C. Zheng1,2, R. Zeng1, G. Wu2, Y. Hu2, H. Yu2,3
1. Shantou University Medical College, Shantou, China; 2. Guangdong Eye Institute, Department of Ophthalmology, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, China; 3. Guangdong Provincial Key Laboratory of Artificial Intelligence in Medical Image Analysis and Application, Guangzhou, China
Corresponding Author: Prof. Honghua Yu, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou 510080, China. Tel: 86-186-8888-8422.Fax: 86-8382-7812, E-mail: yuhonghua@gdph.org.cn; Prof. Yijun Hu, Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou 510080, China. Tel: 86-137-1052-6990. Fax: 86-8382-7812; E-mail: huyijun2014@163.com
J Prev Alz Dis 2024;2(11):469-483
Published online September 15, 2023, http://dx.doi.org/10.14283/jpad.2023.118
Abstract
With the aging of the global population, the health care burden of Alzheimer’s disease (AD) and dementia is considered to increase dramatically in the coming decades. Given the insufficiency of effective interventions for AD and dementia, clinical research on identifying potentially modifiable risk factors and early diagnostic biomarkers becomes a public health priority. Currently, extracerebral manifestations with a large proportion of ocular involvement are usually recognized to precede the symptoms of AD and dementia. Growing epidemiologic evidence also suggests that eye disorders, such as cataracts, age-related macular degeneration, glaucoma, diabetic retinopathy, and so on, are closely associated with and even have a higher incidence of AD and dementia. The eye, as an extension of the central nervous system, therefore has the potential to provide a feasible approach to detecting structural and functional abnormalities of the brain. Numerous new imaging modalities are developed and give novel insights into the detection of several neurodegenerative, vascular, neuropathological, and other ocular abnormalities of AD and dementia in scientific research and clinical application. This review provides an overview of the epidemiologic associations between eye disorders and AD or dementia and summarizes the recent advances in ocular examinations and techniques employed for the detection of AD and dementia. With more brain-and-eye interconnections being identified, the eye is becoming a noninvasive and easily accessible window for the early diagnosis and prevention of AD and dementia.
Key words: Eye, ocular disorder, ocular examination; Alzheimer’s disease, dementia.
Introduction
With an increase in life expectancy, the prevalence of neurodegenerative diseases is projected to increase substantially. As a typical neurodegenerative disease, Alzheimer’s disease (AD) is considered the most familiar type of dementia, accounting for about 60-80% of dementia cases globally, and compromises the care delivery for the aging population as a public health issue (1, 2). Hallmarked by decline in cognition and impediment of independence with a population over the age of 65, AD is imposing unprecedented stress on both family and society worldwide, and has significant social and economic implications (3, 4). Given the current insufficiency of effective strategies to restrain the progression of the disease, research on identifying and intervening with potentially modifiable risk factors, as well as developing an early and accurate diagnosis of AD, becomes a public health priority.
Similar to AD, the prevalence of visual impairment is also projected to increase with the aging population. It is particularly noteworthy that those elderly with a co-occurrence of cognitive and visual impairments compose a more general and vulnerable population who have a potentiated risk of hospitalization and lead to higher healthcare spending (5-7). The tenth annual American Geriatrics Society and National Institute on Aging conference reported that the comorbidity of cognitive and sensory impairments, especially vision and hearing impairments, is far more prevalent than expected (8). Visual impairment is also supposed to increase dementia risk via several mechanisms similar to hearing impairments, such as increasing cognitive demands, leading to social isolation and physical inactivity, and even causing structural and functional changes in the brain (8, 9). Compared to the irreversible processes of AD and dementia, it is usually possible to prevent and cure visual impairment via common medications and interventions, such as cataract surgery and eyeglasses. Therefore, eye disorders are considered potentially modifiable and preventable risk factors for AD, and the investigation of the association between them is especially important.
Currently, AD and dementia are found to have unexpected extracerebral correlations with a large proportion of ocular involvement instead of simply being restricted to the brain. Generally, visual impairment was found to be associated with an increased incidence of dementia in a meta-analysis of 14 prospective cohort studies (10). Several eye disorders, such as cataracts, age-related macular degeneration (AMD), glaucoma, diabetic retinopathy, and so on, are also reported to be associated with AD and dementia (11-14). Although findings from the current epidemiologic research that studied the interconnections between eye disorders and AD were inconsistent, these findings indicated that the evaluation of visual impairments and eye disorders may be beneficial to identify the elderly with a high risk of developing AD and dementia. With increasing evidence of brain-and-eye interconnections being identified, the elderly with both visual impairment and dementia may benefit from ocular interventions that could improve vision and cognition.
In addition to the epidemiologic associations, there are increasing studies indicating that ophthalmological assessments have detected a number of ocular changes and biomarkers that have implications for the early diagnosis of dementia and AD. Over the past decades, besides traditional physical examinations, numerous new approaches, including optical coherence tomography (OCT), OCT angiography (OCTA), dynamic vessel analyzer (DVA), and so on, have been developed to offer quantitative assessment of ocular indicators. Various studies have reported several neurodegenerative, vascular, neuropathological, and other ocular abnormalities experienced by AD patients (15-18). The ocular manifestations, especially those of the retina, show many correlations with the central nervous system and often mirror dementia processes ongoing elsewhere in the brain (19). With deeper understandings of the epidemiological findings and great advances in technologies, ocular abnormalities have been increasingly recognized as potentially modifiable risk factors and novel non-invasive biomarkers for AD in the general population. It could also have a considerable impact on utilizing ocular examinations and biomarkers for the early detection and prevention of AD and dementia in the near future.
In this review, we would like to provide an overview of the plausible association between visual impairments and eye diseases in AD and dementia since the last decade, and summarize the recent advances in clinical application of ocular examinations and techniques employed to investigate the ocular indicators for the detection of AD and dementia.
Interconnection between eye disorders and Alzheimer’s disease or dementia
Visual Impairment and dementia
Similar to dementia, visual impairment is another serious concern among the elderly, and has significant negative impacts on both physical and psychosocial health (20). It is gradually being considered a comorbidity that increases the risk of disability in patients with cognitive impairment. Current studies usually employ different metrics to make a classification of cognitive impairment, but generally, dementia denotes a more severe and general pathology that impairs numerous cognitive domains and disturbs daily activities. In this context, as a general manifestation and indicator of having eye disorders, visual impairment is typically defined by visual acuity, and its association with dementia is popularly discussed in many epidemiological studies.
Prior cross-sectional studies have suggested that there was a positive association between visual impairment and cognitive decline. A study from Court and his colleagues with a representative sample of older UK adults found that patients with visual impairment were more likely to have multiple health comorbidities than those without. After being standardized by age, gender, and social deprivation, dementia was more prevalent in patients with visual impairment (20). Using a nationally representative sample of older US adults, Chen and his colleagues reported that distance visual impairment and subjective visual impairment were associated with lower cognitive function scores compared to those with normal vision, which remained significant even after adjusting confounders (21). Similarly, in Chinese older adults with visual impairment, there was supposed to be an increased risk of severe to extremely severe dementia, which was more prominent with a combination of hearing impairment (22). Moderate and poor self-rated visions were was found to be independently associated with dementia both cross-sectionally and longitudinally by Davies-Kershaw et al. in individuals aged 50 and older (23). Patients with visual impairment were also shown to have a 1.5 times increased risk of dementia in a recently published brief report (24).
In addition to the cross-sectional studies, several longitudinal studies examined the potential risk of visual impairment on cognitive decline, though some of them reported inconsistent evidence. In a retrospective cohort study by Rogers et al., the development of dementia was found to be associated with poor vision, the risk of which was observed to be 63% lower in individuals with very good or excellent baseline vision during a mean follow-up period of 8.5 years (25). Likewise, the US Medicare population with baseline self-reported visual impairment was considered to have a greater likelihood of dementia over subsequent follow-up, and vice versa, which meant that the association was bidirectional and multifactorial (26). In prospective studies, Zheng et al. and Lim et al. assessed cognitive status using scales and both indicated that older individuals who suffered from visual impairment over the years were thought to experience a greater magnitude of cognitive decline, further highlighting the significance of vision preservation in elderly people (27, 28). Interestingly, a study found that moderate to severe near visual impairment could increase the risk of dementia by 2 times in the first 2 years and 1.8 times from 2 to 4 years of follow-up, though the association was not significant beyond 4 years (29). While mild near visual impairment was associated with an increased risk of dementia only in the first 2 years of follow-up, indicating that near visual impairment might be an indicator of dementia risk in the short and middle term (29). Poor vision was also found to be associated with the transition to cognitive impairment in Ehrlich’s study (30). Moreover, several prospective studies concluded similar findings that a more severe visual impairment was associated with a higher incidence of dementia (31-34). When considered together with visual and hearing impairment, dual sensory impairment was found to have a higher hazard of dementia incidence both cross-sectionally and longitudinally (35, 36). Previously, Pabst et al. debated that in their population-based prospective cohort study, only hearing impairment was associated with an increased risk of all-cause dementia, while either the additional presence or absence of visual impairment had no excess risk or risk compensation (37). However, the latest findings of Kuo et al. suggested that patients with dual sensory impairment have a higher 7-year hazard of dementia compared to those without (35). A multicenter study from the US further confirmed that dual sensory impairment has a 160% greater risk for all-cause dementia and a 267% greater risk for AD (36).
Recent meta-analyses were performed to verify a clear association between visual impairment and dementia. By pooling data from prospective cohort studies, Shang et al. reported that the pooled relative risk associated with visual impairment was 1.47 for incident dementia and 1.35 for incident cognitive impairment, respectively (10). Another meta-analysis performed by Kuźma et al. also indicated that visual impairment was associated with a 1.38-fold increased risk of all-cause dementia (38). In addition, some specific types of visual impairment, such as visual field defects, vision-specific functioning, visual contrast sensitivity, and vision-specific mobility, were reported to be associated with cognitive impairment, dementia, and AD (39-41). Therefore, these studies indicate that worsening vision could be adversely associated with future cognitive functioning, and maintaining good vision is a significant strategy for mitigating cognitive decline in the elderly.
