H. Jun1, Z. Shi2, S. Mattke2
1. Sol Price School of Public Policy, University of Southern California, Los Angeles, USA; 2. Center for Economic and Social Research, University of Southern California, Los Angeles, USA
Corresponding Author: Dr. Soeren Mattke, Center for Economic and Social Research, University of Southern California, 635 Downey Way, #505N, Los Angeles, CA 90089, USA, Email: mattke@usc.edu; Phone: (202) 468-5797
J Prev Alz Dis 2024;1(11):179-184
Published online August 16, 2023, http://dx.doi.org/10.14283/jpad.2023.95
Abstract
BACKGROUND: A disease-modifying Alzheimer’s treatment could provide budgetary savings to Canadian provinces from a reduction in long-term care home use, yet we do not know the magnitude of those potential savings.
OBJECTIVE: We project savings to each Canadian province’s budget from 2023 to 2043.
DESIGN: Annual savings are projected using a Markov model. We account for reduction in long-term care home use and in use of Alternative Level of Care (ALC) beds, which are hospital beds occupied by care home-eligible patients on the wait list for admission.
RESULTS: A treatment that delays disease progression by 40% is projected to avoid 142,507 long-term care home and ALC years, resulting in $17.2 billion cumulative savings across all Canadian provinces, a 21% relative reduction among treatment eligible patients. Average per capita savings were $1,132, ranging from $734 (Alberta) to $2,895 (Prince Edward Island). Cumulative savings could increase to $22.7 billion with enhanced triage of patients in primary care stages and to $25.6 billion if all capacity constraints for diagnosis and treatment were removed.
CONCLUSION: A disease-modifying treatment could create budgetary savings from lower long-term care home use, offsetting part of the treatment cost. With the increasing demand for long-term care home beds and the high rates of patients being held in hospitals while wait-listed, such a treatment could additionally provide relief to the overburdened long-term care system in Canada.
Key words: Alzheimer’s disease, budget impact, disease-modifying therapy, long-term care home.
Introduction
The recent announcement that the amyloid-targeting drug lecanemab met its primary and secondary endpoints in a phase 3 trial makes it likely that a disease-modifying Alzheimer’s treatment will become available soon (1). In light of the large populations with potential eligibility and likely cost of the treatment, the question arises of which cost offsets to payers from reduced disease progression could help mitigate treatment costs. A recent paper estimated that the largest direct cost offset will come from a reduction of long-term care home utilization (2). The authors reported savings of 2.62 trillion USD from 2021 to 2041 for the U.S., whereas savings in direct medical cost were only 52.5 billion USD.
Such decreases in long-term care home costs would translate into direct savings to provincial governments in Canada as the main payer (3). In 2018, approximately $27 billion was spent on long-term care homes in Canada, with 75% being publicly financed and the rest funded through co-payment mechanisms (3, 4). For example, Ontario spent $6.2 billion in 2020 with 74% being funded by the government (5).
Aside from cost savings, reducing the need for long-term care home admission would ease the strain on already overcrowded facilities. It is estimated that by 2035, 199,000 new long-term care beds are needed across Canada leading to $64 billion in capital spending and $130 billion in operating spending (6). Because of their older population, Prince Edward Island has the highest need for new beds followed by New Brunswick and Nova Scotia (6). A disease delaying treatment could offset the burden by reducing the number of people moving into long-term care homes.
The objective of this article is to project potential cost savings from reduced long-term care home years resulting from a disease-modifying Alzheimer’s treatment. We use a simulation model to predict the results of different scenarios from the perspective of each provincial government in Canada.
Methods
Model Structure
Overall, the model projects the number of patients in each stage of Alzheimer’s disease and their natural progression, reduction in long-term care home utilization with a disease-modifying treatment, and the resulting budgetary saving from 2023 to 4023, using the population aged 50 and over in Canada. The model was programmed in Visual Basic Applications for Microsoft Excel.
