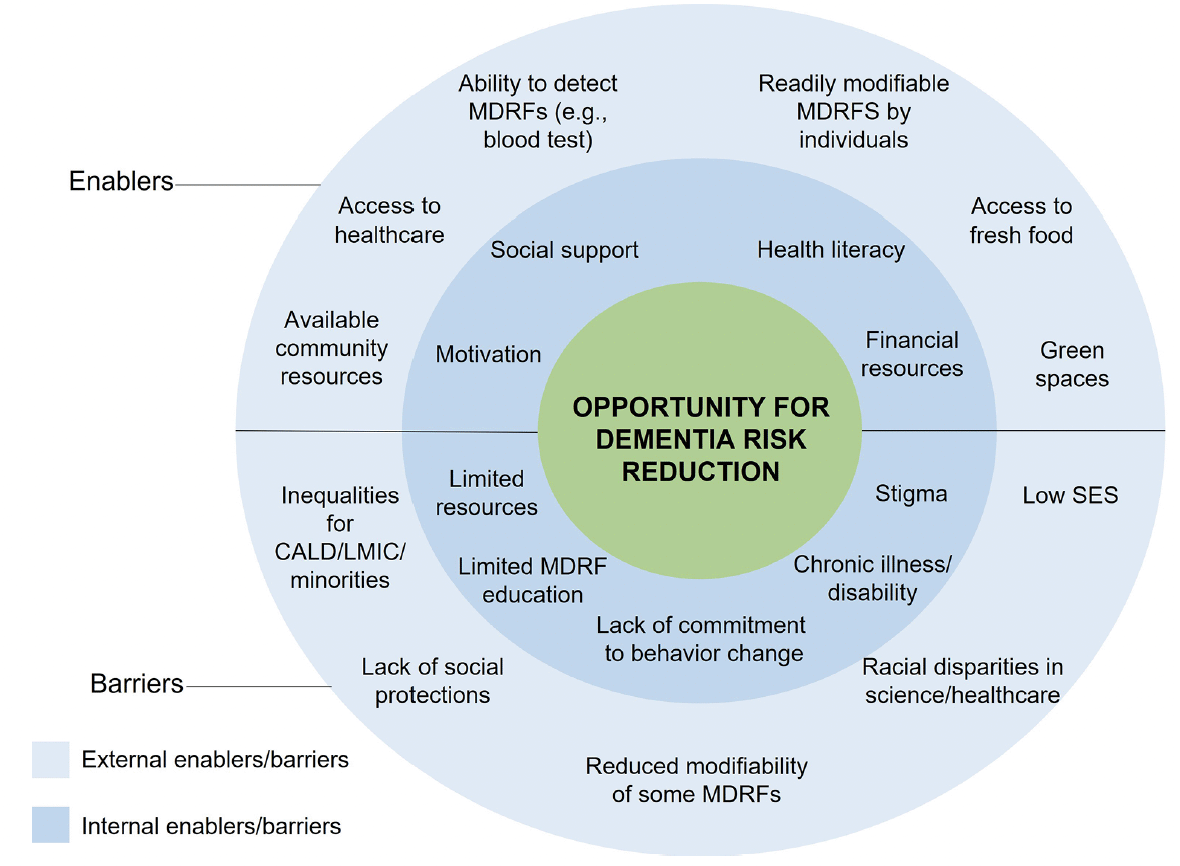
Figure 1. External and internal barriers and enablers that influence opportunity for individuals to engage in dementia risk reduction behavior
Note. MDRF=modifiable dementia risk factor; SES=socioeconomic status; CALD=Culturally and Linguistically Diverse; LMIC=low-middle income countries.
Internal barriers to dementia risk reduction
A key internal, or individual-based, barrier to dementia risk reduction is an individual’s knowledge and beliefs surrounding MDRFs. Large-scale surveys suggest that many in the general population are unaware of MDRFs and have relatively low dementia literacy (109, 110) which may lead to non-evidence-based beliefs and attitudes (e.g., dementia is a normal part of ageing) (111) and limit opportunity for MDRF identification which, for many MDRFs, relies on self-assessment. The limited knowledge of dementia and its risk factors may also contribute to internalized stigma and fear towards dementia, which may deter individuals from learning about and engaging in dementia risk reduction strategies (112–114). Individual barriers may also include limited resources such as lack of time or finances to dedicate towards dementia risk reduction strategies, limited support structures and diminished self-efficacy (i.e., individual’s belief in their abilities to accomplish a goal) (115). Chronic illness or difficulties with mobility may also act as barriers to targeting some MDRFs (i.e., physical inactivity) at the individual level (116). Lastly, motivation and commitment to long-term behavior change is essential to achieve and sustain dementia risk reduction, however, is difficult to maintain for many individuals (36, 117–119). Person-centred interventions that address these barriers will be essential to promoting long-term behaviour change, and, in turn, reducing dementia risk (18, 120).
Internal enablers to dementia risk reduction
Some enablers that are internal to the individual may increase the likelihood of engaging in and maintaining dementia risk reduction behavior, such as autonomous motivation (i.e., engaging in behavior which is perceived as consistent with one’s goals) (121). For example, higher autonomous motivation was a key mediator of successful outcomes across a range of obesity-related lifestyle interventions (122). Further, having social support, as a key social determinant of health, may also increase motivation and engagement in certain health promoting behaviors, such as physical activity (123). Additionally, having personal financial resources to expend may also increase the likelihood of individuals reducing their MDRFs (e.g., joining a gym, engaging in psychological therapy). Finally, an important internal enabler is health literacy, which is an individual’s cognitive and social ability to consume and understand health promoting information (124). Higher health literacy is associated with increased engagement in health behaviors, such as physical activity and healthy diet, as well as engagement in social activities (125), each of which are associated with dementia risk. Thus, individuals with higher health literacy may be more likely to engage in and maintain dementia risk reduction behavior.
External barriers to dementia risk reduction
While agency is important for individuals to modify and maintain healthy lifestyle patterns and behaviors, external and structural barriers can influence opportunity for engagement in dementia risk reduction behavior (126). These barriers include SES both at the personal (i.e., financial) and neighborhood level which influence access to healthcare, education, fresh and nutritional food and green spaces or facilities that promote physical activity and other healthy behaviors (24, 37, 127). Additionally, interventions for certain MDRFs can be expensive and not always accessible (e.g., CPAP machines, hearing aids, psychological treatment) (128, 129). Accessing these interventions can depend on the health care system and governmental support available within one’s country, and individuals’ ability to afford private health insurance. Further, ability to choose health promoting behaviors is dependent on basic social protections such as housing and welfare, which creates barriers for dementia risk reduction in homeless populations (130). Ability for individuals to choose health promoting behaviors is also dependent on the environment in which they live, which suggests that barriers to choosing health-promoting behaviors may increase with the increasing threat of the climate crisis (131).