With a deeper understanding, the association between visual impairment and dementia is supposed to be interrelated and multifactorial, which potentially reflects that greater cognitive load is related to lower vision, underlying brain structural and functional changes, and social isolation caused by poor vision. This is well-discussed and there have been several possibilities proposed to explain the association. Firstly, visual disturbances have been described as one of the earliest manifestations of dementia, since AD is thought to affect the visual pathway and cause further visual impairment (26), and both of them may have common risk factors and share the same spectrum of diseases that are involved in cognitive performance decline. Secondly, patients with visual impairment may reduce social engagement, leading to less cognitive stimulation and higher rates of depression, which then increases the risk of dementia (42, 43). Alternatively, inadequate sensory input would cause neuronal atrophy. Patients usually suffer from visual recognition defects due to damage in the associative visual areas (44, 45). Finally, more perception and interpretation were needed for sensory information in patients with visual impairment, and therefore more cognition burden would be taxed and increase the risk of dementia (26). The combination of visual impairment and dementia has even greater functional defects than either alone due to the interaction between them (7). With further investigation of the association between visual impairment and dementia, it will be beneficial when dealing with the growing population of older people affected by each.
Cataracts and Alzheimer’s disease or dementia
Cataracts are characterized by the opacity of the lens, which is thought to be heavily age-related and has remained the largest contributor to blindness in adults aged over 50 globally. It was also estimated by the WHO that there were 95 million visually impaired people due to cataracts in 2020 (46). Although the typical manifestation of dementia and AD is memory impairment, recent studies have indicated that cataracts might be a non-memory manifestation of AD.
There are several population-based cohort studies that explore the association between cataracts and dementia or AD. In a retrospective cohort study conducted by Lai et al. in Taiwan, individuals with cataracts were found to have a 1.43-fold increased risk of developing AD compared to the non-cataracts group (11). In subgroup analyses, even after 8 years of diagnosing cataract, the risk of developing AD remained markedly high, with an adjusted hazard ratio (HR) of 2.41 and a 95% confidence interval (CI) of 1.29 to 4.49 (11). More recently, in another population-based cohort study, when cataracts were combined with a systemic condition, such as stroke, heart disease, obesity, hypertension, diabetes, or depression, it was reported to have 1.19 to 2.29 times increased risk of developing dementia compared with individuals without either cataracts or systemic condition (47). Similar findings were also demonstrated by a prospective study of Hwang et al. that cataracts were strongly positively associated with an increased risk of AD and all-cause dementia during an average of 4.1 years of follow-up (48). After variable adjustment, cataracts were reported to significantly increase 34% risk of developing AD (48). Moreover, another prospective cohort study with an 8.4-year follow-up time also indicated that patients with non-surgical cataracts had a greater risk of all-cause dementia and AD (49). However, in patients from the Adult Changes in Thought study, cataracts were not a significant risk factor for probable and possible AD development either within 5 years or over 5 years after cataracts were diagnosed (50). The authors suggested that using a simplistic model to assess the association between eye diseases and the risk of AD was insufficient and further study was needed (50).
Early in the last decade, the coexistence of cataracts and dementia aroused the interest of scientists. Approximately 5% of ophthalmology outpatient attendances over 60 years old in the UK each year would be expected to develop dementia, and the considerable overlaps of visual symptoms in patients with cataracts and dementia caused difficulty in diagnosis (51). A meta-analysis conducted by Kuźma et al. generated pooled estimates that cataracts were associated with an increased risk of all-cause dementia and AD (38). Since cataracts are an avoidable cause of blindness, cataract surgery is a key focus of eye health services and will contribute to an impactful reduction in the prevalence of vision loss (52).
Age-related macular degeneration and Alzheimer’s disease or dementia
As a leading cause of central vision loss in the elderly, AMD is projected to affect 288 million patients by 2040 (53). Characterized by the accumulation of drusen at the central retina, AMD is a multifactorial disease with a combination of aging, genes, and environmental factors. The pathogenesis of AMD is still unclear, and it is a prevalent neurodegenerative disease strongly associated with multiple risk factors that are similar to dementia and AD (54). However, in recent observational epidemiological studies, the association between AMD and dementia is still a subject of debate, and controversy exists about the potential associations between AMD and dementia.
Several longitudinal studies have suggested a positive association between AMD and dementia. Early in 2012, Woo and his colleagues reported that lower global cognition scores were found in patients with AMD compared to normal controls, especially those of the geographic atrophy subtype, and they also had a 3.127 times higher risk of developing mild cognitive impairment (55). The study also found a trend that the severity of AMD was positively correlated to the worsening of cognition function (55). A case-control study based on the Taiwan population similarly found that during a mean follow-up time of 4.4 years, patients with AMD had a significantly higher incidence of both senile dementia and AD compared with normal controls (56). Under stratified analyses, non-exudative AMD, rather than exudative AMD, was found to have a 1.44-fold higher risk of senile dementia and AD (56). Lee’s cohort study also mentioned that both recent and established AMD were significantly associated with all-cause dementia (50). For the incidence of AD, there was a 50% increased risk of AD for those with established AMD (50). Similar results were found in the Korean population, where participants with AMD had a 1.48-fold higher incidence of AD compared to those without (57). Additionally, AMD was found to be associated with an 87% greater risk of AD in Hwang’s study (48). Another population-based cohort study also reported a 1.26-fold greater risk of incident dementia after adjusting multiple variables (47). It is worth noting that comorbidity of AMD with any systemic disease had a higher incidence of dementia compared to having AMD or a systemic disease only, and of all the combinations of AMD and a systemic condition, AMD-diabetes had the highest risk (47). Therefore, potential cognitive impairment should be noticed in the visual rehabilitation of patients with AMD.
Although the abovementioned studies suggested a potentially positive association, other investigations cast doubt on it. Based on gold-standard histopathology, a cross-sectional study used 157 autopsy specimens with postmortem diagnoses of AMD and AD failed to support an increased prevalence of AD among patients with AMD, even when the severity of AMD was categorized (58). It also found that the incidence of AD was significantly lower in patients with AMD after adjusting multiple variables (58). In the latest cross-sectional study by Chua and his colleagues, no association was found between AMD and cognitive impairment or AD (59). For longitudinal studies, Keenan’s study suggested that there was no association between AMD and the risk of subsequently developing dementia or AD, and vice versa, which provided observational evidence that patients with dementia in England may have a substantially lower likelihood of receiving AMD treatment (60). In a 10-year multicenter randomized trial that enrolled participants from the Age-Related Eye Disease Study 2 and assessed the bidirectional associations, the authors concluded that cognitive impairment was prospectively and positively associated with late AMD progression, however, the association between baseline AMD severity and the incidence of cognitive impairment was not observed (12).
Finally, meta-analyses have found inconsistent results. A recent meta-analysis by Rong and his colleagues summarized 21 studies and found that patients with AMD were significantly associated with an increased risk of cognitive impairment and AD (61). When assessing cognitive function using scales, patients with AMD were found to have poorer cognitive functions than healthy people (61). On the contrary, Kuźma et al. enrolled 7,800,692 participants with more than 2,559 cases reported that there was no evidence of a significant association between AMD and dementia or AD under pooled estimation (38). Although there have been a profusion of studies investigating their associations, there is, as yet, no definitive conclusion to whether AMD is significantly associated with the incidence of dementia or AD, and their clinical links are even contradictory presently. The heterogeneity of the current observational results is potentially due to inevitable discrepancies in the study population, study designs, AMD subtypes, and so on in different studies. Additionally, reverse causality is commonly seen, and Mendelian randomization analysis was performed to investigate the causal association between AMD and AD in Jiang’s study, in which they found no statistical causal effect of advanced AMD on AD risk (62). Nonetheless, research on the association between these two diseases is still of great clinical significance.
Glaucoma and Alzheimer’s disease or dementia
Glaucoma is composed of a heterogeneous group of optic nerve diseases that are characterized by retinal ganglion cell death and irreversible visual field loss, which is the second leading cause of blindness globally (63). Although it is considered a neurodegenerative disease that shares several common characteristics with dementia or AD, there have been inconsistent results demonstrated by different observational studies.
Generally, a population-based case-control study conducted by Lai and his colleagues found that older people with glaucoma were associated with a 1.5-times greater risk of AD in Taiwan (64). A 1.46-fold increased AD risk for glaucoma diagnoses within 5 years was also reported in Lee’s cohort study (50). Glaucoma diagnosed within 5 years was also found to be a risk factor for all-cause dementia (50). However, there were inconsistent findings reported by other studies. Hwang’s population-based prospective study found that glaucoma was not significantly associated with an increased incidence of dementia (48). In addition, it explained that this null association may be due to the limitations of data sources (48). In a population-based cohort study based on the UK Biobank, glaucoma was not associated with a higher risk of all-cause dementia or AD even after adjusting confounders (47). However, when glaucoma is comorbid with a systemic disease, there is a significantly increased incidence of dementia (47).
