A. Nwosu1, M. Qian2, J. Phillips3,4, C.A. Hellegers1, S. Rushia5, J. Sneed6, J.R. Petrella7, T.E. Goldberg3,4, D.P. Devanand3,4, P.M. Doraiswamy1,8
1. Neurocognitive Disorders Program, Department of Psychiatry and Behavioral Sciences, Duke University School of Medicine, Durham, NC, USA; 2. Department of Biostatistics, Mailman School of Public Health, Columbia University Medical Center, New York, NY, USA; 3. Area Brain Aging and Mental Health, New York State Psychiatric Institute, New York, NY, USA; 4. Department of Psychiatry, Columbia University Medical Center, New York, NY, USA; 5. Department of Psychiatry, University of Massachusetts Chan Medical School, Worcester, MA, USA; 6. Queens College, City University of New York, New York, NY, USA; 7. Department of Radiology, Duke University School of Medicine, Durham, NC, USA; 8. Center for the Study of Aging and Human Development and the Division of Geriatrics, Duke School of Medicine, Durham, NC, USA
Corresponding Author: Adaora Nwosu, Neurocognitive Disorders Program, Department of Psychiatry and Behavioral Sciences, Duke University School of Medicine, Durham, NC, USA, adaora.nwosu@duke.edu
J Prev Alz Dis 2024;1(11):149-154
Published online June 13, 2023, http://dx.doi.org/10.14283/jpad.2023.80
Abstract
BACKGROUND: African Americans with MCI may be at increased risk for dementia compared to Caucasians. The effect of race on the efficacy of cognitive training in MCI is unclear.
METHODS: We used data from a two-site, 78-week randomized trial of MCI comparing intensive, home-based, computerized training with Web-based cognitive games or Web-based crossword puzzles to examine the effect of race on outcomes. The study outcomes were changes from baseline in cognitive and functional scales as well as MRI-measured changes in hippocampal volume and cortical thickness. Analyses used linear models adjusted for baseline scores. This was an exploratory study.
RESULTS: A total of 105 subjects were included comprising 81 whites (77.1%) and 24 African Americans (22.8%). The effect of race on the change from baseline in ADAS-Cog-11 was not significant. The effect of race on change from baseline to week 78 in the Functional Activities Questionnaire (FAQ) was significant with African American participants’ FAQ scores showing greater improvements at weeks 52 and 78 (P = 0.009, P = 0.0002, respectively) than white subjects. Within the CCT cohort, FAQ scores for African American participants showed greater improvement between baseline and week 78, compared to white participants randomized to CCT (P = 0.006). There was no effect of race on the UPSA. There was no effect of race on hippocampal or cortical thickness outcomes.
CONCLUSIONS: Our preliminary findings suggest that web-based cognitive training programs may benefit African Americans with MCI at least as much as Caucasians, and highlight the need to further study underrepresented minorities in AD prevention trials. (Supported by the National Institutes of Health, National Institute on Aging; ClinicalTrials.gov number, NCT03205709.)
Key words: Race, ethnicity, health disparities, brain health, Alzheimer’s.
Introduction
African American communities are disproportionately affected by Alzheimer’s disease (AD) with the development of clinical AD being two times more likely than in Caucasians (1-5). Mild cognitive impairment (MCI) is a condition that precedes dementia, and those with amnestic MCI are at an increased likelihood of developing AD (6). African Americans may be at increased likelihood of developing MCI (7), and may experience a faster rate of cognitive decline, when diagnosed with MCI, in comparison to other races (1-5, 8). Although the reasons behind this disparity are not fully known, possible explanations range from racial differences in socio-economic factors such as access to education, lifestyle factors, genetics, cardio-metabolic disease risks as well as neurobiological processes such as cortical amyloid deposition (1-5, 8-14).
There has been increased interest in the use of computerized cognitive training through web or phone-based video games (CCT) as a therapeutic for mild cognitive impairment, as it has shown the potential to improve cognitive domains in older patients with both normal and impaired cognition (15-19). Additionally, further studies have demonstrated improvements in gait, cognitive processing, delayed memory, attention, and executive functioning in MCI patients engaging with CCT interventions (15-26). In a 78-week, controlled trial, we recently reported that computerized crossword puzzle training (CPT) was superior to computerized games in improving cognition and daily functioning and slowing atrophy in MCI patients (18).
Until a few years ago, clinical trials exploring cognitive benefits due to online brain training have largely been completed in Caucasians and Asians (20-26), with many studies failing to include data on the racial characteristics of the sample. In one systematic review of 31 studies on cognitive training, only 12 were found to report on the racial background of study samples (27) with the inclusion of non-white participants relatively limited to 75% of these studies. One study of a cognitive training program specific to African Americans at risk of falling found a clinically meaningful improvement in balance and gait (28). Another study, a sub-group analysis of the benefit of cognitive training in minority elder adults enrolled in the SeniorWISE trial (29), found that African Americans and Hispanic-identifying individuals had greater gains on the visuospatial memory test than white participants. Furthermore, African American participants improved in instrumental activities of daily living over the course of the study (29). To our knowledge, there are no other studies that have examined Caucasian versus African American differences in cognitive brain training outcomes in older adults.