The incidence of MDRFs will vary across countries and cultures, but many MDRFs cluster around inequalities – disproportionally affecting Culturally and Linguistically Diverse (CALD) populations and residents of low- or middle-income countries (LMICs) (132–134). Barriers to engaging in dementia risk reduction behaviors have in turn disproportionately affected these populations (135, 136). Most observational studies and intervention trials studying or targeting dementia risk typically recruit white, highly educated, and affluent participants. Whilst important insights are obtained from these studies, they are limited in their generalizability and do not account for these barriers (137, 138). This creates racial and socioeconomic disparities in dementia science and healthcare with potential to significantly interfere with dementia risk reduction practices for many individuals (139, 140). For example, whilst the current dementia risk reduction literature is replete with recommendations for the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet, this recommendation may not be appropriate for individuals from CALD populations who are unfamiliar with or whose culture does not align with foods recommended in the MIND diet. Additionally, foods recommended in the MIND diet may not be accessible on a global level and can be highly dependent on where one lives. Greater efforts to address barriers to dementia risk reduction, particularly in CALD populations, racial minorities and LMICs, will be essential, as this remains an important but underrecognized aspect of the dementia risk reduction field.
External enablers to dementia risk reduction
Many external enablers to dementia risk reduction relate to access to facilities or resources that promote healthy behaviors in the neighborhood (i.e., neighborhood advantage) (127) and/or country in which an individual lives. For example, having access to resources in the community such as gyms or libraries, access to fresh food in stores or supermarkets, and green spaces (e.g., parks in urban environments) (141) may increase the likelihood that individuals will engage in and sustain health promoting behaviors and dementia risk reduction strategies (142). Living in a country with universal healthcare may also increase access to medical interventions for many MDRFs including CPAP, medications for cardiovascular conditions, psychological treatment and hearing aids, particularly for those who may not be able to afford private health insurance (143). Further, access to healthcare would also increase detection of some MDRFs through more affordable and routine health checks such as blood pressure monitoring, blood tests for glucose and cholesterol, and hearing and eyesight checks.
Finally, the higher level of modifiability (i.e., modifiable at the individual level) of many MDRFs may also act as an external enabler as this increases feasibility of these MDRFs being targeted by individuals seeking to reduce their dementia risk.
Conclusion
The current review extends previous reports such as the Lancet Commission Report by providing a formal framework to characterize the different levels of modifiability of MDRFs for application in studies aiming to inform dementia risk reduction and prevention strategies, such as intervention trials, guidelines and implementation into clinical practice. This framework also highlighted that the identification of MDRFs also requires different resources which can also influence engagement and achievement of MDRF modification and dementia risk reduction. MDRFs that are modifiable with intervention at the societal or community level require population-based strategies such as government policy change. MDRFs that are modifiable with intervention at the individual level have greater potential for reducing dementia risk and can be targeted more readily in intervention trials and in individuals’ daily lives. This review also proposes that the ideal life stage for intervention of most MDRFs by individuals seeking to reduce their dementia risk is midlife (e.g., aged 45-65 years) (10) or earlier. However, there remain important external (population-based) and internal (individual-based) barriers to reducing MDRFs. It has been proposed that a population-based approach is essential to achieve meaningful and equitable dementia risk reduction (144). It may take a village to prevent dementia. However, large-scale governmental and policy changes take time, effort, and resources to implement and may not address important internal barriers to dementia risk reduction (i.e., motivation and commitment for behavior change). It is thus essential to take both a population-based (144) and a person-centred (119) approach to ensuring that individuals are empowered, supported, and have the means, knowledge and tools necessary to reduce their readily modifiable dementia risk factors.
Funding acknowledgement: L Bransby is supported by a Dementia Australia Research Foundation PhD Scholarship. YY Lim is supported by a National Health and Medical Research Council (NHMRC) Career Development Fellowship (GNT1162645) and an NHMRC Emerging Leadership Grant (GNT2009550). E Rosenich is supported by an NHMRC Boosting Dementia Research Initiative grant (GNT1171816) and an Alzheimer’s Association Research Fellowship (23AARF-1025519).
Conflict of interest disclosure: On behalf of all authors, the corresponding author states that there is no conflict of interest.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: Triad of risk of Alzheimer’s disease. J Steroid Biochem Mol Biol. 2016;160:134-147. doi:10.1016/j.jsbmb.2016.03.012
2. Franks KH, Bransby L, Saling MM, Pase MP. Association of Stress with Risk of Dementia and Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2021;82(4):1573-1590. doi:10.3233/JAD-210094
3. Cao L, Tan L, Wang HF, et al. Dietary Patterns and Risk of Dementia: a Systematic Review and Meta-Analysis of Cohort Studies. Mol Neurobiol. 2016;53(9):6144-6154. doi:10.1007/s12035-015-9516-4
4. Moran C, Beare R, Wang W, Callisaya M, Srikanth V, Initiative (ADNI) for the ADN. Type 2 diabetes mellitus, brain atrophy, and cognitive decline. Neurology. 2019;92(8):e823-e830. doi:10.1212/WNL.0000000000006955
5. Wysocki M, Luo X, Schmeidler J, et al. Hypertension is Associated With Cognitive Decline in Elderly People at High Risk for Dementia. Am J Geriatr Psychiatry. 2012;20(2):179-187. doi:10.1097/JGP.0b013e31820ee833