The first component of the model projects the annual number of patients formally diagnosed with mild cognitive impairment (MCI) due to Alzheimer’s disease, the patient pool that will likely be treated with the treatment (7, 8). Patients at this stage would be diagnosed by a dementia specialist and go through either a positron emission tomography (PET) scan or a cerebrospinal fluid (CSF) test to confirm the Alzheimer’s pathology. Limited diagnostic capacity would lead to wait times as previously shown for Canada (9). The model assumes that patients waiting to receive a diagnosis and treatment would progress into more severe disease stages.
The second part of the model simulates disease progression from MCI to mild, moderate, and severe Alzheimer’s disease and estimates the annual number of patients at each disease stage in both community and institutional settings. The risk of mortality and long-term care home admission increases with disease progression (10, 11). The treatment is assumed to reduce progression rates from MCI to mild Alzheimer’s disease and from mild to moderate Alzheimer’s disease by 40%, based on clinical results of a composite measure for Activities of Daily Living (ADCS-ADL-MCI) in a high-dose cohort of the EMERGE trial (12). The disease-modifying treatment delays progression rates which translates into lower deaths and fewer long-term care home admissions. For the model component, we assume the following based on expert input:
• When a treatment becomes available, 25% of Canadians 50 years and older, without an established diagnosis of cognitive impairment, will see their Family Physician for a brief cognitive test each year.
o Each subsequent year, 5% of those who previously tested negative return for another evaluation.
• The Family Physician will identify those with manifest dementia, i.e., a disease stage in which the treatment would no longer be effective, and those with obvious explanation for cognitive impairment (depression, prior stroke, etc.).
• 80% of those with suspected MCI will get referred to a dementia specialist for confirmatory neurocognitive testing.
• Specialists will identify those who were false positive on the brief cognitive test and order biomarker testing for true positives.
o 42% of biomarker tests will be based on CSF examination (13) and 58% based on a PET scan.
• 55% will be amyloid-positive based on data from the IDEAS study (14).
• 80% will have a confirmed treatment indication after full diagnostic evaluation, as specialists might determine a different etiology to be mainly responsible for cognitive impairment or a different life-limiting disease, making a clinical benefit unlikely.
The final component of our model calculates budget impact resulting from reduced long-term care home utilization when a disease-modifying treatment would be available. The magnitude of the savings and the speed with which they are realized depend on a province’s diagnostic capacity today and over the 20-year horizon of the model. As Newfoundland and Labrador, Prince Edward Island, Nova Scotia, New Brunswick, Manitoba, and Saskatchewan have only few or no dementia specialists and PET scanners because of their comparatively low population, a stable projection of diagnostic capacity was not possible. Therefore, we estimated the sum of avoided long-term care home utilization for the four most populous provinces, for which we could project diagnostic capacity, as well as for Canada as a nation. We subtracted the four provinces’ avoided utilization from that of Canada’s and allocated the residual to the remaining provinces based on their 2023 population aged 50 and over.
Model Parameters
Age and sex-specific population projections from 2023 to 2043 of the population aged 50 and older were obtained from Statistics Canada (15). We derived the number of future dementia specialist visits and PET scanners using a process described in a previously published study.10 For each province, data on dementia specialists (16) were available from 2014 to 2019 and that on PET scanners (17) were available from 2008 to 2019. We assumed the capacity to perform lumbar punctures and infusion treatments were unconstrained.
Transition probabilities for the disease progression model were from Neumann et al.’s study (11). The study also reported transition rates into death and into long-term care homes from each dementia stage. We applied general mortality rates in Canada by age and sex from Statistics Canada (18).
For the third component of the model, we applied government spending data on long-term care homes. In 2020 CAD, the average long-term care home rate per day per resident was $185 in Ontario, $225 in British Columbia, $115 in Alberta, and $164 in Quebec. Only co-payment rates rather than the full rates were publicly available for the less-populated provinces. We thus used the fact that nationally the co-payment rates are 26% (3) and conducted a backward calculation of the full-payment rates.