The aim of this paper was to use data from a 78-week randomized clinical trial of cognitive training in MCI (Cognitive Training and Neuroplasticity in Mild Cognitive Impairment trial [COG-IT]) to examine differences between Caucasians and African Americans in cognitive, functional and brain atrophy outcomes.
Methods
Study Design and Subjects
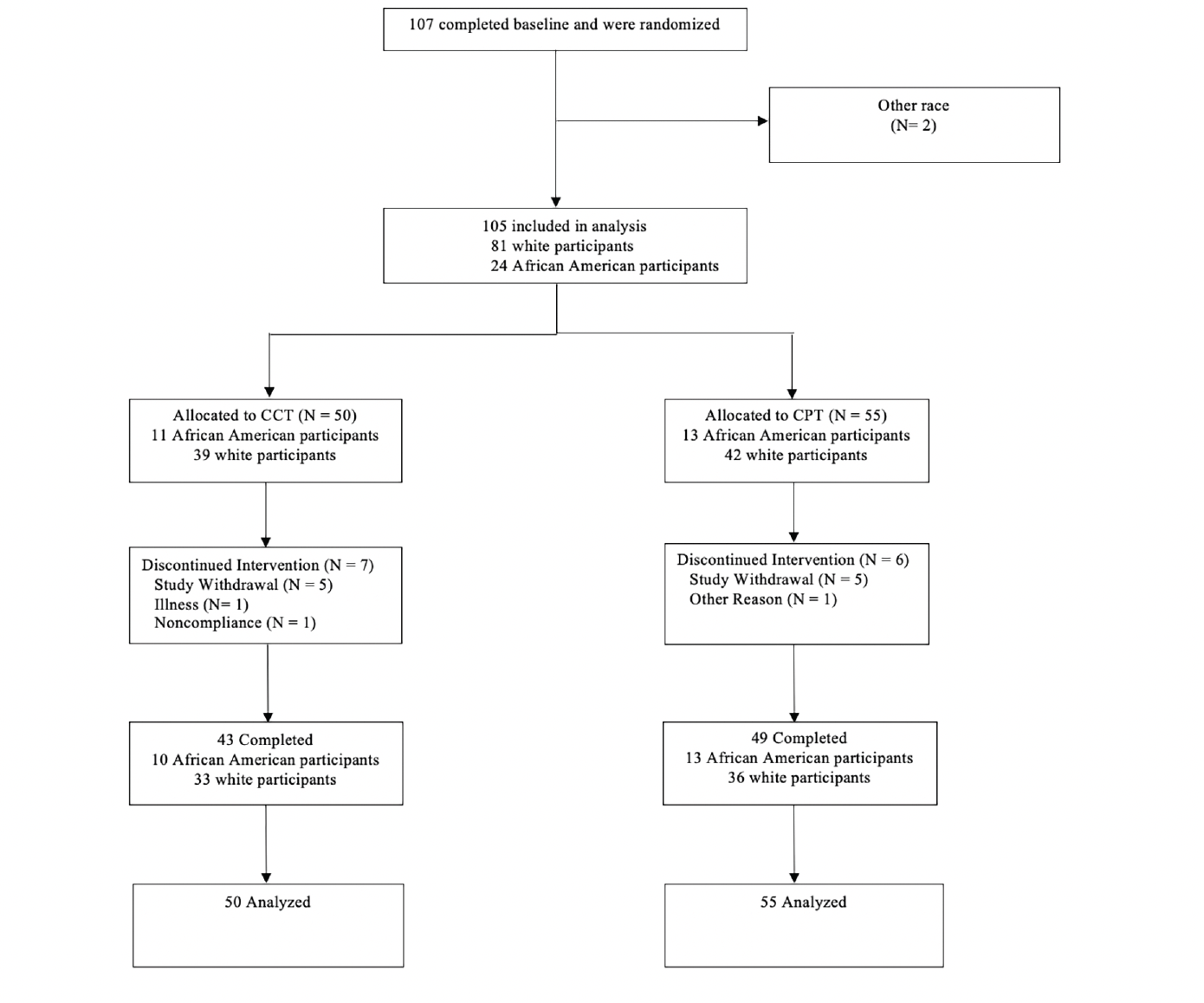
The COG-IT study design (18) and primary findings have been described elsewhere (18, 30). All enrolled participants provided written consent, and the study was approved by each site’s respective institutional IRBs. MCI participants were recruited using criteria described previously (18) and stratified by age and severity. Participants were then randomly assigned to either CCT or CPT training arms for this 78-week, single-blind, two-site study. The training was delivered through Lumosity, a web-based platform. In both study arms, participants completed four 30-minute training sessions per week during the first 12 weeks. After this period, participants completed booster sessions at predetermined time points. Participants were scheduled for five in-clinic visits (Weeks 0, 12, 32, 52, and 78) to complete one 30-minute training session and neuropsychological testing. At baseline and at week 78, participants completed a brain MRI scan for assessment of cortical thickness and hippocampal volume (18). Race was self-identified by participants. Of the 107 participants from the original study that met the trial’s inclusion criteria, 105 who were racially categorized as African American or Caucasian (white) were included in the analysis, irrespective of ethnicity. Data were available from four groups: African American participants assigned to CPT, African American participants assigned to CCT, white participants assigned to CPT, and white participants assigned to CCT.