6. He F, Tang JJ, Zhang T, et al. Impact of Air Pollution on Cognitive Impairment in Older People: A Cohort Study in Rural and Suburban China. J Alzheimers Dis. 2020;77(4):1671-1679. doi:10.3233/JAD-200587
7. Wang HF, Zhang W, Rolls ET, et al. Hearing impairment is associated with cognitive decline, brain atrophy and tau pathology. EBioMedicine. 2022;86:104336. doi:10.1016/j.ebiom.2022.104336
8. Jia L, Xu H, Chen S, et al. The APOE ε4 exerts differential effects on familial and other subtypes of Alzheimer’s disease. Alzheimers Dement. 2020;16(12):1613-1623. doi:10.1002/alz.12153
9. van der Lee SJ, Wolters FJ, Ikram MK, et al. The effect of APOE and other common genetic variants on the onset of Alzheimer’s disease and dementia: a community-based cohort study. Lancet Neurol. 2018;17(5):434-444. doi:10.1016/S1474-4422(18)30053-X
10. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet Lond Engl. 2020;396(10248):413-446. doi:10.1016/S0140-6736(20)30367-6
11. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet Lond Engl. 2015;385(9984):2255-2263. doi:10.1016/S0140-6736(15)60461-5
12. Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14(11):653-666. doi:10.1038/s41582-018-0070-3
13. Coley N, Giulioli C, Aisen PS, Vellas B, Andrieu S. Randomised controlled trials for the prevention of cognitive decline or dementia: A systematic review. Ageing Res Rev. 2022;82:101777. doi:10.1016/j.arr.2022.101777
14. Rosenberg A, Mangialasche F, Ngandu T, Solomon A, Kivipelto M. Multidomain Interventions to Prevent Cognitive Impairment, Alzheimer’s Disease, and Dementia: From FINGER to World-Wide FINGERS. J Prev Alzheimers Dis. 2020;7(1):29-36. doi:10.14283/jpad.2019.41
15. World Health Organization. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. World Health Organization; 2019. Accessed April 29, 2023. https://apps.who.int/iris/handle/10665/312180
16. Altomare D, Molinuevo JL, Ritchie C, et al. Brain Health Services: organization, structure, and challenges for implementation. A user manual for Brain Health Services-part 1 of 6. Alzheimers Res Ther. 2021;13(1):168. doi:10.1186/s13195-021-00827-2
17. Heffernan M, Andrews G, Fiatarone Singh MA, et al. Maintain Your Brain: Protocol of a 3-Year Randomized Controlled Trial of a Personalized Multi-Modal Digital Health Intervention to Prevent Cognitive Decline Among Community Dwelling 55 to 77 Year Olds. J Alzheimers Dis JAD. 2019;70(s1):S221-S237. doi:10.3233/JAD-180572
18. Lim YY, Ayton D, Perin S, et al. An Online, Person-Centered, Risk Factor Management Program to Prevent Cognitive Decline: Protocol for A Prospective Behavior-Modification Blinded Endpoint Randomized Controlled Trial. J Alzheimers Dis. 2021;83(4):1603-1622. doi:10.3233/JAD-210589
19. Milne R, Altomare D, Ribaldi F, et al. Societal and equity challenges for Brain Health Services. A user manual for Brain Health Services—part 6 of 6. Alzheimers Res Ther. 2021;13(1):173. doi:10.1186/s13195-021-00885-6
20. Kröger E, Andel R, Lindsay J, Benounissa Z, Verreault R, Laurin D. Is complexity of work associated with risk of dementia? The Canadian Study of Health And Aging. Am J Epidemiol. 2008;167(7):820-830. doi:10.1093/aje/kwm382
21. Xu C, Apostolova LG, Oblak AL, Gao S. Association of Hypercholesterolemia with Alzheimer’s Disease Pathology and Cerebral Amyloid Angiopathy. J Alzheimers Dis JAD. 2020;73(4):1305-1311. doi:10.3233/JAD-191023
22. Santabárbara J, Lipnicki DM, Villagrasa B, Lobo E, Lopez-Anton R. Anxiety and risk of dementia: Systematic review and meta-analysis of prospective cohort studies. Maturitas. 2019;119:14-20. doi:10.1016/j.maturitas.2018.10.014
23. Machado A, Herrera AJ, de Pablos RM, et al. Chronic stress as a risk factor for Alzheimer’s disease. Rev Neurosci. 2014;25(6):785-804. doi:10.1515/revneuro-2014-0035
24. George KM, Lutsey PL, Kucharska-Newton A, et al. Life-Course Individual and Neighborhood Socioeconomic Status and Risk of Dementia in the Atherosclerosis Risk in Communities Neurocognitive Study. Am J Epidemiol. 2020;189(10):1134-1142. doi:10.1093/aje/kwaa072
25. Solfrizzi V, Panza F, Frisardi V, et al. Diet and Alzheimer’s disease risk factors or prevention: the current evidence. Expert Rev Neurother. 2011;11(5):677-708. doi:10.1586/ern.11.56
26. Akbaraly TN, Portet F, Fustinoni S, et al. Leisure activities and the risk of dementia in the elderly: results from the Three-City Study. Neurology. 2009;73(11):854-861. doi:10.1212/WNL.0b013e3181b7849b
27. Sabia S, Fayosse A, Dumurgier J, et al. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. 2021;12(1):2289. doi:10.1038/s41467-021-22354-2
28. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373-377. doi:10.1126/science.1241224
29. Winer JR, Deters KD, Kennedy G, et al. Association of Short and Long Sleep Duration With Amyloid-β Burden and Cognition in Aging. JAMA Neurol. 2021;78(10):1187-1196. doi:10.1001/jamaneurol.2021.2876
30. Andrade AG, Bubu OM, Varga AW, Osorio RS. The Relationship between Obstructive Sleep Apnea and Alzheimer’s Disease. J Alzheimers Dis JAD. 2018;64(s1):S255-S270. doi:10.3233/JAD-179936
31. Ma LZ, Zhang YR, Li YZ, et al. Cataract, Cataract Surgery, and Risk of Incident Dementia: A Prospective Cohort Study of 300,823 Participants. Biol Psychiatry. 2023;93(9):810-819. doi:10.1016/j.biopsych.2022.06.005
32. Kremen WS, Beck A, Elman JA, et al. Influence of young adult cognitive ability and additional education on later-life cognition. Proc Natl Acad Sci. 2019;116(6):2021-2026. doi:10.1073/pnas.1811537116
33. Dotson VM, Kitner-Triolo MH, Evans MK, Zonderman AB. Effects of race and socioeconomic status on the relative influence of education and literacy on cognitive functioning. J Int Neuropsychol Soc JINS. 2009;15(4):580-589. doi:10.1017/S1355617709090821
34. Boots EA, Schultz SA, Almeida RP, et al. Occupational Complexity and Cognitive Reserve in a Middle-Aged Cohort at Risk for Alzheimer’s Disease. Arch Clin Neuropsychol Off J Natl Acad Neuropsychol. 2015;30(7):634-642. doi:10.1093/arclin/acv041
35. Darin-Mattsson A, Fors S, Kåreholt I. Different indicators of socioeconomic status and their relative importance as determinants of health in old age. Int J Equity Health. 2017;16(1):173. doi:10.1186/s12939-017-0670-3