There were several studies investigating the associations between different glaucoma subtypes and dementia. In a population-based cohort study with a 3-year follow-up, the results found that patients with open-angle glaucoma (OAG) had a 4 times higher incidence of developing dementia after adjusting confounders (13). Nevertheless, other researchers reached different conclusions. In a cross-sectional study that recruited individuals resided in Stockholm found that in both males and females, the prevalence of primary open-angle glaucoma (POAG) in dementia decreased with an adjusted odd ratio of 0.653 and 0.714 respectively, though the authors explained that their findings were probably due to an underestimation of glaucoma in dementia patients (65). A cohort study performed by Keenan and his colleagues also supported the findings that patients with POAG did not have an elevated risk of AD as expected (66). As a subtype of POAG, normal-tension glaucoma (NTG) is characterized by glaucomatous changes without ocular hypertension (67). In the participants recruited from the Australian and New Zealand Registry of Advanced Glaucoma, Mullany and his colleagues conducted a case-control and cross-sectional cognitive screening and found a significantly positive association between NTG status and cognitive impairment (68). Moreover, they also indicated that, compared to high-tension glaucoma, cognitive impairment was 2.2-fold more prevalent in the NTG cohort (68). As one type of secondary open-angle glaucoma, pseudoexfoliation glaucoma was also assessed and showed that the prevalence of it was not significantly correlated with dementia (69). There is a recent meta-analysis summarizing the association between glaucoma and dementia or AD, but no evidence was found on the association between glaucoma and the risk of dementia among the 175,357 participants and 44,144 cases enrolled (38).
The abovementioned findings from individual studies remind us that despite the fact that glaucoma shares similar neurodegenerative characteristics with dementia or AD, there are several features that set glaucoma apart and further studies are warranted to classify their associations.
Diabetic retinopathy and Alzheimer’s disease or dementia
Although the explicit mechanisms underlying diabetes mellitus and dementia remain elusive, increasing findings suggest that it is the role of microvascular dysfunction and normal neurovascular coupling disruption that accounts for their closed correlation (70). Given that the presence of diabetic retinopathy (DR) is due to neurovascular dysfunction of the retina and the retina is thought to be an extension of the brain, the association between the presence of DR and dementia has gradually become a popular area of interest.
There is a certain amount of longitudinal research showing a significantly positive association. In the Edinburgh Type 2 Diabetes Study, increasing severity of retinopathy was suggested to be independently associated with cognitive decline (71). In a cohort study with a median of 4 years of follow-up, DR was reported to be positively correlated with the increased risk of mild cognitive impairment, and this result remained significant after confounder adjustments (72). Similarly, in a recent prospective cohort study recruiting patients in southern Brazil, DR was shown to have a 2.5-fold greater possibility of having cognitive impairment (73). Patients with DR were estimated to have a 42% increased incidence of dementia in another cohort study by Exalto and his colleagues, including 29,961 patients with type 2 diabetes aged over 60 years old, and further consideration of diabetes-specific and vascular-related disease conditions still came out with a positive association (74). Increased dementia and AD risks were found in both recent 5 years and more than 5 years earlier of DR diagnosed in a population-based cohort study by Lee and his colleagues (50). A recent study based on the UK Biobank also indicated that, generally, diabetes-related eye diseases in patients with a systemic disease were correlated with an increased risk for incident dementia (47). Interestingly, a cross-sectional study enrolled patients from the South East London Diabetic Retinopathy Study and showed that patients with minimal DR had more severe cognitive impairment than those with advanced DR, which indicated that there was an inverse association between the severity of DR and cognitive impairment (75). Nevertheless, similar to other eye disorders, findings are not always consistent. In a multiethnic Asian population, a cross-sectional study showed that although patients with AD were 3-fold more likely to develop DR compared to those with no cognitive impairment in several multivariable models, this association was no longer significant after adjusting for the presence of diabetes (59). This might suggest a false positive result. In a population-based cohort study, there was no significant association between DR and all-cause dementia or AD after adjustment for confounders (48).
Contrary to the conflicting conclusions of the individual studies, pooled estimations in several systemic reviews and meta-analyses draw a consistently positive conclusion. In a systemic review summarized data on 2094 patients, results showed that there was an increased risk of developing cognitive impairment in DR patients compared to controls (76). Especially, authors also reported that DR was more strongly correlated with impairment in the verbal learning and recent memory cognitive domains (76). Similarly, the association between DR and cognitive impairment was considered to be significantly positive in Cheng’s study, which included 15 studies for systemic review and 10 studies for meta-analysis (77). A pooled estimation of a 34% increase in all-cause dementia risk was suggested to be associated with DR among 43,658 participants, and the corresponding estimation was 29% for AD among 9,955 participants compared to patients without retinopathy (38). Moreover, Chan and coworkers included 17 cross-sectional and 6 longitudinal studies to demonstrate comprehensively the association between DR and different stages of cognitive impairment (14). Their results showed that the existence of DR in patients with diabetes mellitus would cause a higher prevalence of cognitive impairment cross-sectionally and escalate the risks of cognitive impairment development longitudinally (14).
Since DR is considered a non-negligible risk factor for incident dementia, determining their observational and pathological associations is of great significance. If DR is proven to be a modifiable factor in future dementia development, therapeutic interventions should be taken to achieve early diagnosis and disease modification.
Other eye disorders and Alzheimer’s disease or dementia
Despite the abovementioned eye disorders that are commonly considered to be associated with dementia or AD, there are other types of eye disorders that are less discussed but still worth attention.
Retinal vein occlusion (RVO) is the second most common retinal vascular disease following DR, which is reported to have a higher incidence in elderly patients (78). Since RVO also affects the retinal blood flow as dementia does, it is becoming noteworthy and has been recently found to confer an increased dementia risk observationally. A cross-sectional study conducted by Chan and coworkers reported a significantly higher prevalence of dementia among patients with RVO compared to those without, which was still significant after adjustment for sex, hypertension, or diabetes (79). However, when adjusting for either age or stroke, significance no longer existed, which means that these were shared risk factors that affected their association (79). In the South Korean population, Nam and his colleagues conducted a retrospective cohort study and obtained a similar conclusion that patients with RVO had increased risks of subsequent all-cause dementia and AD after adjustment of all the confounding variables (80). However, whether it is the RVO per se that implies a cause-and-effect relationship with the incidence of dementia still needs further validation.
Generally, retinopathy, which was defined as the presence of one or more dot/blot hemorrhages, microaneurysms, cotton wool spots, or evidence of laser treatment for retinopathy by Schrijvers and coworkers, was found to have nearly a 2-times higher prevalence in patients with dementia or AD cross-sectionally (81). When the authors further investigated the association between retinopathy and dementia or AD longitudinally, however, there was no significant association detected at baseline with incident dementia or AD (81). By contrast, in a cross-sectional study of 3,906 patients recruited from the AGES-Reykjavik Study, results showed that retinopathy lesions had significant association with dementia or AD (82). Specifically, they reported that there was no significant association between retinal focal arteriolar narrowing, arteriovenous nicking, and dementia (82).
With the improvement and deeper investigation of clinical epidemiological research, the association between eye disorders and dementia or AD would be better understood, and having eye disorders may become one of the indicators and modifiable risk factors of potential dementia or AD development.
Intervention to eye disorders for Alzheimer’s disease or dementia
As dementia imposes unprecedented pressure on patients and society around the world, it becomes increasingly crucial to identify modifiable risk factors to detect, prevent, or postpone the development of cognitive impairment and dementia. Increasing numbers of epidemiological studies provide evidence that visual disturbances usually precede the symptoms of cognitive decline, and many eye disorders play an important role in the prevalence and incidence of dementia or AD.
To estimate the proportion of dementia risk that may be caused by visual impairment, a recent study by Ehrlich et al. calculated the population attributable fraction of dementia associated with visual impairment and found that the population attributable fraction of visual impairment was 1.8%, suggesting that more than 100,000 prevalent dementia cases in the US could potentially have been prevented through healthy vision (24). Rogers and his colleagues suggested that for patients with poorer vision who did not visit an ophthalmologist, the risk of developing AD was increased by 9.5-fold and by 5-fold for cognitive impairment (25). The risk of AD could remain a 5-fold increase in poorer vision without prior eye surgery (25). Moreover, among Americans aged 90 or older who maintained normal cognition, 77.9% of them had received at least one eye procedure, compared to only 51.7% among those with AD (25). Different from AD and dementia, there are effective interventions for eye disorders, since most of their causes are treatable or curable, such as wearing corrective lenses and having cataract surgery. These important findings indicated that addressing visual impairment may be a potential preventive strategy, and treating eye disorders may be modifiable risk factors for dementia and AD.
Currently, surgery is the only curable treatment for cataracts. Cataract surgery is the most common surgery in ophthalmology and has brought certain health benefits to patients and society. Early in 2014, Yu and his colleagues performed a population-based cohort study in the Taiwan population and found that the rate of dementia was significantly lower in patients who underwent cataract surgery, with a HR of 0.77 (83). And a shorter interval between the date of the first diagnosis of cataracts and the date of cataract surgery, especially within 365 days, was significantly associated with a reduced risk of subsequent dementia (83). Later in 2021, Lee and his colleagues conducted a prospective, longitudinal cohort study and showed that patients who underwent cataract extraction were significantly associated with a reduced risk of dementia with a HR of 0.71 compared to those without after the control of additional factors (84). Similar findings were also indicated for the incidence of AD in Lee’s study (84). In a recent study published in 2022, Ma and his colleagues reported that patients who underwent cataract surgery had a 50% lower incidence of all-cause dementia and AD compared with non-surgical patients (49). Interestingly, although patients with cataracts had an increased risk of dementia, there was no difference between patients who underwent cataract surgery and healthy people, suggesting that cataract surgery may even reverse the risk of future dementia development (49). Therefore, since reversible visual impairment, such as cataracts, lacks specific measurements to effectively treat dementia and AD, interventions that could reduce their incidence deserve a great deal of attention.
There were also other vision interventions, such as wearing glasses, rehabilitation training, and other eye surgeries. Nevertheless, there is yet no precise evidence for their positive impact on dementia or AD (85). In Lee’s study, glaucoma surgery, which did not restore vision, was not detected to have a significant association with the decreased risk of dementia, though they explained that this may be probably due to insufficient power in the analysis (84). However, since visual impairment and eye disorders are widely considered to be closely associated with dementia development, it is of great significance to perform future interventional research and trials about visual interventions or treatments for effectively alleviating cognitive impairment or even dementia.