Cognitive, Functional and MRI Outcomes
Our analysis evaluated the effect of race on cognitive, functional, and MRI outcomes. The cognitive outcome was the change in the Alzheimer’s Disease Assessment Scale-Cognitive subscale (ADAS-Cog-11) total score from baseline to week 78 (18). Functional outcomes included the change from baseline to week 78 on the University of California, San Diego Performance-Based Skills Assessment (UPSA) and the informant-reported Functional Activities Questionnaire (FAQ) (18). The UPSA measures performance in everyday activities such as financial and planning skills, while the FAQ assesses activities of daily living, including the participant’s ability to shop, manage bills, and meal preparation. MRI outcomes, collected via imaging at baseline and at week 78, were changes in cortical thickness and hippocampal volume. FAQ and UPSA were selected to include one informant based and one performance based measure, respectively. The MRI sample was slightly smaller due to the exclusion of subjects who failed imaging QC criteria (18); (Table 4).
Statistical Analysis
Using Wilcoxon’s rank sum test for continuous variables and Fisher’s exact test for categorical variables, baseline demographic, cognitive, and imaging data were compared between African American and white participants. As such this was an exploratory, secondary, data analysis from the COG-IT study. Linear mixed-effects models were employed to evaluate the effect of race, treatment, and their interaction on change in the cognitive and functional outcome measures (baseline minus week 78). Linear regression models evaluated the effect of race on MRI outcomes, including the change from baseline to week 78 (i.e., baseline minus week 78), on the hippocampal volume and cortical thickness. Both the linear mixed effects models and linear regression models were adjusted for baseline values of the outcome measures, site, sex, age, and years of education.
Results
Baseline Characteristics of Participants
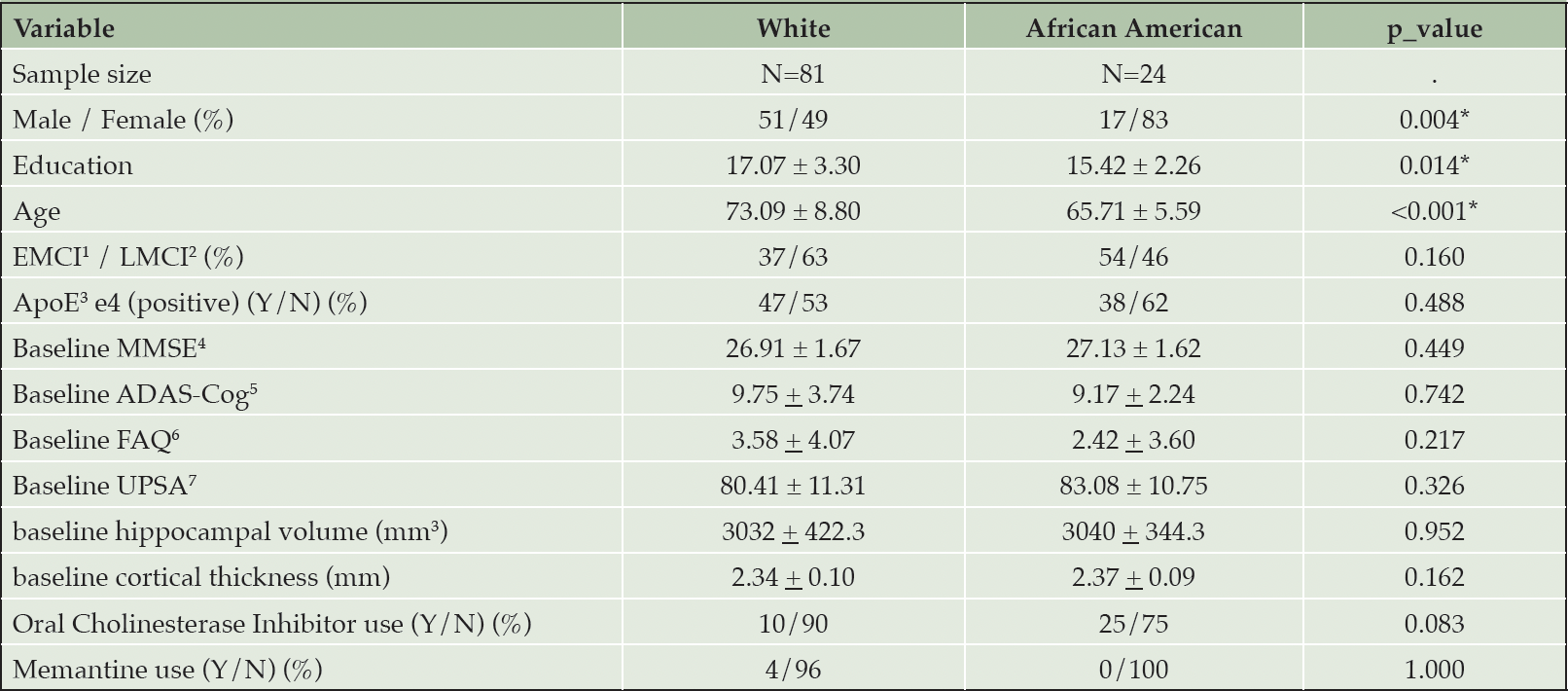