36. Stormacq C, Van den Broucke S, Wosinski J. Does health literacy mediate the relationship between socioeconomic status and health disparities? Integrative review. Health Promot Int. 2019;34(5):e1-e17. doi:10.1093/heapro/day062
37. Röhr S, Rodriguez FS, Siemensmeyer R, Müller F, Romero-Ortuno R, Riedel-Heller SG. How can urban environments support dementia risk reduction? A qualitative study. Int J Geriatr Psychiatry. 2022;37(1). doi:10.1002/gps.5626
38. Zahodne LB. Biopsychosocial pathways in dementia inequalities: Introduction to the Michigan Cognitive Aging Project. Am Psychol. 2021;76:1470-1481. doi:10.1037/amp0000936
39. Majoka MA, Schimming C. Effect of Social Determinants of Health on Cognition and Risk of Alzheimer Disease and Related Dementias. Clin Ther. 2021;43(6):922-929. doi:10.1016/j.clinthera.2021.05.005
40. Deckers K, Cadar D, van Boxtel MPJ, Verhey FRJ, Steptoe A, Köhler S. Modifiable Risk Factors Explain Socioeconomic Inequalities in Dementia Risk: Evidence from a Population-Based Prospective Cohort Study. J Alzheimers Dis JAD. 2019;71(2):549-557. doi:10.3233/JAD-190541
41. Wang B, Ho SSH, Ho KF, et al. An Environmental Chamber Study of the Characteristics of Air Pollutants Released from Environmental Tobacco Smoke. Aerosol Air Qual Res. 2012;12(6):1269-1281. doi:10.4209/aaqr.2011.11.0221
42. Kilian J, Kitazawa M. The emerging risk of exposure to air pollution on cognitive decline and Alzheimer’s disease – Evidence from epidemiological and animal studies. Biomed J. 2018;41(3):141-162. doi:10.1016/j.bj.2018.06.001
43. Brønnum-Hansen H, Bender AM, Andersen ZJ, et al. Assessment of impact of traffic-related air pollution on morbidity and mortality in Copenhagen Municipality and the health gain of reduced exposure. Environ Int. 2018;121:973-980. doi:10.1016/j.envint.2018.09.050
44. Schraufnagel DE, Balmes JR, De Matteis S, et al. Health Benefits of Air Pollution Reduction. Ann Am Thorac Soc. 2019;16(12):1478-1487. doi:10.1513/AnnalsATS.201907-538CME
45. Erickson LD, Gale SD, Anderson JE, Brown BL, Hedges DW. Association between Exposure to Air Pollution and Total Gray Matter and Total White Matter Volumes in Adults: A Cross-Sectional Study. Brain Sci. 2020;10(3):164. doi:10.3390/brainsci10030164
46. Galgano M, Toshkezi G, Qiu X, Russell T, Chin L, Zhao LR. Traumatic Brain Injury. Cell Transplant. 2017;26(7):1118-1130. doi:10.1177/0963689717714102
47. Peeters W, van den Brande R, Polinder S, et al. Epidemiology of traumatic brain injury in Europe. Acta Neurochir (Wien). 2015;157(10):1683-1696. doi:10.1007/s00701-015-2512-7
48. Sarmiento K, Thomas KE, Daugherty J, et al. Emergency Department Visits for Sports- and Recreation-Related Traumatic Brain Injuries Among Children — United States, 2010–2016. Morb Mortal Wkly Rep. 2019;68(10):237-242. doi:10.15585/mmwr.mm6810a2
49. Nordström A, Nordström P. Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study. PLOS Med. 2018;15(1):e1002496. doi:10.1371/journal.pmed.1002496
50. Walsh S, Wallace L, Kuhn I, et al. Are Population-Level Approaches to Dementia Risk Reduction Under-Researched? A Rapid Review of the Dementia Prevention Literature. J Prev Alzheimers Dis. Published online May 15, 2023. doi:10.14283/jpad.2023.57
51. Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. 2020;30(3):160-164. doi:10.1016/j.tcm.2019.05.003
52. Birtcher KK, Ballantyne CM. Measurement of Cholesterol. Circulation. 2004;110(11):e296-e297. doi:10.1161/01.CIR.0000141564.89465.4E
53. Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet Lond Engl. 1998;352(9137):1347-1351. doi:10.1016/s0140-6736(98)03086-4
54. Zhang X, Wen J, Zhang Z. Statins use and risk of dementia: A dose-response meta analysis. Medicine (Baltimore). 2018;97(30):e11304. doi:10.1097/MD.0000000000011304
55. Ndanuko RN, Tapsell LC, Charlton KE, Neale EP, Batterham MJ. Dietary Patterns and Blood Pressure in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv Nutr Bethesda Md. 2016;7(1):76-89. doi:10.3945/an.115.009753
56. Najjar RS, Moore CE, Montgomery BD. A defined, plant-based diet utilized in an outpatient cardiovascular clinic effectively treats hypercholesterolemia and hypertension and reduces medications. Clin Cardiol. 2018;41(3):307-313. doi:10.1002/clc.22863
57. Li XY, Zhang M, Xu W, et al. Midlife Modifiable Risk Factors for Dementia: A Systematic Review and Meta-analysis of 34 Prospective Cohort Studies. Curr Alzheimer Res. 2019;16(14):1254-1268. doi:10.2174/1567205017666200103111253
58. Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28(1):75-80. doi:10.1159/000231980
59. Walker KA, Sharrett AR, Wu A, et al. Association of Midlife to Late-Life Blood Pressure Patterns With Incident Dementia. JAMA. 2019;322(6):535-545. doi:10.1001/jama.2019.10575
60. US Preventive Services Task Force. Screening for Prediabetes and Type 2 Diabetes: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;326(8):736-743. doi:10.1001/jama.2021.12531
61. Alkhatib A, Tsang C, Tiss A, et al. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients. 2017;9(12):E1310. doi:10.3390/nu9121310
62. Ninomiya T. Diabetes Mellitus and Dementia. Curr Diab Rep. 2014;14(5):487. doi:10.1007/s11892-014-0487-z
63. Swift DL, McGee JE, Earnest CP, Carlisle E, Nygard M, Johannsen NM. The Effects of Exercise and Physical Activity on Weight Loss and Maintenance. Prog Cardiovasc Dis. 2018;61(2):206-213. doi:10.1016/j.pcad.2018.07.014
64. Chao AM, Quigley KM, Wadden TA. Dietary interventions for obesity: clinical and mechanistic findings. J Clin Invest. 2021;131(1):140065. doi:10.1172/JCI140065
65. Wolfe BM, Kvach E, Eckel RH. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ Res. 2016;118(11):1844-1855. doi:10.1161/CIRCRESAHA.116.307591
66. Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66(3):336-342. doi:10.1001/archneurol.2008.582
67. Koliaki C, Liatis S, Kokkinos A. Obesity and cardiovascular disease: revisiting an old relationship. Metabolism. 2019;92:98-107. doi:10.1016/j.metabol.2018.10.011
68. Erickson KI, Hillman C, Stillman CM, et al. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med Sci Sports Exerc. 2019;51(6):1242-1251. doi:10.1249/MSS.0000000000001936
69. Kaczynski AT, Henderson KA. Environmental Correlates of Physical Activity: A Review of Evidence about Parks and Recreation. Leis Sci. 2007;29(4):315-354. doi:10.1080/01490400701394865
70. Lanier JB, Bury DC, Richardson SW. Diet and Physical Activity for Cardiovascular Disease Prevention. Am Fam Physician. 2016;93(11):919-924.