We further incorporated the fact that the government pays for the cost of so-called Alternative Level of Care (ALC) beds in nearly all cases. ALC patients are patients held in the hospital after acute care treatment because there is a wait to get allocated to a long-term care bed and their state of health does not allow them to be discharged to the community. The daily cost of an ALC bed in 2020 CAD was $524 in Ontario, $562 in British Columbia, $665 in Alberta, and $572 in Quebec. Based on the prevalence of long-term care home stays with dementia (19) and with the number of ALC days (20), where half of which were attributed to older adults living with dementia (21), we calculated each provincial spending on dementia patients eligible for long-term care by adding cost of dementia related long-term care and ALC. Details on the model parameters and sources are documented in the technical appendix.
All monetary values were inflated to 2022 using the Canadian Health & Personal Care Consumer Price Index (CPI) (22). A 2% growth rate was applied to future cost from 2023 based on the Canadian projected CPI values (23).
Scenario Analysis
We assumed for our baseline scenario that referrals to a dementia specialist would be based on results from a cognitive exam, such as the Mini-Mental State Examination. We further used two hypothetical scenarios that would allow diagnosing patients faster, since treatment access in Canada is most likely to be affected by the lack of dementia specialist capacity. In the first scenario, we assumed a blood-based biomarker test for Alzheimer’s disease pathology would be conducted if the cognitive test was positive. Only if both the cognitive and blood test were positive, patients would be referred to a dementia specialist. The second alternative scenario assumed no capacity constraints, i.e., all patients would be evaluated without any wait times for specialist visits and biomarker testing.
Results
Impact on long-term care home years and related cost savings
Our model projects that, without a disease-modifying treatment, long-term care home or ALC years among patients with MCI due to Alzheimer’s disease in all Canadian provinces would increase to 73,076 patient-years in 2043 and 673,006 cumulatively from 2023 to 2043. Applying long-term care home and ALC rates to these patient-years show that the cumulative cost sums up to $80.3 billion. If a disease-modifying treatment were utilized, it would prevent long-term care home and ALC years annually resulting in a lower cumulative spending of $63.1 billion, a 21% relative reduction among treatment eligible patients.
Figure 1 illustrates the annual long-term care home years prevented and the corresponding cost savings for all Canadian provinces. At the peak of the effect in 2038, a disease-modifying treatment is projected to prevent 12,207 long-term care home and ALC years. Cumulatively from 2023 to 2043, 142,507 long-term care home years would be avoided resulting in $17.2 billion savings.
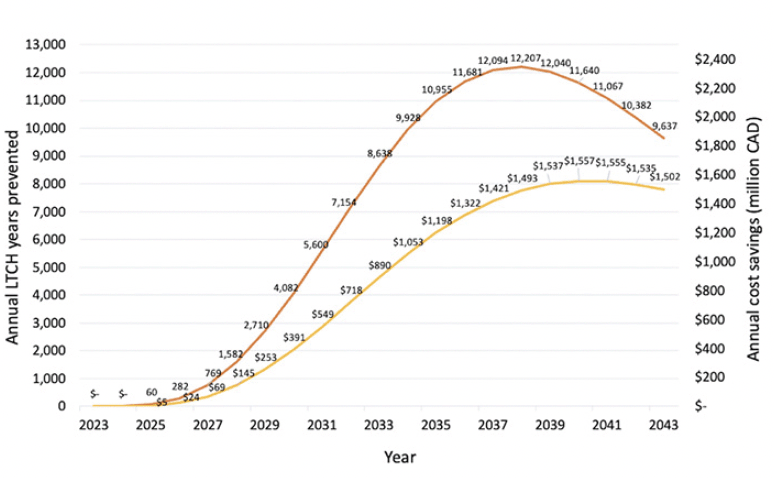
Figure 1. Annual long-term care home years prevented and related cost savings for all Canadian provinces
Cumulative cost savings per population aged 50 and over for each province is presented in Figure 2 with a darker shade indicating higher per capita savings. Average per capita savings rate for all Canadian provinces was $1,132; Alberta had the lowest per capita saving of $734 and Prince Edward Island had the highest of $2,895. Per capita savings in Ontario ($1,038), British Columbia ($1,050), and Quebec ($947) did not differ greatly, and were higher in the less populous provinces because of higher long-term care home occupancy per capita.