A total of 105 subjects were included, comprising 81 whites (77.1%) and 24 African Americans (22.8%) (Figure 1). African American participants were on average, eight years younger than white participants (Table 1) and had fewer years of education. There was also a greater percentage of males in the white cohort. Although there was a numerically greater percentage of EMCI participants among African Americans than whites, this was not statistically significant. Figure 1 depicts the flow of subjects throughout the study. Additional baseline comparisons of neuropsychological tests and brain morphometric features are shown in Table 1.
1. EMCI, early mild cognitive impairment; 2. LMCI, late mild cognitive impairment; 3. ApoE4, apolipoprotein E gene; 4. MMSE, Mini-Mental State Examination; 5. ADAS-Cog, Alzheimer’s Disease Assessment Scale-Cognitive subscale; 6. FAQ, Functional Activity Questionnaire; 7. the University of California, San Diego Performance-Based Skills Assessment
Cognitive and Functional Outcomes
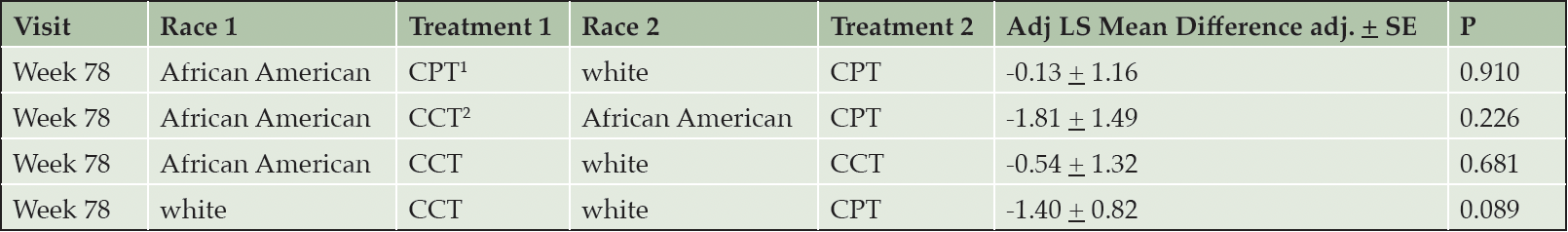
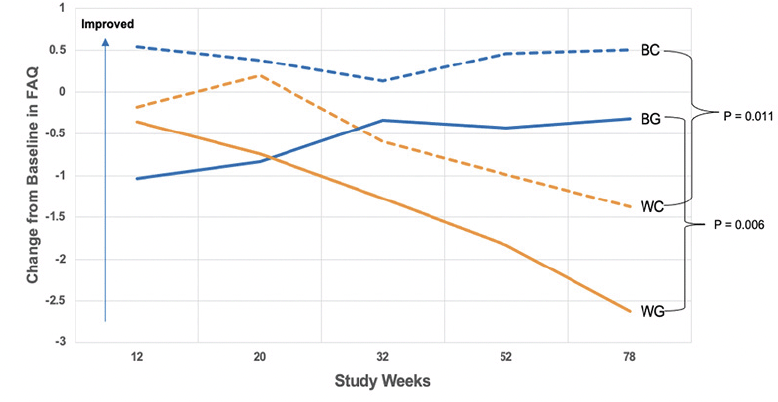
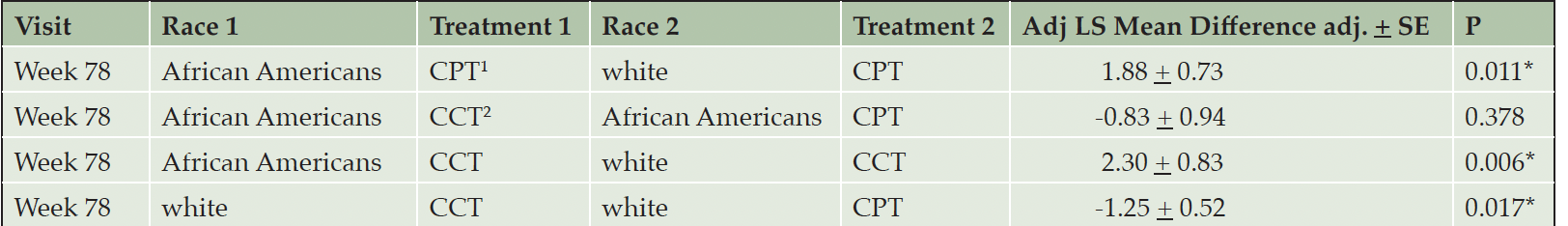
The cognitive outcome – change in ADAS-Cog score from baseline to week 78 – did not differ significantly between treatments within each race cohort (p=0.226 for African Americans, p=0.089 for white), nor between races within each treatment group (p=0.910 for CPT; p=0.681 for CCT; Table 2). There was an effect of race on the change in FAQ from baseline to week 78. Irrespective of treatment intervention, African American participants’ FAQ scores were consistent over the course of the study, with notable improvement from week 32 to week 78. Conversely, white participants worsened substantially, with statistically significant differences between the two cohorts at weeks 52 and 78 (P = 0.009, P = 0.0002, respectively). Within the CCT cohort, FAQ scores for African American participants improved between baseline and week 78, compared to white participants randomized to CCT (Figure 2, Table 3). This difference was also notable in the CPT cohort (Figure 2, Table 3). Within the white cohort, the CCT group worsened more than CPT patients(Table 3). There was no effect of race on the UPSA.