71. Kotz D, Batra A, Kastaun S. Smoking Cessation Attempts and Common Strategies Employed. Dtsch Arzteblatt Int. 2020;117(1-2):7-13. doi:10.3238/arztebl.2020.0007
72. Debanne SM, Bielefeld RA, Cheruvu VK, Fritsch T, Rowland DY. Alzheimer’s disease and smoking: bias in cohort studies. J Alzheimers Dis JAD. 2007;11(3):313-321. doi:10.3233/jad-2007-11308
73. Zwar N, Richmond R, Borland R, Stillman S, Cunningham M, Litt J. Smoking cessation guidelines for Australian general practice. Aust Fam Physician. 2005;34(6):461-466.
74. Charisis S, Ntanasi E, Yannakoulia M, et al. Diet Inflammatory Index and Dementia Incidence: A Population-Based Study. Neurology. 2021;97(24):e2381-e2391. doi:10.1212/WNL.0000000000012973
75. Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2015;11(9):1007-1014. doi:10.1016/j.jalz.2014.11.009
76. Szczechowiak K, Diniz BS, Leszek J. Diet and Alzheimer’s dementia – Nutritional approach to modulate inflammation. Pharmacol Biochem Behav. 2019;184:172743. doi:10.1016/j.pbb.2019.172743
77. Badimon L, Chagas P, Chiva-Blanch G. Diet and Cardiovascular Disease: Effects of Foods and Nutrients in Classical and Emerging Cardiovascular Risk Factors. Curr Med Chem. 2019;26(19):3639-3651. doi:10.2174/0929867324666170428103206
78. Wallhed Finn S, Bakshi AS, Andréasson S. Alcohol Consumption, Dependence, and Treatment Barriers: Perceptions Among Nontreatment Seekers with Alcohol Dependence. Subst Use Misuse. 2014;49. doi:10.3109/10826084.2014.891616
79. Knox J, Hasin DS, Larson FRR, Kranzler HR. Prevention, screening, and treatment for heavy drinking and alcohol use disorder. Lancet Psychiatry. 2019;6(12):1054-1067. doi:10.1016/S2215-0366(19)30213-5
80. Zhang A, Franklin C, Jing S, et al. The effectiveness of four empirically supported psychotherapies for primary care depression and anxiety: A systematic review and meta-analysis. J Affect Disord. 2019;245:1168-1186. doi:10.1016/j.jad.2018.12.008
81. Fournier JC, DeRubeis RJ, Hollon SD, Gallop R, Shelton RC, Amsterdam JD. Differential change in specific depressive symptoms during antidepressant medication or cognitive therapy. Behav Res Ther. 2013;51(7):392-398. doi:10.1016/j.brat.2013.03.010
82. Carek PJ, Laibstain SE, Carek SM. Exercise for the treatment of depression and anxiety. Int J Psychiatry Med. 2011;41(1):15-28. doi:10.2190/PM.41.1.c
83. Hofmann SG, Gómez AF. Mindfulness-Based Interventions for Anxiety and Depression. Psychiatr Clin North Am. 2017;40(4):739-749. doi:10.1016/j.psc.2017.08.008
84. Olaya B, Moneta MV, Miret M, Ayuso-Mateos JL, Haro JM. Course of depression and cognitive decline at 3-year follow-up: The role of age of onset. Psychol Aging. 2019;34(4):475-485. doi:10.1037/pag0000354
85. Santabárbara J, Lopez-Anton R, de la Cámara C, et al. Clinically significant anxiety as a risk factor for dementia in the elderly community. Acta Psychiatr Scand. 2019;139(1):6-14. doi:10.1111/acps.12966
86. Ouanes S, Popp J. High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front Aging Neurosci. 2019;11. Accessed July 28, 2023. https://www.frontiersin.org/articles/10.3389/fnagi.2019.00043
87. Zollars I, Poirier TI, Pailden J. Effects of mindfulness meditation on mindfulness, mental well-being, and perceived stress. Curr Pharm Teach Learn. 2019;11(10):1022-1028. doi:10.1016/j.cptl.2019.06.005
88. Johansson L, Guo X, Waern M, et al. Midlife psychological stress and risk of dementia: a 35-year longitudinal population study. Brain. 2010;133(8):2217-2224. doi:10.1093/brain/awq116
89. Shen C, Rolls E, Cheng W, et al. Associations of Social Isolation and Loneliness With Later Dementia. Neurology. Published online June 8, 2022:10.1212/WNL.0000000000200583. doi:10.1212/WNL.0000000000200583
90. Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11(11):1006-1012. doi:10.1016/S1474-4422(12)70191-6
91. Burke SL, Hu T, Spadola CE, Burgess A, Li T, Cadet T. Treatment of Sleep Disturbance May Reduce the Risk of Future Probable Alzheimer’s Disease. J Aging Health. 2019;31(2):322-342. doi:10.1177/0898264318795567
92. Irish LA, Kline CE, Gunn HE, Buysse DJ, Hall MH. The role of sleep hygiene in promoting public health: A review of empirical evidence. Sleep Med Rev. 2015;22:23-36. doi:10.1016/j.smrv.2014.10.001
93. Gandhi KD, Mansukhani MP, Silber MH, Kolla BP. Excessive Daytime Sleepiness. Mayo Clin Proc. 2021;96(5):1288-1301. doi:10.1016/j.mayocp.2020.08.033
94. Jc O, R M, Z S, Y X, S S, Jk W. A randomized controlled trial of mindfulness meditation for chronic insomnia. Sleep. 2014;37(9). doi:10.5665/sleep.4010
95. Wang C, Holtzman DM. Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2020;45(1):104-120. doi:10.1038/s41386-019-0478-5
96. Smagula SF, Jia Y, Chang CCH, Cohen A, Ganguli M. Trajectories of daytime sleepiness and their associations with dementia incidence. J Sleep Res. 2020;29(6):e12952. doi:10.1111/jsr.12952
97. Dunietz GL, Chervin RD, Burke JF, Conceicao AS, Braley TJ. Obstructive sleep apnea treatment and dementia risk in older adults. Sleep. 2021;44(9):zsab076. doi:10.1093/sleep/zsab076
98. Liguori C, Mercuri NB, Izzi F, et al. Obstructive Sleep Apnea is Associated With Early but Possibly Modifiable Alzheimer’s Disease Biomarkers Changes. Sleep. 2017;40(5). doi:10.1093/sleep/zsx011
99. Huang X, Tang S, Lyu X, Yang C, Chen X. Structural and functional brain alterations in obstructive sleep apnea: a multimodal meta-analysis. Sleep Med. 2019;54:195-204. doi:10.1016/j.sleep.2018.09.025
100. Yeo BSY, Song HJJMD, Toh EMS, et al. Association of Hearing Aids and Cochlear Implants With Cognitive Decline and Dementia: A Systematic Review and Meta-analysis. JAMA Neurol. 2023;80(2):134-141. doi:10.1001/jamaneurol.2022.4427
101. Yoo SH, Zein M. Vision Restoration: Cataract Surgery and Surgical Correction of Myopia, Hyperopia, and Presbyopia. Med Clin North Am. 2021;105(3):445-454. doi:10.1016/j.mcna.2021.01.002