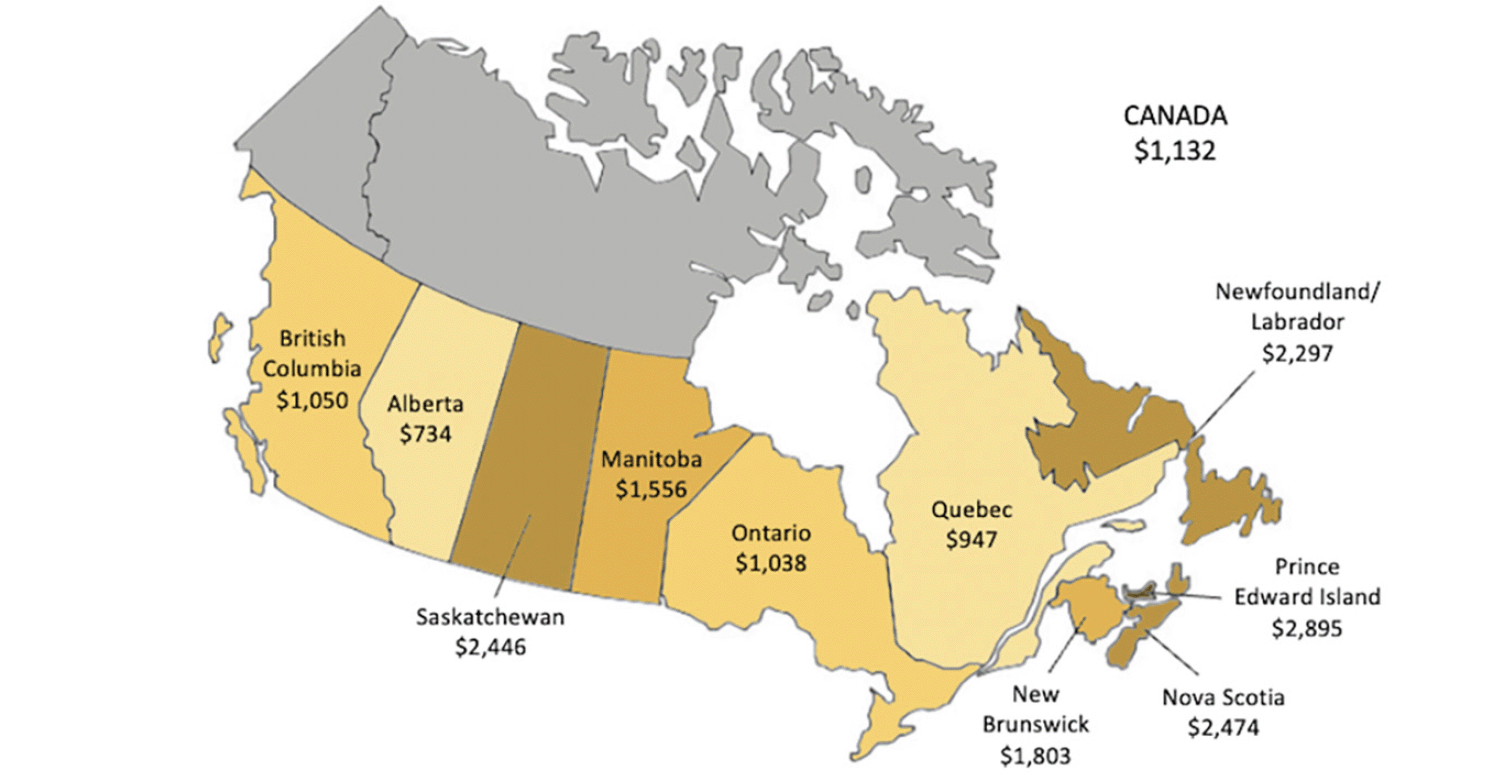
Figure 1. Cumulative cost savings per capita from long-term care home stays prevented by province, 2023-2043
Scenario Analysis
Figure 3 presents the change in cumulative savings under different scenarios. In addition to our main analysis, we assumed two different scenarios that could eliminate barriers in diagnosing and testing patients: adopting a blood-based biomarker test and removing all capacity constraints. An additional blood test will reduce diagnosis wait times by improving triage of patients in primary care settings and the hypothetical case of removing all constraints will remove all wait times. A blood-based biomarker test, compared to the base case scenario, would increase cost savings by 45% in Ontario, 26% in British Columbia, 24% in Quebec, 17% in Alberta, and 32% across all Canadian provinces. Removing all constraints is projected to increase savings relative to the base case, ranging from 31% to 63% across provinces.