Implication of ocular examinations to detect Alzheimer’s disease or dementia
Besides the increasing observational epidemiological studies that implied the potential associations between certain eye disorders and dementia or AD, with the great advances in examination equipment, numerous structural and functional ocular changes are detected to be closely related or even of potential predictive values for dementia or AD assessment and diagnosis.
Previously, diagnostic methods for dementia or AD were usually invasive, expensive, and time-consuming, including cerebrospinal fluid analysis, neuroimaging examination, neuropsychological assessment, blood examination, and so on (86). Although cerebrospinal fluid analysis and neuroimaging markers are gold standards for the diagnosis of dementia or AD, neither of them is cost-effective enough to be utilized as clinical screening and early diagnostic tools (87). Additionally, many clinical trials have been undertaken and some promising agents, such as lecanemab and donanemab, were supposed to contribute to slowing the cognition decline in patients with early symptomatic AD (88-91). These great advances in AD therapeutics have prompted a higher requirement for the earlier, possibly preclinical stage detection of the disease. Only with the redoubled efforts of early diagnosis of dementia using noninvasive, widely available, and sensitive methods or techniques could preventive therapy exert its maximum efforts on the therapeutic effect.
Over the past few decades, numerous new imaging technologies, especially retinal imaging modalities, have been developed and hold promising fields in scientific research and clinical application. Ocular changes often precede brain symptoms, and the eye, as an extension of the central nervous system (CNS), therefore provides a noninvasive and easily accessible window for high-resolution imaging of CNS tissue. Meanwhile, many neurodegenerative, vascular, neuropathological, and other ocular abnormalities can now be imaged at unprecedented resolution and demonstrated in many different forms (15-18). With the general application and increased accuracy of new imaging modalities, the eye becomes a promising window for biomarker development and gives novel insights for the early diagnosis of dementia or AD.
Detection of ocular neurodegenerative changes in Alzheimer’s disease or dementia
Growing evidence shows that the abnormalities in the brain of patients with AD share many similarities with those in the eyes. AD is represented by the loss of neurons and the weakening of synaptic plasticity in the cortical and specific subcortical regions (92). Patients with AD were also observed to have some neurodegenerative changes, such as loss of retinal ganglion cells (RGCs), thinning of the retinal nerve fiber layer (RNFL), and other structural and functional changes (93). All of these pathological changes serve as the basis for clinical application in determining the association between ocular changes in patients with AD.
Among the commonly detected neurodegenerative changes, RNFL is most often measured and determined to have associations with the progression of AD. RNFL is composed of axons of the ganglion cells projected to the CNS through the optic nerve. Detected by OCT, the reduction of RNFL thickness was significantly found in patients with mild cognitive impairment, dementia, or AD when compared to controls (19, 94-98). A study in Spain reported that RNFL thickness were significantly thinner in all quadrants in patients with mild cognitive impairment and AD (94). In mild-moderate AD patients, a significant reduction of RNFL thickness was reported to be more evident in the superior quadrant (95). The risk of dementia and AD was increased and associated with thinner RNFL at baseline in a prospective population-based cohort study conducted by Mutlu and his colleagues (97). In a Japanese study, the reduced thickness of the macular RNFL rather than the peripapillary RNFL (ppRNFL) was reported to be associated with dementia (99). Patients with AD were also reported to have a thinner average global circumpapillary RNFL thickness (96, 100). A cross-sectional imaging study reported that there were generalized decreases in the whole and individual retinal thickness among carriers of familial AD who were cognitively unimpaired years before clinical onset (101). Similarly, in patients at high genetic risk of sporadic AD, a significant volume reduction was observed (102). When performing the preclinical stage of AD screening in patients who are cognitively normal but have in vivo AD pathologic abnormalities, a reduction of RNFL thickness, particularly in the inferior quadrant, was detected (103). When combined with the use of magnetic resonance imaging for screening tests, another showed that thinner RNFL was associated with atrophy of visual and limbic networks that correlated with AD progression (104). When combined with the detection of cerebrospinal fluid, the RNFL and retinal ganglion cell layer were thinner in patients with the AD biomarker Tau protein (16). Subtle thickness changes in the inner nasal macular region were also detected in patients with subjective cognitive decline (17). A greater decline in processing speed from childhood to adulthood was suggested to be associated with a thinner RNFL (105). In contrast, a few studies did not identify significant differences in the RNFL thickness of cognitively impaired, dementia, or AD patients compared to controls (106-108).
While the compositions of retinal ganglion cell-inner plexiform layer (GC-IPL) correspond to gray matter components of the brain. GC-IPL thickness was found to be inversely associated with the presence of dementia (99). Interestingly, in Mutlu’s cohort study, thinner GC-IPL was associated with prevalent dementia but not with incident dementia (97). All macular and foveal thickness measurements were also thicker in the mild cognitive impairment group and controls than in the AD group (94). Byun and his colleagues reported a reduction of inner nasal macular thickness in patients who are cognitively normal but have in vivo AD pathologic abnormalities (103). In a study that randomly sampled community-dwelling Korean individuals, a thinner baseline total macular RNFL thickness was found to be associated with greater cognitive decline and a higher prevalence of cognitive impairment and AD (109). Recently, meta-analyses were conducted to generate pooled estimations between retinal measurements and dementia or AD. Chan’s study suggested that patients with AD showed significant differences in the thickness of GC-IPL, ganglion cell complex, ppRNFL, macular inner and outer sectors, and choroidal layers under the pooled estimation of 30 eligible studies (110). Ge and his colleagues also suggested thinner ppRNFL, total macular, and subfoveal choroid thickness in patients with AD (111).
Detection of the pathological changes in the retina and optic nerve of patients with dementia or AD provides a critical approach to finding surrogate markers of the pathology in the central nervous system. With the subsequent evolution and general application of the updated imaging technology, there would be a greater characterization of the retinal layer changes associated with AD.
Detection of ocular vasculature changes in Alzheimer’s disease or dementia
With the invention of ocular vascular imaging technology, ocular vasculature changes are visualized and measured easily and objectively, which are considered significant components in triggering the development of dementia and AD (17, 112). With the increasing clues of vascular changes being identified, it is of great significance to understand the specific pathophysiological processes that confer the development and progression of dementia and AD.
Early in 2013, Frost’s study measured the retinal vascular changes concerning brain amyloid plaque burden and AD (113). The authors investigated 19 retinal vascular parameters from digital retinal color photographs, and a number of them were found to be significantly different in patients with AD (113). Later in 2014, Cheung and coworkers conducted a case-control study and measured a spectrum of quantitative retinal microvascular parameters on retinal photographs (114). Their results suggested that patients with a narrower venular caliber and sparser and more tortuous retinal vessels were at higher risk of AD development (114). Similar findings were reported in another recent study (112). In a nested case-control study, generalized arteriolar narrowing was reported to be associated with a higher risk of disabling dementia after multivariable adjustment (115). Using the Dynamic Vessel Analysis system, the amplitude of retinal venous and arterial pulsations was detected and found to correlate with Aβ plaque load, which was related to the subsequent clinical dementia (19). Alterations in retinal arterial oscillations were also detected in patients with mild cognitive impairment and AD (116).
With the application of OCTA, retinal vascular pathologies could be detected, and lower retinal vascular density, larger foveal avascular zone (FAZ), and lower choroidal thickness were suggested to be significantly associated with AD (98, 117-119). O’Bryhim and his colleagues persisted in a more than 3-year follow-up case-control study and proved that the FAZ was enlarged in cognitively healthy individuals with preclinical AD (98). The report of their first longitudinal follow-up further confirmed their previous results (117). In a cross-sectional study enrolled patients with POAG and AD, significantly reduced vessel density in the deep vascular plexus and an enlarged FAZ were seen in patients with AD when compared to those in the POAG and control groups (119). When compared to controls, a significantly reduced macular vessel density in both superficial and deep capillary plexuses was seen in patients with AD (120). Another study measuring vessel length density also came out with a similar result (18). In a study investigating the choroidal structures, results showed that patients with AD were more likely to have a greater total choroidal area, greater luminal area, and lower choroidal vascularity index (121). Moreover, patients with retinal hemorrhages and arteriovenous nicking were reported to have higher odds of mild cognitive impairment/dementia with a primary or secondary diagnosis of cerebrovascular disease in a biracial population-based study (122).
However, in another study that assessed patients with preclinical AD, the authors found no significant differences in FAZ between groups (123). No associations of vessel density and FAZ with global cognitive function or incident dementia were found in another population-based study (124). Recent meta-analyses have also reported conflicting results. Katsimpris and his colleagues generated a pooled estimation of retinal vessel density for patients with AD and healthy controls, and they suggested that the vessel density of the whole and parafoveal superficial vascular plexus was significantly lower in AD (125). In an umbrella review performed by Costanzo and his colleagues, those ocular biomarkers with the best evidence were shown to have poor to moderate diagnostic accuracy (126). Nonetheless, it is valuable to investigate the association between vascular changes and the development of dementia or AD, since the retinal blood flow offers a unique and feasible window for measurements and potential biomarker identification.
Detection of neuropathological features in Alzheimer’s disease or dementia
Besides neurogenerative and vascular changes detected in the eye, the typical neuropathological features of dementia or AD, amyloid β-protein (Aβ) plaques and tau deposits, are also suggested to be present in the degeneration of inner retinal layers.