1. CPT, crossword puzzle training; 2. CCT, computerized cognitive training. LS mean differences are depicting baseline minus week 78. None of the comparisons were significant. Please see text for details.
BC = African American participants randomized to CPT; BG = African American participants randomized to CCT; WC = white participants randomized to CPT; WG = white participants randomized to CCT. The horizontal axis illustrates study time points going from Week 12 to Week 78, and the vertical axis depicts the mean change on the FAQ from baseline to Week 78, with a higher value on the y-axis meaning improvement. At week 78, BC had a higher mean change in FAQ scores than WC. Similarly, BG experienced a greater improvement in FAQ than WG at week 78.
1. CPT, crossword puzzle training; 2. CCT, computerized cognitive training. LS mean differences are depicting baseline minus week 78. Within the CPT cohort, African Americans showed significant improvement in FAQ outcomes at week 78 than white participants. At week 78, white participants randomized to CPT significantly improved on FAQ outcomes than white individuals randomized to CCT.
MRI Outcomes
There was no effect of race on the MRI outcomes. Regarding hippocampal volume change, there were no significant between African American and white participants. Within the white cohort, participants randomized to CCT exhibited a greater decrease in hippocampal volume than those in the CPT intervention from baseline to week 78 (Table 4). Similarly, there was no effect of race on cortical thickness. Within the white cohort, those randomized to CCT showed a greater decrease in cortical thickness from baseline to week 78 than those randomized to CPT (Table 4).
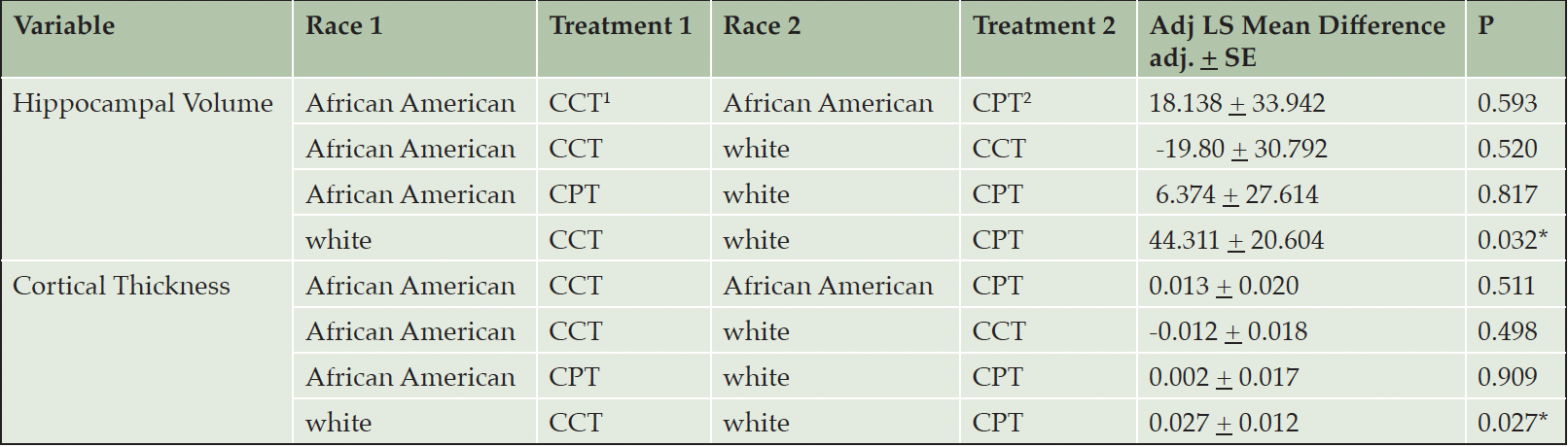
Table 4. Changes in Hippocampal Volume and Cortical Thickness across race and study intervention at week 78
1. CCT, computerized cognitive training; 2. CPT, crossword puzzle training. LS mean differences are depicting baseline minus week 78. There was no effect of race on hippocampal volume. However, within the white cohort, those randomized to CPT had significant improvements in hippocampal volume and cortical thickness at week 78 than those randomized to CCT.