102. Sarabandi A, Vatankhah S, Kamali M, Aryankhesal A. Essential components of rehabilitation services provided to visually impaired people. Clin Exp Optom. 2021;104(2):215-221. doi:10.1111/cxo.13121
103. Jiang F, Mishra SR, Shrestha N, et al. Association between hearing aid use and all-cause and cause-specific dementia: an analysis of the UK Biobank cohort. Lancet Public Health. 2023;8(5):e329-e338. doi:10.1016/S2468-2667(23)00048-8
104. Helfer KS, Jesse A. Hearing and speech processing in midlife. Hear Res. 2021;402:108097. doi:10.1016/j.heares.2020.108097
105. Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357-367. doi:10.1016/S1474-4422(13)70044-9
106. Sutphen CL, Jasielec MS, Shah AR, et al. Longitudinal Cerebrospinal Fluid Biomarker Changes in Preclinical Alzheimer Disease During Middle Age. JAMA Neurol. 2015;72(9):1029-1042. doi:10.1001/jamaneurol.2015.1285
107. Lioret S, Campbell KJ, McNaughton SA, et al. Lifestyle Patterns Begin in Early Childhood, Persist and Are Socioeconomically Patterned, Confirming the Importance of Early Life Interventions. Nutrients. 2020;12(3):724. doi:10.3390/nu12030724
108. Kim S, Sargent-Cox K, Cherbuin N, Anstey KJ. Development of the Motivation to Change Lifestyle and Health Behaviours for Dementia Risk Reduction Scale. Dement Geriatr Cogn Disord Extra. 2014;4(2):172-183. doi:10.1159/000362228
109. Vrijsen J, Matulessij TF, Joxhorst T, de Rooij SE, Smidt N. Knowledge, health beliefs and attitudes towards dementia and dementia risk reduction among the Dutch general population: a cross-sectional study. BMC Public Health. 2021;21(1):857. doi:10.1186/s12889-021-10913-7
110. Pugh E, Stewart J, Carter L, Calamia M, Carmichael O, Newton RL. Beliefs, Understanding, and Barriers Related to Dementia Research Participation Among Older African Americans. Alzheimer Dis Assoc Disord. 2022;36(1):52-57. doi:10.1097/WAD.0000000000000476
111. Siddiqui F, Nistala KRY, Quek CWN, et al. Knowledge, Attitudes, and Perceptions Toward Dementia Among Middle-Aged Singapore Residents. J Alzheimers Dis. 2022;86(1):231-244. doi:10.3233/JAD-215262
112. Hamieh N, Sharara E, Salibi N, Mrad P, Chaaya M. Public Knowledge of, Perceptions About and Attitudes Towards Dementia: A Cross-Sectional Survey Among Lebanese Primary Health Care Attenders. Community Ment Health J. 2019;55(8):1362-1368. doi:10.1007/s10597-019-00436-2
113. Hurzuk S, Farina N, Pattabiraman M, et al. Understanding, experiences and attitudes of dementia in India: A qualitative study. Dement Lond Engl. Published online August 14, 2022:14713012221118774. doi:10.1177/14713012221118774
114. Li X, Fang W, Su N, Liu Y, Xiao S, Xiao Z. Survey in Shanghai communities: the public awareness of and attitude towards dementia. Psychogeriatr Off J Jpn Psychogeriatr Soc. 2011;11(2):83-89. doi:10.1111/j.1479-8301.2010.00349.x
115. Strecher VJ, DeVellis BM, Becker MH, Rosenstock IM. The role of self-efficacy in achieving health behavior change. Health Educ Q. 1986;13(1):73-92. doi:10.1177/109019818601300108
116. Vancampfort D, Koyanagi A, Ward PB, et al. Chronic physical conditions, multimorbidity and physical activity across 46 low- and middle-income countries. Int J Behav Nutr Phys Act. 2017;14(1):6. doi:10.1186/s12966-017-0463-5
117. Kim S, Sargent-Cox KA, Anstey KJ. A qualitative study of older and middle-aged adults’ perception and attitudes towards dementia and dementia risk reduction. J Adv Nurs. 2015;71(7):1694-1703. doi:10.1111/jan.12641
118. Heger I, Deckers K, van Boxtel M, et al. Dementia awareness and risk perception in middle-aged and older individuals: baseline results of the MijnBreincoach survey on the association between lifestyle and brain health. BMC Public Health. 2019;19(1):678. doi:10.1186/s12889-019-7010-z
119. Bosco A, Jones KA, Di Lorito C, Stephan BCM, Orrell M, Oliveira D. Changing lifestyle for dementia risk reduction: Inductive content analysis of a national UK survey. PloS One. 2020;15(5):e0233039. doi:10.1371/journal.pone.0233039
120. Barker A, Cameron P, Flicker L, et al. Evaluation of RESPOND, a patient-centred program to prevent falls in older people presenting to the emergency department with a fall: A randomised controlled trial. PLoS Med. 2019;16(5):e1002807. doi:10.1371/journal.pmed.1002807
121. Hagger MS, Hardcastle SJ, Chater A, Mallett C, Pal S, Chatzisarantis NLD. Autonomous and controlled motivational regulations for multiple health-related behaviors: between- and within-participants analyses. Health Psychol Behav Med. 2014;2(1):565-601. doi:10.1080/21642850.2014.912945
122. Teixeira PJ, Carraça EV, Marques MM, et al. Successful behavior change in obesity interventions in adults: a systematic review of self-regulation mediators. BMC Med. 2015;13(1):84. doi:10.1186/s12916-015-0323-6
123. Lindsay Smith G, Banting L, Eime R, O’Sullivan G, van Uffelen JGZ. The association between social support and physical activity in older adults: a systematic review. Int J Behav Nutr Phys Act. 2017;14(1):56. doi:10.1186/s12966-017-0509-8
124. Friis K, Lasgaard M, Rowlands G, Osborne RH, Maindal HT. Health Literacy Mediates the Relationship Between Educational Attainment and Health Behavior: A Danish Population-Based Study. J Health Commun. 2016;21(sup2):54-60. doi:10.1080/10810730.2016.1201175
125. Geboers B, Reijneveld SA, Jansen CJM, de Winter AF. Health Literacy Is Associated With Health Behaviors and Social Factors Among Older Adults: Results from the LifeLines Cohort Study. J Health Commun. 2016;21(sup2):45-53. doi:10.1080/10810730.2016.1201174
126. Cockerham WC. Health Lifestyle Theory and the Convergence of Agency and Structure. J Health Soc Behav. 2005;46(1):51-67. doi:10.1177/002214650504600105
127. Pase MP, Rowsthorn E, Cavuoto MG, et al. Association of Neighborhood-Level Socioeconomic Measures With Cognition and Dementia Risk in Australian Adults. JAMA Netw Open. 2022;5(3):e224071. doi:10.1001/jamanetworkopen.2022.4071