The two classical neuropathological features of AD are neuronal and glial abnormal protein deposits of misfolded endogenous proteins, which were hallmarked as extracellular Aβ plaques and intracellular neurofibrillary tangles that generated from aggregation of hyperphosphorylated tau protein (pTau) (127, 128). In postmortem retinas of patients with AD, the accumulation of Aβ plaques was remarkably observed within the inner layers and around the melanopsin retinal ganglion cells (95, 129-131). As for the aggregation of tau protein, several studies revealed disparate results, while others were just performed on the animal level, and further investigation should be conducted (132-135).
In general, there is no consensus regarding the presence of Aβ and tau proteins in the retinas of patients with AD. Retinal Aβ and tau protein imaging has not yet been applied for clinical population-based screening. Future studies are needed to clarify the distribution, manifestation, and prevalence of them among AD and other diseases prior to widespread application. Moreover, the following studies are also important to determine if retinal Aβ loads persist over time and how these deposits change with disease progression (136).
Detection of other potential ocular modalities in Alzheimer’s disease or dementia
Other ocular detection modalities also provide important information about some ocular assessments of dementia or AD.
Ocular motility was assessed and potentially supported a diagnosis of dementia or AD (137, 138). Pupillary light response may also be a biomarker in preclinical AD detection, since it is considered the integrity of the locus coeruleus, where the earliest AD neuropathology is detected (139, 140). Retinal oximetry was developed to detect retinal vessel oxygen saturation, and retinal vessel oxygen saturation was significantly different in patients with dementia and AD (141, 142). Detection of Apoptosing Retinal Cells, a recent advancement in retinal imaging technology, was used in conjunction with fluorescent annexin A5 to detect degenerating RGCs, allowing early visualization of AD and the association between eye disorders and the brain (143-145). Electroretinography was used to detect RCG dysfunction and investigate retinal electrophysiologic function, which could help document retinal functional changes that precede cognitive decline in AD (146). Additionally, tear fluid, which consists of an aqueous-lipidic layer, provides proteomics and lipidomics analysis to detect biomarkers for numerous ocular and systemic conditions (147, 148). Corneal confocal microscopy was used to investigate corneal nerve fiber loss, which was found to be associated with cognitive function decline and loss of functional independence in patients with cognitive impairment and dementia (149). Recently, the retinal hyperspectral imaging technique was also used to identify AD-related early pathological changes in the retina (150, 151). The progression of new techniques provides a more visible and wider spectrum of ocular information for dementia and AD evaluation.
Application of artificial intelligence
The development of next-generation computational techniques such as artificial intelligence (AI) and deep learning (DL) algorithms has further enhanced the potential of data-rich retinal imaging as a promising tool and a source of biomarkers for AD, particularly for individuals at the preclinical AD stage. AI, especially DL, has been trained to manage many layers of convolutional and non-linear operations based on deep neural networks to address previously unfeasible amounts of data. Coupled with the non-invasive, cost-effective, and widely available retinal imaging equipment, such as retinal photographs, OCT, and OCTA images, AL and DL are thought to have the ability to screen and discover discriminative latent information associated with AD or other neurodegenerative diseases based on a large number of retinal images (152-154). Currently, the DL algorithm for dementia and AD detection is of great research value and is still being optimized, and it has the potential to be adopted in future clinical practice for screening AD and major eye disorders in a community setting.
Future directions
Through integrating the current evidence for the associations between ocular disorders and AD or dementia, as well as the exploration and applications of different early-detection modalities, our review highlights that (1) ocular disorders are closely related to the occurrence of AD and dementia; (2) the treatment of ocular diseases might be beneficial to the development of AD and dementia; and (3) emerging research has been focused on detecting AD and dementia through ocular biomarkers and examinations, which is non-invasive, convenient, and promising (Figure 1).
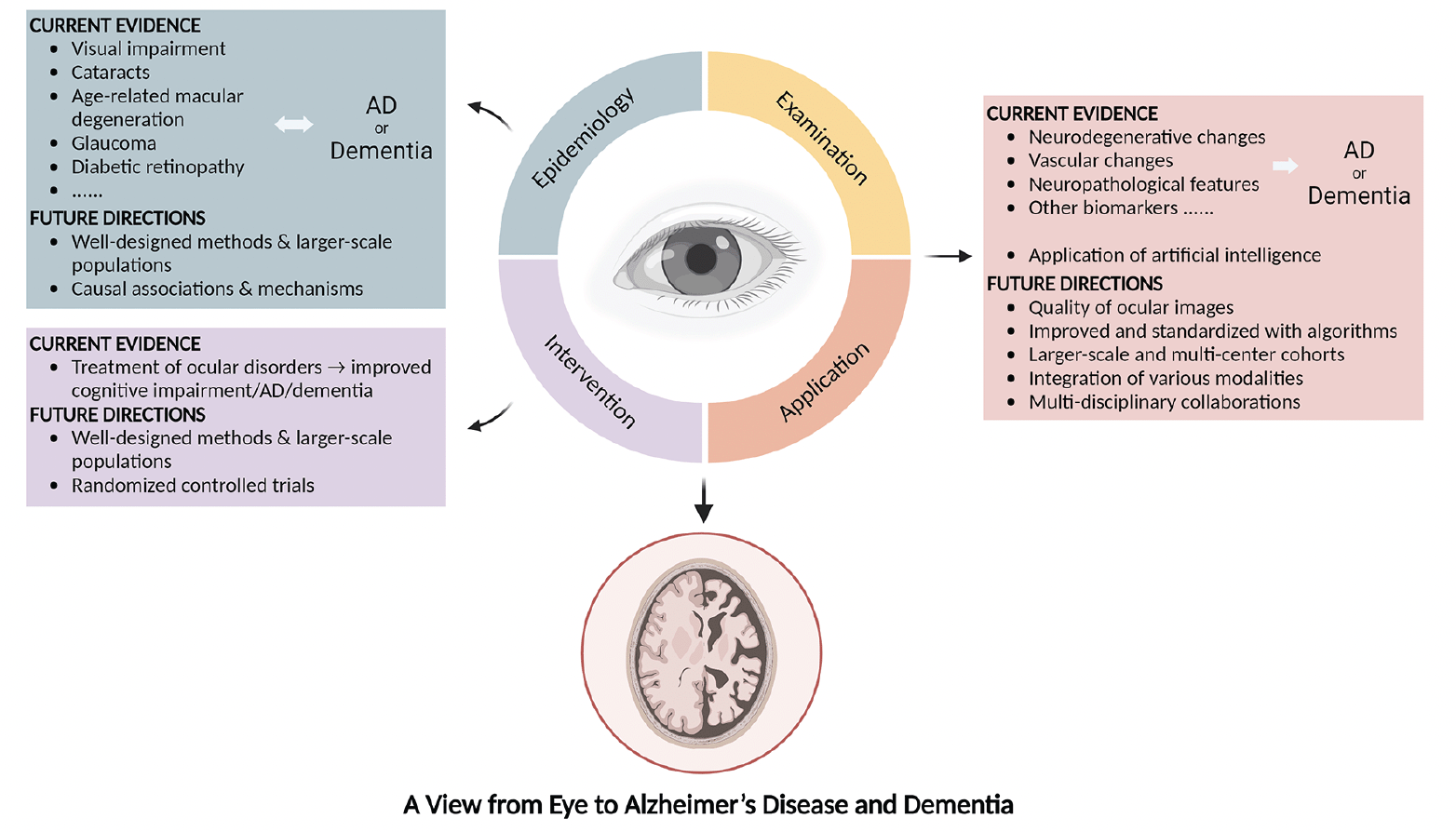
Figure 1. Current evidence and future directions on the associations between the eye and Alzheimer’s disease or dementia
AD: Alzheimer’s disease.
Findings from observational studies are still controversial, especially on the chronological order of ocular diseases and AD or dementia. Patients with AD or dementia might not be timely diagnosed, followed, and treated for ocular and metabolic diseases. Further investigations with well-designed methods and larger-scale populations are required. In addition, causal associations and shared pathogenetic mechanisms could be explored.
Detecting AD and dementia through ocular biomarkers and examinations obviously advantageous. Ocular imaging is non-invasive and cost-effective; thus, it is acceptable to most patients and can be provided in a variety of settings, including primary care centers. In addition, detection of AD or dementia at an early stage contributes to timely intervention before AD or dementia advances, and improves the quality of life. Nevertheless, the quality of ocular images, for example, the detailed architecture and morphology of the retina, needs to be improved when detecting, and standardized with algorithms. Studies on larger-scale and multi-center cohorts, multi-disciplinary collaborations, and the integration of various techniques, for instance, comparison across results from different examinations, are warranted to improve the accuracy of models.
Research exploring the associations between the eye and AD or dementia is marching forward, and effective screening and early detection of AD and dementia by ocular examinations are around the corner. As a window to AD and dementia, the eye deserves further exploration for future developments of novel disease-management strategies.
Conflict of interest: The authors declared no conflict of interest.
Funding Information: This study was funded by the National Natural Science Foundation of China (grant number 82171075, 81870663); the Science and Technology Program of Guangzhou (grant number 20220610092); the Outstanding Young Talent Trainee Program of Guangdong Provincial People’s Hospital (grant number KJ012019087); the Guangdong Provincial People’s Hospital Scientific Research Funds for Leading Medical Talents and Distinguished Young Scholars in Guangdong Province (grant number KJ012019457); the Talent Introduction Fund of Guangdong Provincial People’s Hospital (grant number Y012018145); the launch fund of Guangdong Provincial People’s Hospital for National Natural Science Foundation of China (grant number 8217040546).
Acknowledgements: The authors sincerely thank the funders for their support. The figure was created with BioRender.com.
Conflict of interest: The authors declare that they have no conflicts of interest.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Gupta VB, Chitranshi N, den Haan J, Mirzaei M, You Y, Lim JK, et al. Retinal changes in Alzheimer’s disease—integrated prospects of imaging, functional and molecular advances. 2021;82:100899.
2. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700-89. doi: 10.1002/alz.12638.