A summary of cumulative costs, savings, and per capita savings for each province, under all three scenarios is reported in Table 1.
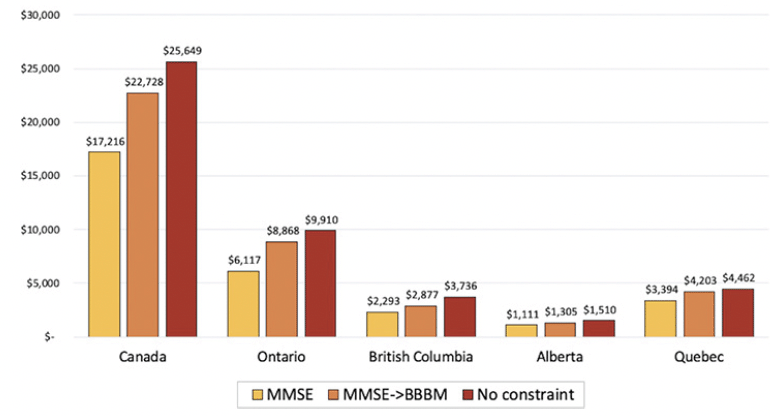
Figure 3. Cumulative cost savings from reduced long-term care home stays across scenarios by province, 2023-2043 (million CAD)
Noted are the cumulative cost savings from 2023 to 2043 under three different scenarios: (1) MMSE: referrals to a dementia specialist are based on results from the Mini-Mental State Examination; (2) MMSE→BBBM: a blood-based biomarker test for Alzheimer’s disease pathology is conducted if the cognitive test is positive and specialist referrals occur if both tests are positive; and (3) there are no capacity constraints for specialist visits and biomarker testing.
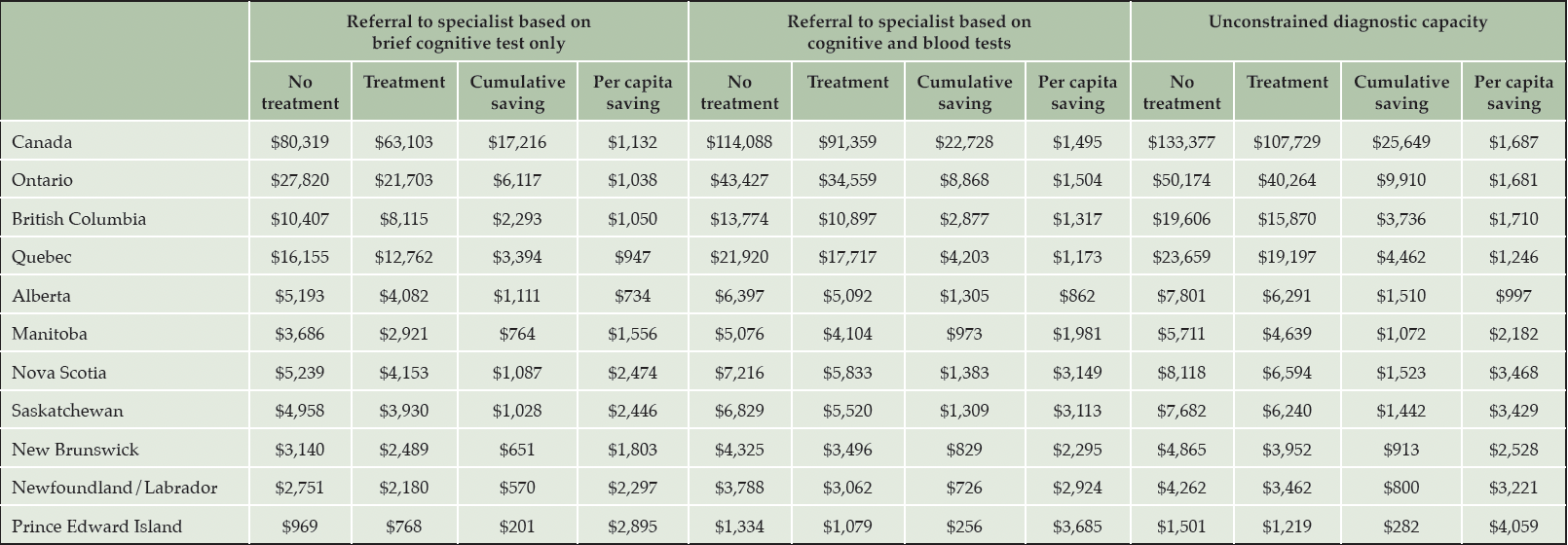
Table 1. Cumulative savings in patients with early-stage Alzheimer’s disease from reduced long-term care home stays by province, 2023-2043 (million CAD)
Discussion
Our study projects the potential cost savings from providing an Alzheimer’s disease modifying treatment to eligible patients from long-term care utilization in Canada. We estimate cumulative savings of $17.2 billion from 2023 to 2043 across all provinces, a relative reduction of 21%. Cumulative savings range from $201 million in Prince Edward Island to $6.1 billion in Ontario. Per capita savings are similar in British Columbia, Ontario, and Quebec, and are slightly higher in less-populated provinces.
Our scenario analyses show that introducing a blood-based test, which would allow primary care physicians to more accurately triage patients for specialist referral, could substantially increase cost savings. Compared to referring solely based on a cognitive test, combining it with a blood-based test is projected to increase cumulative savings from $17.2 billion to $22.7 billion across all provinces, a 32% increase. This finding illustrates that provinces would benefit from adopting cost-effective and scalable technologies for diagnosis of Alzheimer’s disease, because they would reduce wait times and potentially lead to higher budgetary savings.