Discussion
To our knowledge, this is the first study to examine the effects of race on the cognitive, functional, and MRI outcomes in a randomized trial of MCI subjects engaging in either CCT or CPT. We did not find an effect of race on the cognitive or MRI outcomes but found that African American participants showed greater functional improvements on an informant-reported scale (FAQ) over the 78 weeks compared to white participants. Combined with our prior finding of the beneficial effects of crossword puzzle training (18), these initial data suggest that African Americans are likely to benefit at least as much as Caucasians from such training.
The mechanism(s) underlying the effect of race on functional outcomes is unknown. Our findings that African American participants received greater functional benefits (on FAQ) than Caucasians from computerized cognitive training appears concordant with a prior trial that also showed greater improvements of IADLs in African American participants (26) – suggesting they may represent a true differential functional benefit. Our observation of racial differences in outcomes on the informant-based FAQ but not on the performance-based UPSA as well as the worsening seen in Caucasians was unexpected. A study of 4284 MCI participants and their informants from the National Alzheimer’s Coordinating Center Uniform Data Set found that FAQ scores were rated lowest (less impaired) among Black/African American informants as compared to all other racial/ethnic groups (31). This study also found that FAQ scores were higher among informants with higher levels of education (31). Another study found that black caregivers generally overrated, and white caregivers underrated, their care recipient’s cognitive ability (32). Hence, in addition to true differences, there is also the possibility of an informant bias by race and educational level.
Overall, these findings must be interpreted as preliminary, as the two ethnic groups in the sample were not equivalent in size, and at baseline, the proportion of EMCI was numerically (but not statistically) higher in African American participants than in white participants. Additionally, we did not have enough statistical power to test other moderator variables by race such as engagement, socioeconomic status, genetics, or medical comorbidities. Further studies in larger samples are needed to replicate and elucidate possible mechanisms.
The strengths of our study are the use of data from prospective clinical trial, rigorous selection of study subjects, relatively low attrition rates, long study duration of 78-weeks, use of well-validated outcomes measures and study design comparing two cognitive training strategies in wide use by older adults. Limitations including the relatively small sample size, failure to study other races, exploratory nature of analyses and the lack of a control condition. Hence, our findings must be interpreted in this context and must be viewed as preliminary pending replication.
To summarize, this is the first study to examine the effect of race on cognitive, functional, and imaging outcomes in MCI participants following two types of computerized cognitive training. Our study suggests that African Americans are likely to receive at least as much benefit as white participants and highlights the need to further study underrepresented people in AD preventive trials.
Funding statement: This work is supported by National Institute on Aging NIH grant number 1R01AG052440. Lumos Labs provided the computerized web-based platform at no cost, and was not involved in the final design, analyses, or drafting of the manuscript.
Competing interest: DPD has received research grants from the National Institute on Aging and the Alzheimer’s Assocation and has served as a consultant on scientific advisory boards to Acadia, Corium, Genentech, TauRx, and a DSMB for BioXcel. PMD has received research grants from the National Institute on Aging, DARPA, DOD, ONR, Salix, Avanir, Avid, Cure Alzhaimer’s Fund, Karen L. Wrenn Trust, Steve Aoki Foundation, and advisory fees from Apollo, Brain Forum, Clearview, Lumos, Neuroglee, Otsuka, Verily, Vitakey, Sermo, Lilly, Nutricia, and Transposon. PMD is a co-inventor on patents for the diagnosis or treatment of Alzheimer disease and a patent for infection detection. PMD owns shares in several biotechnology companies whose products are not discussed here. Other authors have received grant support from NIH and report no other competing interests. Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Acknowlexgment: We thank all the participants and their informants in the trial. We thank Jessica D’Antonio, Laura Simon-Pearson, Charlie Ndouli and Kaylee Bodner for their assistance in data collection and conduct of the study. We thank Dr. William McDonald of Emory University, Dr. Anand Kumar of University of Illinois and Dr. Stephen Rapp of Wake Forest University for their valuable oversight as members of the Data Safety and Monitoring Board. We thank Lumos Labs for providing the computerized web-based platform for the interventions in this trial.