3. Nicholas LH, Langa KM, Bynum JP, Hsu JWJJim. Financial presentation of Alzheimer disease and related dementias. 2021;181(2):220-7.
4. Hansson OJNm. Biomarkers for neurodegenerative diseases. 2021;27(6):954-63.
5. Alzheimer’s AsAJ, dementia. 2019 Alzheimer’s disease facts and figures. 2019;15(3):321-87.
6. Deardorff WJ, Liu PL, Sloane R, Van Houtven C, Pieper CF, Hastings SN, et al. Association of Sensory and Cognitive Impairment With Healthcare Utilization and Cost in Older Adults. J Am Geriatr Soc. 2019;67(8):1617-24. doi: 10.1111/jgs.15891.
7. Patel N, Stagg BC, Swenor BK, Zhou Y, Talwar N, Ehrlich JR. Association of Co-occurring Dementia and Self-reported Visual Impairment With Activity Limitations in Older Adults. JAMA Ophthalmol. 2020;138(7):756-63. doi: 10.1001/jamaophthalmol.2020.1562.
8. Whitson HE, Cronin-Golomb A, Cruickshanks KJ, Gilmore GC, Owsley C, Peelle JE, et al. American Geriatrics Society and National Institute on Aging Bench-to-Bedside Conference: Sensory Impairment and Cognitive Decline in Older Adults. J Am Geriatr Soc. 2018;66(11):2052-8. doi: 10.1111/jgs.15506.
9. Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev. 2015;23(Pt B):154-66. doi: 10.1016/j.arr.2015.06.002.
10. Shang X, Zhu Z, Wang W, Ha J, He M. The Association between Vision Impairment and Incidence of Dementia and Cognitive Impairment: A Systematic Review and Meta-analysis. Ophthalmology. 2021;128(8):1135-49. doi: 10.1016/j.ophtha.2020.12.029.
11. Lai S-W, Lin C-L, Liao K-F. Cataract may be a non-memory feature of Alzheimer’s disease in older people. Eur J Epidemiol. 2014;29(6):405-9. doi: 10.1007/s10654-014-9903-6.
12. Le JT, Agrón E, Keenan TDL, Clemons TE, Brenowitz WD, Yaffe K, et al. Assessing bidirectional associations between cognitive impairment and late age-related macular degeneration in the Age-Related Eye Disease Study 2. Alzheimers Dement. 2022;18(7):1296-305. doi: 10.1002/alz.12473.
13. Helmer C, Malet F, Rougier M-B, Schweitzer C, Colin J, Delyfer M-N, et al. Is there a link between open-angle glaucoma and dementia? The Three-City-Alienor cohort. Ann Neurol. 2013;74(2):171-9. doi: 10.1002/ana.23926.
14. Chan RNF, Tang Z, Chan VTT, Chan RNC, Cheng ETW, Ng NCY, et al. The cross-sectional and longitudinal relationship of diabetic retinopathy to cognitive impairment: a systematic review and meta-analysis. Eye (Lond). 2023;37(2):220-7. doi: 10.1038/s41433-022-02033-2.
15. Cheung CY-L, Ong Y-T, Ikram MK, Chen C, Wong TY. Retinal microvasculature in Alzheimer’s disease. J Alzheimers Dis. 2014;42 Suppl 4:S339-S52. doi: 10.3233/JAD-141596.
16. López-de-Eguileta A, López-García S, Lage C, Pozueta A, García-Martínez M, Kazimierczak M, et al. The retinal ganglion cell layer reflects neurodegenerative changes in cognitively unimpaired individuals. Alzheimers Res Ther. 2022;14(1):57. doi: 10.1186/s13195-022-00998-6.
17. Marquié M, Valero S, Castilla-Marti M, Martínez J, Rodríguez-Gómez O, Sanabria Á, et al. Association between retinal thickness and β-amyloid brain accumulation in individuals with subjective cognitive decline: Fundació ACE Healthy Brain Initiative. Alzheimers Res Ther. 2020;12(1):37. doi: 10.1186/s13195-020-00602-9.
18. Biscetti L, Lupidi M, Luchetti E, Eusebi P, Gujar R, Vergaro A, et al. Novel noninvasive biomarkers of prodromal Alzheimer disease: The role of optical coherence tomography and optical coherence tomography-angiography. Eur J Neurol. 2021;28(7):2185-91. doi: 10.1111/ene.14871.
19. Golzan SM, Goozee K, Georgevsky D, Avolio A, Chatterjee P, Shen K, et al. Retinal vascular and structural changes are associated with amyloid burden in the elderly: ophthalmic biomarkers of preclinical Alzheimer’s disease. Alzheimers Res Ther. 2017;9(1):13. doi: 10.1186/s13195-017-0239-9.
20. Court H, McLean G, Guthrie B, Mercer SW, Smith DJ. Visual impairment is associated with physical and mental comorbidities in older adults: a cross-sectional study. BMC Med. 2014;12:181. doi: 10.1186/s12916-014-0181-7.
21. Chen SP, Bhattacharya J, Pershing S. Association of Vision Loss With Cognition in Older Adults. JAMA Ophthalmol. 2017;135(9):963-70. doi: 10.1001/jamaophthalmol.2017.2838.
22. Luo Y, He P, Guo C, Chen G, Li N, Zheng X. Association Between Sensory Impairment and Dementia in Older Adults: Evidence from China. J Am Geriatr Soc. 2018;66(3):480-6. doi: 10.1111/jgs.15202.
23. Davies-Kershaw HR, Hackett RA, Cadar D, Herbert A, Orrell M, Steptoe A. Vision Impairment and Risk of Dementia: Findings from the English Longitudinal Study of Ageing. J Am Geriatr Soc. 2018;66(9):1823-9. doi: 10.1111/jgs.15456.
24. Ehrlich JR, Goldstein J, Swenor BK, Whitson H, Langa KM, Veliz P. Addition of Vision Impairment to a Life-Course Model of Potentially Modifiable Dementia Risk Factors in the US. JAMA Neurol. 2022;79(6):623-6. doi: 10.1001/jamaneurol.2022.0723.
25. Rogers MAM, Langa KM. Untreated poor vision: a contributing factor to late-life dementia. Am J Epidemiol. 2010;171(6):728-35. doi: 10.1093/aje/kwp453.
26. Chen SP, Azad AD, Pershing S. Bidirectional Association between Visual Impairment and Dementia Among Older Adults in the United States Over Time. Ophthalmology. 2021;128(9):1276-83. doi: 10.1016/j.ophtha.2021.02.021.
27. Zheng DD, Swenor BK, Christ SL, West SK, Lam BL, Lee DJ. Longitudinal Associations Between Visual Impairment and Cognitive Functioning: The Salisbury Eye Evaluation Study. JAMA Ophthalmol. 2018;136(9):989-95. doi: 10.1001/jamaophthalmol.2018.2493.
28. Lim ZW, Chee M-L, Soh ZD, Cheung N, Dai W, Sahil T, et al. Association Between Visual Impairment and Decline in Cognitive Function in a Multiethnic Asian Population. JAMA Netw Open. 2020;3(4):e203560. doi: 10.1001/jamanetworkopen.2020.3560.
29. Naël V, Pérès K, Dartigues J-F, Letenneur L, Amieva H, Arleo A, et al. Vision loss and 12-year risk of dementia in older adults: the 3C cohort study. Eur J Epidemiol. 2019;34(2):141-52. doi: 10.1007/s10654-018-00478-y.
30. Ehrlich JR, Swenor BK, Zhou Y, Langa KM. The Longitudinal Association of Vision Impairment With Transitions to Cognitive Impairment and Dementia: Findings From the Aging, Demographics and Memory Study. J Gerontol A Biol Sci Med Sci. 2021;76(12):2187-93. doi: 10.1093/gerona/glab157.
31. Lee ATC, Richards M, Chan WC, Chiu HFK, Lee RSY, Lam LCW. Higher Dementia Incidence in Older Adults with Poor Visual Acuity. J Gerontol A Biol Sci Med Sci. 2020;75(11):2162-8. doi: 10.1093/gerona/glaa036.
32. Tran EM, Stefanick ML, Henderson VW, Rapp SR, Chen J-C, Armstrong NM, et al. Association of Visual Impairment With Risk of Incident Dementia in a Women’s Health Initiative Population. JAMA Ophthalmol. 2020;138(6):624-33. doi: 10.1001/jamaophthalmol.2020.0959.
33. Zhu Z, Shi D, Liao H, Ha J, Shang X, Huang Y, et al. Visual Impairment and Risk of Dementia: The UK Biobank Study. Am J Ophthalmol. 2022;235. doi: 10.1016/j.ajo.2021.08.010.
34. Littlejohns TJ, Hayat S, Luben R, Brayne C, Conroy M, Foster PJ, et al. Visual Impairment and Risk of Dementia in 2 Population-Based Prospective Cohorts: UK Biobank and EPIC-Norfolk. J Gerontol A Biol Sci Med Sci. 2022;77(4):697-704. doi: 10.1093/gerona/glab325.
35. Kuo P-L, Huang AR, Ehrlich JR, Kasper J, Lin FR, McKee MM, et al. Prevalence of Concurrent Functional Vision and Hearing Impairment and Association With Dementia in Community-Dwelling Medicare Beneficiaries. JAMA Netw Open. 2021;4(3):e211558. doi: 10.1001/jamanetworkopen.2021.1558.
36. Hwang PH, Longstreth WT, Thielke SM, Francis CE, Carone M, Kuller LH, et al. Longitudinal Changes in Hearing and Visual Impairments and Risk of Dementia in Older Adults in the United States. JAMA Netw Open. 2022;5(5):e2210734. doi: 10.1001/jamanetworkopen.2022.10734.