A disease-modifying treatment could also provide relief to overburdened long-term care systems in Canada by reducing the number of patients progressing into long-term care homes. With the aging population, it is estimated that an additional 199,000 long-term care beds will be needed in Canada by 2035, nearly double the size of current capacity.6 Even now, more than 40,000 Canadians are waiting for a long-term care bed (3). The average wait time for a bed in 2017-18 was 146 days in Ontario and as high as 223 days in the Greater Toronto Area (24). The less populous provinces particularly lack such infrastructure. For instance, in 2006 the 122 public and private long-term care homes in Manitoba and the 71 in Nova Scotia were both near full occupancy (25). Among all provinces, Prince Edward Island is projected to have the largest need for new beds relative to population size (6).
The shortage of long-term care beds puts pressure on hospitals. Approximately 14% of hospital beds are occupied by ALC patients across Canada (26). In Ontario, around 20% of patients on the waitlist for a long-term care home bed waited in hospital (24), with the median wait time being 28 days (27). In British Columbia, the median wait in hospital was 32 days (27). Pressures are higher in smaller provinces; in Nova Scotia, for example, nearly one-third of hospital beds are occupied by ALC patients (28). Hence, delaying progression into long-term care homes could provide relief by reducing waitlisted patients that prevent hospital beds from being used by patients in need of acute care.
Our study has limitations. First, transition probabilities to long-term care homes by each disease stage were based on a U.S. study because related data for Canada was unavailable. A Canadian study reported an average annual admission risk of 11.76% (29), which is slightly higher than the U.S. estimate of 9.25%, indicating our savings could be underestimated. Second, unlike Alberta, British Columbia, Ontario, Quebec, and Canada as a nation, we were unable to project long-term care home years for less-populated provinces, as mentioned in the methods section, and long-term care home rates for these provinces were estimated from co-payment rates due to lack of data. Hence, projected long-term care home years for these provinces were allocated based on the population size aged 50 and older and should be regarded as an approximation. As not all patients in ALC beds may be held because unavailability of a long-term care bed, we might have overestimated those costs, however only around 8% of our adjusted costs were from ALC patients while 92% came from long-term care homes.