Ethical standards: The study was approved by all site IRBs and all subjects gave written informed consent.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Shin J, & Doraiswamy PM. (2016). Underrepresentation of african-americans in alzheimer’s trials: A call for affirmative action. Front Aging Neurosci 8, 123-123.
2. Alzheimer’s Association (2023). 2023 Alzheimer’s disease facts and figures. Alzheimers Dement 19, 1598-1695.
3. Demirovic, J, Prineas R, Loewenstein D, Bean J, Duara R, Sevush S, Szapocznik J. (2003). Prevalence of dementia in three ethnic groups: The south florida program on aging and health. Ann Epidemiol 13, 472-478.
4. Fortune DA, Lang R, Cook S, Byrd GS. (2013). African Americans and Alzheimer’s disease: Role of health educators in addressing this silent epidemic. American Journal of Health Studies, 28, 92.
5. Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster A, Ayonayon H, Simonsick E, Hlth ABC Study (2013). Effect of socioeconomic disparities on incidence of dementia among biracial older adults. BMJ(Online) 346, 7051-7051.
6. Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack J, Clifford R, Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R. (2004). Mild Cognitive Impairment Can be Distinguished from Alzheimer’s Disease and Normal Aging for Clinical Trials. Arch Neurol 61, 59-66.
7. Wright CB, DeRosa JT, Moon MP, Strobino K, DeCarli C, Cheung YK, Assuras S, Levin B, Stern Y, Sun X, Rundek T, Elkind MSV, Sacco RL. (2021). Race/Ethnic Disparities in Mild Cognitive Impairment and Dementia: The Northern Manhattan Study. J Alzheimers Dis. 80, 1129-1138.
8. Lee HB, Richardson AK, Black BS, Shore AD, Kasper JD, Rabins V. (2012). Race and cognitive decline among community-dwelling elders with mild cognitive impairment: Findings from the Memory and Medical Care Study. Aging & Mental Health, 16, 372–377.
9. Reitz C, Jun G, Naj A, Rajbhandary R, Vardarajan BN, Wang L, Valladares O, Lin C, Larson EB, Graff-Radford NR, Evans D, De Jager PL, Crane PK, Buxbaum JD, Murrell JR, Raj T, Ertekin-Taner N, Logue M, Baldwin CT, Alzheimer Dis Genetics Consortium. (2013). Variants in the ATP-binding cassette transporter (ABCA7), apolipoprotein E epsilon 4, and the risk of late-onset Alzheimer disease in African Americans. JAMA 309, 1483-1492.
10. Chin AL, Negash S, Hamilton R. (2011). Diversity and disparity in dementia: The impact of ethnoracial differences in alzheimers disease. Alzheimer Dis Assoc Disord 25, 187-195.
11. Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EHP, Mayeux R. (1999). Rates of dementia in three ethnoracial group. Int J Geriatr Psychiatry 14, 481-493.
12. Steenland K, Goldstein FC, Levey A, Wharton W. (2016) A Meta-Analysis of Alzheimer’s Disease Incidence and Prevalence Comparing African-Americans and Caucasians. J Alzheimers Dis 50, 71-76.
13. Deters KD, Napolioni V, Sperling RA, Greicius MD, Mayeuk R, Hohman T, Mormino EC. (2021). Amyloid PET imaging in self-identified non-Hispanic black participants of the anti-amyloid in asymptomatic alzheimer’s disease (A4) study. Neurology 96, 1491-1500.
14. Wilkins CH, Windon CC, Dilworth-Anderson P, Romanoff J, Gastonis C, Hanna L, Apgar C, Gareen IF, Hill CV, Hillner BE, March A, Siegel BA, Whitmer RA, Carrillo MC, Rabinovici GD. (2022). Racial and Ethnic Differences in Amyloid PET Positivity in Individuals With Mild Cognitive Impairment or Dementia A Secondary Analysis of the Imaging Dementia-Evidence for Amyloid Scanning (IDEAS) Cohort Study. JAMA Neurol 79, 1139-1147.
15. Bodner KA, Goldberg TE, Devanand DP, Doraiswamy PM. (2020). Advancing computerized cognitive training for MCI and Alzheimer’s disease in a pandemic and post-pandemic world. Front Psychiatry 11, 557571.