37. Pabst A, Bär J, Röhr S, Löbner M, Kleineidam L, Heser K, et al. Do self-reported hearing and visual impairments predict longitudinal dementia in older adults? J Am Geriatr Soc. 2021;69(6):1519-28. doi: 10.1111/jgs.17074.
38. Kuźma E, Littlejohns TJ, Khawaja AP, Llewellyn DJ, Ukoumunne OC, Thiem U. Visual Impairment, Eye Diseases, and Dementia Risk: A Systematic Review and Meta-Analysis. J Alzheimers Dis. 2021;83(3):1073-87. doi: 10.3233/JAD-210250.
39. Pelak VS, Smyth SF, Boyer PJ, Filley CM. Computerized visual field defects in posterior cortical atrophy. Neurology. 2011;77(24):2119-22. doi: 10.1212/WNL.0b013e31823e9f2a.
40. Fenwick EK, Gan ATL, Man REK, Gupta P, Sabanayagam C, Cheng C-Y, et al. Vision, vision-specific functioning and mobility, and their relationship with clinically assessed cognitive impairment. Age Ageing. 2021;50(4):1236-42. doi: 10.1093/ageing/afaa276.
41. Risacher SL, Wudunn D, Pepin SM, MaGee TR, McDonald BC, Flashman LA, et al. Visual contrast sensitivity in Alzheimer’s disease, mild cognitive impairment, and older adults with cognitive complaints. Neurobiol Aging. 2013;34(4):1133-44. doi: 10.1016/j.neurobiolaging.2012.08.007.
42. Hong T, Mitchell P, Burlutsky G, Gopinath B, Liew G, Wang JJ. Visual impairment and depressive symptoms in an older Australian cohort: longitudinal findings from the Blue Mountains Eye Study. Br J Ophthalmol. 2015;99(8):1017-21. doi: 10.1136/bjophthalmol-2014-306308.
43. Carrière I, Delcourt C, Daien V, Pérès K, Féart C, Berr C, et al. A prospective study of the bi-directional association between vision loss and depression in the elderly. J Affect Disord. 2013;151(1):164-70. doi: 10.1016/j.jad.2013.05.071.
44. Marchesi N, Fahmideh F, Boschi F, Pascale A, Barbieri A. Ocular Neurodegenerative Diseases: Interconnection between Retina and Cortical Areas. Cells. 2021;10(9). doi: 10.3390/cells10092394.
45. Sivak JM. The aging eye: common degenerative mechanisms between the Alzheimer’s brain and retinal disease. Invest Ophthalmol Vis Sci. 2013;54(1):871-80. doi: 10.1167/iovs.12-10827.
46. Liu Y-C, Wilkins M, Kim T, Malyugin B, Mehta JS. Cataracts. Lancet. 2017;390(10094):600-12. doi: 10.1016/S0140-6736(17)30544-5.
47. Shang X, Zhu Z, Huang Y, Zhang X, Wang W, Shi D, et al. Associations of ophthalmic and systemic conditions with incident dementia in the UK Biobank. Br J Ophthalmol. 2023;107(2):275-82. doi: 10.1136/bjophthalmol-2021-319508.
48. Hwang PH, Longstreth WT, Thielke SM, Francis CE, Carone M, Kuller LH, et al. Ophthalmic conditions associated with dementia risk: The Cardiovascular Health Study. Alzheimers Dement. 2021;17(9):1442-51. doi: 10.1002/alz.12313.
49. Ma L-Z, Zhang Y-R, Li Y-Z, Ou Y-N, Yang L, Chen S-D, et al. Cataract, Cataract Surgery, and Risk of Incident Dementia: A Prospective Cohort Study of 300,823 Participants. Biol Psychiatry. 2023;93(9):810-9. doi: 10.1016/j.biopsych.2022.06.005.
50. Lee CS, Larson EB, Gibbons LE, Lee AY, McCurry SM, Bowen JD, et al. Associations between recent and established ophthalmic conditions and risk of Alzheimer’s disease. Alzheimers Dement. 2019;15(1):34-41. doi: 10.1016/j.jalz.2018.06.2856.
51. Jefferis JM, Mosimann UP, Clarke MP. Cataract and cognitive impairment: a review of the literature. Br J Ophthalmol. 2011;95(1):17-23. doi: 10.1136/bjo.2009.165902.
52. McCormick I, Butcher R, Evans JR, Mactaggart IZ, Limburg H, Jolley E, et al. Effective cataract surgical coverage in adults aged 50 years and older: estimates from population-based surveys in 55 countries. Lancet Glob Health. 2022;10(12):e1744-e53. doi: 10.1016/S2214-109X(22)00419-3.
53. Wong WL, Su X, Li X, Cheung CMG, Klein R, Cheng C-Y, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106-e16. doi: 10.1016/S2214-109X(13)70145-1.
54. Ohno-Matsui K. Parallel findings in age-related macular degeneration and Alzheimer’s disease. Prog Retin Eye Res. 2011;30(4):217-38. doi: 10.1016/j.preteyeres.2011.02.004.
55. Woo SJ, Park KH, Ahn J, Choe JY, Jeong H, Han JW, et al. Cognitive impairment in age-related macular degeneration and geographic atrophy. Ophthalmology. 2012;119(10):2094-101. doi: 10.1016/j.ophtha.2012.04.026.
56. Tsai D-C, Chen S-J, Huang C-C, Yuan M-K, Leu H-B. Age-Related Macular Degeneration and Risk of Degenerative Dementia among the Elderly in Taiwan: A Population-Based Cohort Study. Ophthalmology. 2015;122(11). doi: 10.1016/j.ophtha.2015.07.033.
57. Choi S, Jahng WJ, Park SM, Jee D. Association of Age-Related Macular Degeneration on Alzheimer or Parkinson Disease: A Retrospective Cohort Study. Am J Ophthalmol. 2020;210:41-7. doi: 10.1016/j.ajo.2019.11.001.
58. Schwaber EJ, Thompson AC, Smilnak G, Stinnett SS, Whitson HE, Lad EM. Co-Prevalence of Alzheimer’s Disease and Age-Related Macular Degeneration Established by Histopathologic Diagnosis. J Alzheimers Dis. 2020;76(1):207-15. doi: 10.3233/JAD-200111.
59. Chua J, Zhang Z, Wong D, Tan B, Kulantayan B, Sng CCA, et al. Age-Related Eye Diseases in Individuals With Mild Cognitive Impairment and Alzheimer’s Disease. Front Aging Neurosci. 2022;14:933853. doi: 10.3389/fnagi.2022.933853.
60. Keenan TDL, Goldacre R, Goldacre MJ. Associations between age-related macular degeneration, Alzheimer disease, and dementia: record linkage study of hospital admissions. JAMA Ophthalmol. 2014;132(1):63-8. doi: 10.1001/jamaophthalmol.2013.5696.
61. Rong SS, Lee BY, Kuk AK, Yu XT, Li SS, Li J, et al. Comorbidity of dementia and age-related macular degeneration calls for clinical awareness: a meta-analysis. Br J Ophthalmol. 2019;103(12):1777-83. doi: 10.1136/bjophthalmol-2018-313277.
62. Jiang L, Li J-C, Tang B-S, Guo J-F, Shen L. Lack of bidirectional association between age-related macular degeneration and Alzheimer’s disease: A Mendelian randomization study. Alzheimers Dement. 2022;18(12):2725-9. doi: 10.1002/alz.12775.
63. Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-90. doi: 10.1016/j.ophtha.2014.05.013.
64. Lai S-W, Lin C-L, Liao K-F. Glaucoma may be a non-memory manifestation of Alzheimer’s disease in older people. Int Psychogeriatr. 2017:1-7. doi: 10.1017/S1041610217000801.
65. Wändell PE, Ljunggren G, Wahlström L, Carlsson AC. Psychiatric diseases and dementia and their association with open-angle glaucoma in the total population of Stockholm. Ann Med. 2022;54(1):3349-56. doi: 10.1080/07853890.2022.2148735.
66. Keenan TDL, Goldacre R, Goldacre MJ. Associations between primary open angle glaucoma, Alzheimer’s disease and vascular dementia: record linkage study. Br J Ophthalmol. 2015;99(4):524-7. doi: 10.1136/bjophthalmol-2014-305863.
67. Pan Y, Suga A, Kimura I, Kimura C, Minegishi Y, Nakayama M, et al. METTL23 mutation alters histone H3R17 methylation in normal-tension glaucoma. J Clin Invest. 2022;132(21). doi: 10.1172/JCI153589.
68. Mullany S, Xiao L, Qassim A, Marshall H, Gharahkhani P, MacGregor S, et al. Normal-tension glaucoma is associated with cognitive impairment. Br J Ophthalmol. 2022;106(7):952-6. doi: 10.1136/bjophthalmol-2020-317461.
69. Bikbov MM, Zainullin RM, Gilmanshin TR, Kazakbaeva GM, Yakupova DF, Nuriev IF, et al. Prevalence and Associated Factors of Pseudoexfoliation in a Russian Population: The Ural Eye and Medical Study. Am J Ophthalmol. 2020;210:158-66. doi: 10.1016/j.ajo.2019.10.003.
70. van Sloten TT, Sedaghat S, Carnethon MR, Launer LJ, Stehouwer CDA. Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020;8(4):325-36. doi: 10.1016/S2213-8587(19)30405-X.
71. Ding J, Strachan MWJ, Reynolds RM, Frier BM, Deary IJ, Fowkes FGR, et al. Diabetic retinopathy and cognitive decline in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes. 2010;59(11):2883-9. doi: 10.2337/db10-0752.
72. Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, Baertlein L, et al. Association of diabetes with amnestic and nonamnestic mild cognitive impairment. Alzheimers Dement. 2014;10(1):18-26. doi: 10.1016/j.jalz.2013.01.001.