In summary, a disease-modifying Alzheimer’s treatment could delay admissions into long-term care homes, leading to budgetary savings that would partially offset the treatment cost. Reduced demand for long-term care beds will also lower the burden placed on Canada’s provincial long-term care systems, which are plagued by long wait lists nationwide, and also reduce ALC rates in hospital settings. Incorporating new diagnostic technology could accelerate the process and allow larger savings.
Funding statement: The work was funded by a contract from the Alzheimer Society of Ontario to the University of Southern California. The sponsor provided comments on a draft of the paper but the authors had full control of design, analysis, final manuscript and decision to submit.
Conflict of interest: Soeren Mattke serves on the board of directors of Senscio Systems, Inc., and the scientific advisory board of AiCure Technologies, and Boston Millennia Partners. He has received consulting fees from Biogen, C2N, Eisai, Novartis and Genentech/Roche. Hankyung Jun and Zehao Shi report no potential conflicts.
Ethical standards: As the study did not constitute human subjects research per U.S. federal regulations (45 CFR 46, 102(f))20), it was exempt from IRB review, consent requirements and registration.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Lecanemab confirmatory phase 3 clarity AD study met primary endpoint, showing highly statistically significant reduction of clinical decline in large global clinical study of 1,795 participants with early Alzheimer’s disease. Eisai News Release. Published Sep 28, 2022. Accessed Sep 29, 2022. https://www.eisai.com/news/2022/news202271.html.
2. Prados MJ, Liu Y, Jun H, Lam J, Mattke S. Projecting the long-term societal value of a disease-modifying treatment for Alzheimer’s disease in the United States. Alzheimer’s & Dementia. 2022;18(1):142-151. https://doi.org/10.1002/alz.12578.
3. National Institute on Ageing. Enabling the Future Provision of Long-Term Care in Canada. Toronto, ON: National Institute on Ageing White Paper; 2019.
4. Canadian Institute for Health Information. Public and Private Sector Health Expenditures by Use of Funds. Accessed Dec 15, 2022. https://www.cihi.ca/en/public-and-private-sector-health-expenditures-by-use-of-funds.
5. Ministry of Long-Term Care. Long-Term Care Overview: Funding and Staffing. Published Sep 24 2020. Accessed Apr 15, 2022. https://wayback.archive-it.org/17275/20210810151226/http://www.ltccommission-commissionsld.ca/presentations/pdf/Long-Term_Care_Staffing_and_Funding_Overview.pdf.
6. Gibbard R. Sizing up the challenge: Meeting the demand for long-term care in Canada. Ottawa, Canada: The Conference Board of Canada. 2017.
7. Liu JL, Hlavka JP, Hillestad R, Mattke S. Assessing the Preparedness of the U.S. Health Care System Infrastructure for an Alzheimer’s Treatment. RAND Corporation; 2017. https://www.rand.org/pubs/research_reports/RR2272.html.
8. Mattke S, Cho SK, Bittner T, Hlávka J, Hanson M. Blood-based biomarkers for Alzheimer’s pathology and the diagnostic process for a disease-modifying treatment: Projecting the impact on the cost and wait times. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2020;12(1). https://doi.org/10.1002/dad2.12081.
9. Liu JL, Hlavka JP, Coulter DT, Baxi SM, Mattke S, Gidengil CA. Assessing the Preparedness of the Canadian Health Care System Infrastructure for an Alzheimer’s Treatment. Santa Monica, CA: RAND Corporation;2019. https://www.rand.org/pubs/research_reports/RR2744.html.
10. Lam J, Jun H, Cho SK, Hanson M, Mattke S. Projection of budgetary savings to US state Medicaid programs from reduced nursing home use due to an Alzheimer’s disease treatment. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2021;13(1). https://doi.org/10.1002/dad2.12159.