128. Nogueira JF, Simonelli G, Giovini V, et al. Access to CPAP treatment in patients with moderate to severe sleep apnea in a Latin American City. Sleep Sci. 2018;11(3):174-182. doi:10.5935/1984-0063.20180032
129. Jilla AM, Johnson CE, Huntington-Klein N. Hearing aid affordability in the United States. Disabil Rehabil Assist Technol. 2023;18(3):246-252. doi:10.1080/17483107.2020.1822449
130. Babulal GM, Rani R, Adkins-Jackson P, Pearson AC, Williams MM. Associations between Homelessness and Alzheimer’s Disease and Related Dementia: A Systematic Review. J Appl Gerontol Off J South Gerontol Soc. Published online June 24, 2022:7334648221109747. doi:10.1177/07334648221109747
131. Haines A, Ebi KL, Smith KR, Woodward A. Health risks of climate change: act now or pay later. The Lancet. 2014;384(9948):1073-1075. doi:10.1016/S0140-6736(14)61659-7
132. Mukadam N, Sommerlad A, Huntley J, Livingston G. Population attributable fractions for risk factors for dementia in low-income and middle-income countries: an analysis using cross-sectional survey data. Lancet Glob Health. 2019;7(5):e596-e603. doi:10.1016/S2214-109X(19)30074-9
133. Schaich CL, Yeboah J, Espeland MA, et al. Association of Vascular Risk Scores and Cognitive Performance in a Diverse Cohort: The Multi-Ethnic Study of Atherosclerosis. J Gerontol A Biol Sci Med Sci. 2022;77(6):1208-1215. doi:10.1093/gerona/glab189
134. Patel D, Montayre J, Karamacoska D, Siette J. Progressing dementia risk reduction initiatives for culturally and linguistically diverse older adults in Australia. Australas J Ageing. Published online August 3, 2022. doi:10.1111/ajag.13117
135. Boughtwood D, Shanley C, Adams J, et al. Dementia information for culturally and linguistically diverse communities: sources, access and considerations for effective practice. Aust J Prim Health. 2012;18(3):190-196. doi:10.1071/PY11014
136. Hughson JA, Woodward-Kron R, Parker A, et al. A review of approaches to improve participation of culturally and linguistically diverse populations in clinical trials. Trials. 2016;17(1):263. doi:10.1186/s13063-016-1384-3
137. Scanlon JK, Wofford L, Fair A, Philippi D. Predictors of Participation in Clinical Research. Nurs Res. 2021;70(4):289-297. doi:10.1097/NNR.0000000000000513
138. Mooldijk SS, Licher S, Wolters FJ. Characterizing Demographic, Racial, and Geographic Diversity in Dementia Research: A Systematic Review. JAMA Neurol. 2021;78(10):1255-1261. doi:10.1001/jamaneurol.2021.2943
139. Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on Ethnic and Racial Disparities in Alzheimer’s Disease and Related Dementias: Update and Areas of Immediate Need. Alzheimers Dement J Alzheimers Assoc. 2019;15(2):292-312. doi:10.1016/j.jalz.2018.09.009
140. Wheeler SM, Bryant AS. Racial and Ethnic Disparities in Health and Health Care. Obstet Gynecol Clin North Am. 2017;44(1):1-11. doi:10.1016/j.ogc.2016.10.001
141. Paul LA, Hystad P, Burnett RT, et al. Urban green space and the risks of dementia and stroke. Environ Res. 2020;186:109520. doi:10.1016/j.envres.2020.109520
142. Dintica CS, Bahorik A, Xia F, Kind A, Yaffe K. Dementia Risk and Disadvantaged Neighborhoods. JAMA Neurol. Published online July 19, 2023. doi:10.1001/jamaneurol.2023.2120
143. Moreno-Serra R, Smith PC. Does progress towards universal health coverage improve population health? The Lancet. 2012;380(9845):917-923. doi:10.1016/S0140-6736(12)61039-3
144. Walsh S, Govia I, Wallace L, et al. A whole-population approach is required for dementia risk reduction. Lancet Healthy Longev. 2022;3(1):e6-e8. doi:10.1016/S2666-7568(21)00301-9
145. Hyun J, Hall CB, Katz MJ, et al. Education, Occupational Complexity, and Incident Dementia: A COSMIC Collaborative Cohort Study. J Alzheimers Dis JAD. 2022;85(1):179-196. doi:10.3233/JAD-210627
146. Sesker AA, O’Súilleabháin PS, Lee JH, et al. Pathways From Early-Life SES to Dementia Risk in Old Age: The Role of Personality. J Gerontol Ser B. 2022;77(5):850-859. doi:10.1093/geronb/gbab159
147. Ran J, Schooling CM, Han L, et al. Long-term exposure to fine particulate matter and dementia incidence: A cohort study in Hong Kong. Environ Pollut Barking Essex 1987. 2021;271:116303. doi:10.1016/j.envpol.2020.116303
148. McGrath ER, Beiser AS, DeCarli C, et al. Blood pressure from mid- to late life and risk of incident dementia. Neurology. 2017;89(24):2447-2454. doi:10.1212/WNL.0000000000004741
149. Iwagami M, Qizilbash N, Gregson J, et al. Blood cholesterol and risk of dementia in more than 1·8 million people over two decades: a retrospective cohort study. Lancet Healthy Longev. 2021;2(8):e498-e506. doi:10.1016/S2666-7568(21)00150-1
150. Barbiellini Amidei C, Fayosse A, Dumurgier J, et al. Association Between Age at Diabetes Onset and Subsequent Risk of Dementia. JAMA. 2021;325(16):1640-1649. doi:10.1001/jama.2021.4001
151. Karlsson IK, Lehto K, Gatz M, Reynolds CA, Dahl Aslan AK. Age-dependent effects of body mass index across the adult life span on the risk of dementia: a cohort study with a genetic approach. BMC Med. 2020;18(1):131. doi:10.1186/s12916-020-01600-2
152. Shimizu Y, Sawada N, Ihira H, et al. Alcohol consumption from midlife and risk of disabling dementia in a large population-based cohort study in Japan. Int J Geriatr Psychiatry. 2023;38(3):e5896. doi:10.1002/gps.5896
153. Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA. Midlife vs Late-Life Depressive Symptoms and Risk of Dementia: Differential Effects for Alzheimer Disease and Vascular Dementia. Arch Gen Psychiatry. 2012;69(5):493-498. doi:10.1001/archgenpsychiatry.2011.1481
154. Gimson A, Schlosser M, Huntley JD, Marchant NL. Support for midlife anxiety diagnosis as an independent risk factor for dementia: a systematic review. BMJ Open. 2018;8(4):e019399. doi:10.1136/bmjopen-2017-019399