16. Jenkins E, Koirala B, Rodney T, Lee JW, Cotter VT, Szanton SL, Taylor JL. (2021). Home/community-based interventions to improve function in persons with mild cognitive impairment/early dementia. Geriatric Nursing (New York), 42, 1109-1124.
17. Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Rebok GW, Smith DM, Tennstedt SL, Unverzagt FW, Willis SL, for the ACTIVE Study Group, ACTIVE Study Grp, & Advanced Cognitive Training for Independent and Vital Elderly Study Group. (2002). Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA 288, 2271-2281.
18. Devanand DP, Goldberg TE, Qian M, Rushia SN, Sneed JR, Andrews HF, Nino I, Phillips J, Pence ST, Linares AR, Hellegers CA, Michael AM, Kerner NA, Petrella JR, Doraiswamy PM. (2022). Computerized Games versus Crosswords Training in Mild Cognitive Impairment. NEJM Evidence 1.
19. Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Kivipelto M, (2015). A 2-year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomized controlled trial. Lancet (British Edition), 385, 2255-2263.
20. Corbett A, Owen A, Hampshire A, Grahn J, Stenton R, Dajani S, Burns A, Howard R, Williams N, Williams G, Ballard C. (2015). The effect of an online cognitive training package in healthy older adults: an online randomized controlled trial. J Am Med Dir Assoc 16, 990-997.
21. Barban F, Annicchiarico R, Pantelopoulos S, Federici A, Perri R, Fadda L, Carlesimo GA, Ricci C, Giuli S, Scalici F, Turchetta CS, Adriano F, Lombardi MG, Zaccarelli C, Cirillo G, Passuti S, Mattarelli P, Lymperopoulou O, Sakka P, Caltagirone C. (2016). Protecting cognition from aging and Alzheimer’s disease: a computerized cognitive training combined with reminiscence therapy: Protecting late-life cognition with a combined training. Int J Geriatric Psychiatry 31, 340-348.
22. Gooding AL, Choi J, Fiszdon JM, Wilkins K, Kirwin PD, van Dyck CH, Devanand D, Bell, MD, & Rivera Mindt M. (2016). Comparing three methods of computerised cognitive training for older adults with subclinical cognitive decline. Neuropsychol Rehabil 26, 810-821.
23. Hyer L, Scott C, Atkinson MM, Mullen CM, Lee A, Johnson A, & Mckenzie LC. (2016). Cognitive training program to improve working memory in older adults with MCI. Clin Gerontol 39, 410-427.
24. Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, Zelinski EM. (2009). A Cognitive Training Program Based on Principles of Brain Plasticity: Results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) Study. J Am Geriatr Soc 57, 594-603.
25. Vance D, Dawson J, Wadley V, Edwards J, Roenker D, Rizzo M, Ball K. (2007). The Accelerate Study: The Longitudinal Effect of Speed of Processing Training on Cognitive Performance of Older Adults. Rehabil Psychol. 52, 89-96.
26. Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Martin R, Luger TM, Duff K, Dotson MM. (2011). Interim analyses from a randomized controlled trial to improve visual processing speed in older adults: the Iowa Healthy and Active Minds Study. BMJ open 1, e000225-e000225.
27. Tzuang M, Owusu JT, Spira AP, Albert MS, Rebok, GW. (2018;2017;). Cognitive training for ethnic minority older adults in the united states: A review. Gerontologist 58, e311-e324.
28. Smith-Ray RL, Makowski-Woidan B, Hughes SL. (2014). A Randomized Trial to Measure the Impact of a Community-Based Cognitive Training Intervention on Balance and Gait in Cognitively Intact Black Older Adults. Health Educ Behav. 41, 62S-69S
29. McDougall GJ, Becker H, Pituch K, Acee TW, Vaughan PW, Delville CL. (2010a). Differential benefits of memory training for minority older adults in the SeniorWISE study. Gerontologist, 50, 632–645.
30. D’Antonio J, Simon-Pearson L, Goldberg T, Sneed JR, Rushia SN, Kerner NA, Andrews AM, Hellegers CA, Tolbert S, Perea E, Petrella JR, Doraiswamy PM (2019) Cognitive training and neuroplasticity in mild cognitive impairment (COG-IT): protocol for a two-site, blinded, randomized, controlled treatment trial. BMJ open 9, e028536.
31. Hackett K, Mis R, Drabick DAG, Giovannetti T. (2020). Informant reporting in mild cognitive impairment: Sources of discrepancy on the functional activities questionnaire. J Int Neuropsychol Soc, 26, 503-514.
32. Burns R, Nichols LO, Graney MJ, Martindale-Adams J, Lummus A. (2006). Cognitive abilities of Alzheimer’s patients: Perceptions of black and white caregivers. Int J Aging Hum Dev 62, 209-219.
© The Authors 2023