11. Neumann PJ, Araki SS, Arcelus A, et al. Measuring Alzheimer’s disease progression with transition probabilities. Estimates from CERAD. 2001;57(6):957-964. https://doi.org/10.1212/WNL.57.6.957
12. Budd Haeberlein S, Aisen PS, Barkhof F, et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. The Journal of Prevention of Alzheimer’s Disease. 2022. https://doi.org/10.14283/jpad.2022.30.
13. Alzheimer’s Research UK. Dementia Attitudes Monitor Wave 2. Sep 2021. https://www.dementiastatistics.org/wp-content/uploads/2021/09/ALZ_DAM_long-Report_21_LR_WEB_FINAL2.pdf.
14. Rabinovici GD, Gatsonis C, Apgar C, et al. Association of Amyloid Positron Emission Tomography With Subsequent Change in Clinical Management Among Medicare Beneficiaries With Mild Cognitive Impairment or Dementia. JAMA. 2019;321(13):1286-1294. https://doi.org/10.1001/jama.2019.2000.
15. Statistics Canada. Projected population, by projeciton scenario, age and sex. Accessed April 15, 2022. https://doi.org/10.25318/1710005701-eng.
16. Canadian Medical Association. Number of physicians by province/territory and speciality, Canada. Accessed April 15, 2022. https://www.cma.ca/sites/default/files/2019-11/2019-01-spec-prov_1.pdf.
17. Canadian Agency for Drugs and Technologies in Health. The Canadian Medical Imaging Inventory 2019-2020. Ottawa: CADTH;2021.
18. Statistics Canada. Mortality rates by age group. Accessed Mar 15, 2022. https://doi.org/10.25318/1310071001-eng.
19. Canadian Institute for Health Information. Profile of Residents in Residential and Hospital-Based Continuing Care, 2020–2021. Ottawa, ON: CIHI; 2021. Accessed May 15, 2022.
20. Canadian Institute for Health Information. Inpatient Hospitalization, Surgery and Newborn Statistics, 2020–2021. Accessed May 15, 2022. https://www.cihi.ca/en/hospital-stays-in-canada.
21. Canadian Institute for Health Information. Dementia in hospitals. Accessed May 15, 2022. https://www.cihi.ca/en/dementia-in-canada/dementia-care-across-the-health-system/dementia-in-hospitals.
22. Statistics Canada. Consumer Price Index, annual average, not seasonally adjusted. Accessed May 15, 2022. https://www150.statcan.gc.ca/.
23. USDA Economic Research Service. Projected Consumer Price Indices (CPIs) for Baseline Countries/Regions (in percent) 2011-2031. Accessed April 15, 2022. https://www.ers.usda.gov/.
24. Um SG, Iveniuk J. Waiting for long-term care in the GTA: trends and persistent disparities. Wellesly Institute. 2020. https://www.wellesleyinstitute.com/publications/waiting-for-long-term-care-in-the-gta-trends-and-persistent-disparities/.
25. Banerjee A. An Overview of Long-Term Care in Canada and Selected Provinces and Territories. Women and Health Care Reform. Oct 2007.
26. Sutherland J. Exploring alternative level care (ALC) and the role of funding policies : An evolving evidence base for Canada. Canadian Health Services Research Foundation. 2012.
27. Canadian Institute for Health Information. Seniors in Transition: Exploring Pathways Across the Care Continuum. 2017. Accessed June 15, 2022.
https://www.cihi.ca/sites/default/files/document/seniors-in-transition-report-2017-en.pdf.
28. Picard A. Bedlam over beds: We can no longer ignore our long-term-care crisis. The Globe and Mail. Published May 28, 2019. Accessed July 15, 2022. https://www.theglobeandmail.com/opinion/article-bedlam-over-beds-we-can-no-longer-ignore-our-long-term-care-crisis/.
29. Cloutier DS, Penning MJ, Nuernberger K, Taylor D, Macdonald S. Long-Term Care Service Trajectories and Their Predictors for Persons Living With Dementia: Results From a Canadian Study. Journal of Aging and Health. 2019;31(1):139-164. https://doi.org/10.1177/0898264317725618.
© The Authors 2023