155. Landau SM, Marks SM, Mormino EC, et al. Association of Lifetime Cognitive Engagement and Low β-Amyloid Deposition. Arch Neurol. 2012;69(5):623-629. doi:10.1001/archneurol.2011.2748
156. Sommerlad A, Sabia S, Singh-Manoux A, Lewis G, Livingston G. Association of social contact with dementia and cognition: 28-year follow-up of the Whitehall II cohort study. PLoS Med. 2019;16(8):e1002862. doi:10.1371/journal.pmed.1002862
157. Li M, Wang N, Dupre ME. Association between the self-reported duration and quality of sleep and cognitive function among middle-aged and older adults in China. J Affect Disord. 2022;304:20-27. doi:10.1016/j.jad.2022.02.039
158. Petersen JD, Wehberg S, Packness A, et al. Association of Socioeconomic Status With Dementia Diagnosis Among Older Adults in Denmark. JAMA Netw Open. 2021;4(5):e2110432. doi:10.1001/jamanetworkopen.2021.10432
159. Almetwally AA, Bin-Jumah M, Allam AA. Ambient air pollution and its influence on human health and welfare: an overview. Environ Sci Pollut Res Int. 2020;27(20):24815-24830. doi:10.1007/s11356-020-09042-2
160. Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ. Air Pollution and Dementia: A Systematic Review. J Alzheimers Dis JAD. 2019;70(s1):S145-S163. doi:10.3233/JAD-180631
161. Snowden TM, Hinde AK, Reid HMO, Christie BR. Does Mild Traumatic Brain Injury Increase the Risk for Dementia? A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2020;78(2):757-775. doi:10.3233/JAD-200662
162. Jung H, Yang PS, Kim D, et al. Associations of hypertension burden on subsequent dementia: a population-based cohort study. Sci Rep. 2021;11(1):12291. doi:10.1038/s41598-021-91923-8
163. Anstey KJ, Ashby-Mitchell K, Peters R. Updating the Evidence on the Association between Serum Cholesterol and Risk of Late-Life Dementia: Review and Meta-Analysis. J Alzheimers Dis JAD. 2017;56(1):215-228. doi:10.3233/JAD-160826
164. Petersmann A, Müller-Wieland D, Müller UA, et al. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp Clin Endocrinol Diabetes. 2019;127(S 1):S1-S7. doi:10.1055/a-1018-9078
165. Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63(7):1181-1186. doi:10.1212/01.wnl.0000140291.86406.d1
166. Alford S, Patel D, Perakakis N, Mantzoros CS. Obesity as a risk factor for Alzheimer’s disease: weighing the evidence. Obes Rev Off J Int Assoc Study Obes. 2018;19(2):269-280. doi:10.1111/obr.12629
167. Qizilbash N, Gregson J, Johnson ME, et al. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3(6):431-436. doi:10.1016/S2213-8587(15)00033-9
168. Sabia S, Dugravot A, Dartigues JF, et al. Physical activity, cognitive decline, and risk of dementia: 28 year follow-up of Whitehall II cohort study. BMJ. 2017;357:j2709. doi:10.1136/bmj.j2709
169. Więckowska-Gacek A, Mietelska-Porowska A, Wydrych M, Wojda U. Western diet as a trigger of Alzheimer’s disease: From metabolic syndrome and systemic inflammation to neuroinflammation and neurodegeneration. Ageing Res Rev. 2021;70:101397. doi:10.1016/j.arr.2021.101397
170. Anstey KJ, von Sanden C, Salim A, O’Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007;166(4):367-378. doi:10.1093/aje/kwm116
171. Xu W, Wang H, Wan Y, et al. Alcohol consumption and dementia risk: a dose-response meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(1):31-42. doi:10.1007/s10654-017-0225-3
172. Koch M, Fitzpatrick AL, Rapp SR, et al. Alcohol Consumption and Risk of Dementia and Cognitive Decline Among Older Adults With or Without Mild Cognitive Impairment. JAMA Netw Open. 2019;2(9):e1910319. doi:10.1001/jamanetworkopen.2019.10319
173. Ayuso-Mateos JL, Nuevo R, Verdes E, Naidoo N, Chatterji S. From depressive symptoms to depressive disorders: the relevance of thresholds. Br J Psychiatry J Ment Sci. 2010;196(5):365-371. doi:10.1192/bjp.bp.109.071191
174. Beaudreau SA, O’Hara R. Late-Life Anxiety and Cognitive Impairment: A Review. Am J Geriatr Psychiatry. 2008;16(10):790-803. doi:10.1097/JGP.0b013e31817945c3
175. Cohen S, Janicki-Deverts D, Miller GE. Psychological Stress and Disease. JAMA. 2007;298(14):1685-1687. doi:10.1001/jama.298.14.1685
176. Chen Y, Liang Y, Zhang W, Crawford JC, Sakel KL, Dong X. Perceived Stress and Cognitive Decline in Chinese-American Older Adults. J Am Geriatr Soc. 2019;67(S3):S519-S524. doi:10.1111/jgs.15606
177. Peterson RL, Gilsanz P, George KM, et al. Differences in association of leisure time activities and cognition in a racially/ethnically diverse cohort of older adults: Findings from the KHANDLE study. Alzheimers Dement N Y N. 2020;6(1):e12047. doi:10.1002/trc2.12047
178. Bransby L, Buckley RF, Rosenich E, et al. The relationship between cognitive engagement and better memory in midlife. Alzheimers Dement Amst Neth. 2022;14(1):e12278. doi:10.1002/dad2.12278
179. Kuiper JS, Zuidersma M, Oude Voshaar RC, et al. Social relationships and risk of dementia: A systematic review and meta-analysis of longitudinal cohort studies. Ageing Res Rev. 2015;22:39-57. doi:10.1016/j.arr.2015.04.006
180. Bubu OM, Umasabor-Bubu OQ, Turner AD, et al. Self-reported obstructive sleep apnea, amyloid and tau burden, and Alzheimer’s disease time-dependent progression. Alzheimers Dement J Alzheimers Assoc. Published online October 8, 2020. doi:10.1002/alz.12184
181. Sharma RA, Varga AW, Bubu OM, et al. Obstructive Sleep Apnea Severity Affects Amyloid Burden in Cognitively Normal Elderly. A Longitudinal Study. Am J Respir Crit Care Med. 2018;197(7):933-943. doi:10.1164/rccm.201704-0704OC
182. Chern A, Golub JS. Age-related hearing loss and dementia. Alzheimer Dis Assoc Disord. 2019;33(3):285-290. doi:10.1097/WAD.0000000000000325
183. Shang X, Zhu Z, Wang W, Ha J, He M. The Association between Vision Impairment and Incidence of Dementia and Cognitive Impairment: A Systematic Review and Meta-analysis. Ophthalmology. 2021;128(8):1135-1149. doi:10.1016/j.ophtha.2020.12.029
© The Authors 2023