73. de Almeida Faria ACR, Dall’Agnol JF, Gouveia AM, de Paiva CI, Segalla VC, Baena CP. Risk factors for cognitive decline in type 2 diabetes mellitus patients in Brazil: a prospective observational study. Diabetol Metab Syndr. 2022;14(1):105. doi: 10.1186/s13098-022-00872-3.
74. Exalto LG, Biessels GJ, Karter AJ, Huang ES, Quesenberry CP, Whitmer RA. Severe diabetic retinal disease and dementia risk in type 2 diabetes. J Alzheimers Dis. 2014;42 Suppl 3(0 3):S109-S17. doi: 10.3233/JAD-132570.
75. Crosby-Nwaobi RR, Sivaprasad S, Amiel S, Forbes A. The relationship between diabetic retinopathy and cognitive impairment. Diabetes Care. 2013;36(10):3177-86. doi: 10.2337/dc12-2141.
76. Crosby-Nwaobi R, Sivaprasad S, Forbes A. A systematic review of the association of diabetic retinopathy and cognitive impairment in people with Type 2 diabetes. Diabetes Res Clin Pract. 2012;96(2):101-10. doi: 10.1016/j.diabres.2011.11.010.
77. Cheng D, Zhao X, Yang S, Wang G, Ning G. Association Between Diabetic Retinopathy and Cognitive Impairment: A Systematic Review and Meta-Analysis. Front Aging Neurosci. 2021;13:692911. doi: 10.3389/fnagi.2021.692911.
78. Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2010;117(2). doi: 10.1016/j.ophtha.2009.07.017.
79. Chan AX, Bakhoum CY, Bangen KJ, Bakhoum MF. Relationship between Retinal Vascular Occlusions and Cognitive Dementia in a Large Cross-Sectional Cohort. Am J Ophthalmol. 2021;226:201-5. doi: 10.1016/j.ajo.2021.01.026.
80. Nam GE, Han K, Park SH, Cho KH, Song SJ. Retinal Vein Occlusion and the Risk of Dementia: A Nationwide Cohort Study. Am J Ophthalmol. 2021;221:181-9. doi: 10.1016/j.ajo.2020.07.050.
81. Schrijvers EMC, Buitendijk GHS, Ikram MK, Koudstaal PJ, Hofman A, Vingerling JR, et al. Retinopathy and risk of dementia: the Rotterdam Study. Neurology. 2012;79(4):365-70. doi: 10.1212/WNL.0b013e318260cd7e.
82. Qiu C, Cotch MF, Sigurdsson S, Jonsson PV, Jonsdottir MK, Sveinbjrnsdottir S, et al. Cerebral microbleeds, retinopathy, and dementia: the AGES-Reykjavik Study. Neurology. 2010;75(24):2221-8. doi: 10.1212/WNL.0b013e3182020349.
83. Yu WK, Chen YT, Wang SJ, Kuo SC, Shia BC, Liu CJL. Cataract surgery is associated with a reduced risk of dementia: a nationwide population-based cohort study. Eur J Neurol. 2015;22(10). doi: 10.1111/ene.12561.
84. Lee CS, Gibbons LE, Lee AY, Yanagihara RT, Blazes MS, Lee ML, et al. Association Between Cataract Extraction and Development of Dementia. JAMA Intern Med. 2022;182(2):134-41. doi: 10.1001/jamainternmed.2021.6990.
85. Dawes P, Wolski L, Himmelsbach I, Regan J, Leroi I. Interventions for hearing and vision impairment to improve outcomes for people with dementia: a scoping review. Int Psychogeriatr. 2019;31(2):203-21. doi: 10.1017/S1041610218000728.
86. Blennow K, Zetterberg H. Biomarkers for Alzheimer’s disease: current status and prospects for the future. J Intern Med. 2018;284(6):643-63. doi: 10.1111/joim.12816.
87. Laske C, Sohrabi HR, Frost SM, López-de-Ipiña K, Garrard P, Buscema M, et al. Innovative diagnostic tools for early detection of Alzheimer’s disease. Alzheimers Dement. 2015;11(5):561-78. doi: 10.1016/j.jalz.2014.06.004.
88. Mintun MA, Lo AC, Duggan Evans C, Wessels AM, Ardayfio PA, Andersen SW, et al. Donanemab in Early Alzheimer’s Disease. N Engl J Med. 2021;384(18):1691-704. doi: 10.1056/NEJMoa2100708.
89. Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks J, et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA. 2023;330(6):512-27. doi: 10.1001/jama.2023.13239.
90. van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023;388(1). doi: 10.1056/NEJMoa2212948.
91. Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014;6(4):37. doi: 10.1186/alzrt269.
92. Barthet G, Mulle C. Presynaptic failure in Alzheimer’s disease. Prog Neurobiol. 2020;194:101801. doi: 10.1016/j.pneurobio.2020.101801.
93. Alber J, Goldfarb D, Thompson LI, Arthur E, Hernandez K, Cheng D, et al. Developing retinal biomarkers for the earliest stages of Alzheimer’s disease: What we know, what we don’t, and how to move forward. Alzheimers Dement. 2020;16(1):229-43. doi: 10.1002/alz.12006.
94. Ascaso FJ, Cruz N, Modrego PJ, Lopez-Anton R, Santabárbara J, Pascual LF, et al. Retinal alterations in mild cognitive impairment and Alzheimer’s disease: an optical coherence tomography study. J Neurol. 2014;261(8):1522-30. doi: 10.1007/s00415-014-7374-z.
95. La Morgia C, Ross-Cisneros FN, Koronyo Y, Hannibal J, Gallassi R, Cantalupo G, et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol. 2016;79(1). doi: 10.1002/ana.24548.
96. Chua J, Li C, Ho LKH, Wong D, Tan B, Yao X, et al. A multi-regression framework to improve diagnostic ability of optical coherence tomography retinal biomarkers to discriminate mild cognitive impairment and Alzheimer’s disease. Alzheimers Res Ther. 2022;14(1):41. doi: 10.1186/s13195-022-00982-0.
97. Mutlu U, Colijn JM, Ikram MA, Bonnemaijer PWM, Licher S, Wolters FJ, et al. Association of Retinal Neurodegeneration on Optical Coherence Tomography With Dementia: A Population-Based Study. JAMA Neurol. 2018;75(10):1256-63. doi: 10.1001/jamaneurol.2018.1563.
98. O’Bryhim BE, Apte RS, Kung N, Coble D, Van Stavern GP. Association of Preclinical Alzheimer Disease With Optical Coherence Tomographic Angiography Findings. JAMA Ophthalmol. 2018;136(11):1242-8. doi: 10.1001/jamaophthalmol.2018.3556.
99. Ito Y, Sasaki M, Takahashi H, Nozaki S, Matsuguma S, Motomura K, et al. Quantitative Assessment of the Retina Using OCT and Associations with Cognitive Function. Ophthalmology. 2020;127(1):107-18. doi: 10.1016/j.ophtha.2019.05.021.
100. McCann P, Hogg R, Wright DM, Chakravarthy U, Peto T, Cruise S, et al. Intraocular pressure and circumpapillary retinal nerve fibre layer thickness in the Northern Ireland Cohort for the Longitudinal Study of Ageing (NICOLA): distributions and associations. Br J Ophthalmol. 2021;105(7):948-56. doi: 10.1136/bjophthalmol-2020-316499.
101. Armstrong GW, Kim LA, Vingopoulos F, Park JY, Garg I, Kasetty M, et al. Retinal Imaging Findings in Carriers With PSEN1-Associated Early-Onset Familial Alzheimer Disease Before Onset of Cognitive Symptoms. JAMA Ophthalmol. 2021;139(1):49-56. doi: 10.1001/jamaophthalmol.2020.4909.
102. López-Cuenca I, Marcos-Dolado A, Yus-Fuertes M, Salobrar-García E, Elvira-Hurtado L, Fernández-Albarral JA, et al. The relationship between retinal layers and brain areas in asymptomatic first-degree relatives of sporadic forms of Alzheimer’s disease: an exploratory analysis. Alzheimers Res Ther. 2022;14(1):79. doi: 10.1186/s13195-022-01008-5.
103. Byun MS, Park SW, Lee JH, Yi D, Jeon SY, Choi HJ, et al. Association of Retinal Changes With Alzheimer Disease Neuroimaging Biomarkers in Cognitively Normal Individuals. JAMA Ophthalmol. 2021;139(5):548-56. doi: 10.1001/jamaophthalmol.2021.0320.
104. Méndez-Gómez JL, Pelletier A, Rougier M-B, Korobelnik J-F, Schweitzer C, Delyfer M-N, et al. Association of Retinal Nerve Fiber Layer Thickness With Brain Alterations in the Visual and Limbic Networks in Elderly Adults Without Dementia. JAMA Netw Open. 2018;1(7):e184406. doi: 10.1001/jamanetworkopen.2018.4406.
105. Barrett-Young A, Ambler A, Cheyne K, Guiney H, Kokaua J, Steptoe B, et al. Associations Between Retinal Nerve Fiber Layer and Ganglion Cell Layer in Middle Age and Cognition From Childhood to Adulthood. JAMA Ophthalmol. 2022;140(3):262-8. doi: 10.1001/jamaophthalmol.2021.6082.
106. van de Kreeke JA, Legdeur N, Badissi M, Nguyen HT, Konijnenberg E, Tomassen J, et al. Ocular biomarkers for cognitive impairment in nonagenarians; a prospective cross-sectional study. BMC Geriatr. 2020;20(1):155. doi: 10.1186/s12877-020-01556-1.
107. den Haan J, Csinscik L, Parker T, Paterson RW, Slattery CF, Foulkes A, et al. Retinal thickness as potential biomarker in posterior cortical atrophy and typical Alzheimer’s disease. Alzheimers Res Ther. 2019;11(1):62. doi: 10.1186/s13195-019-0516-x.
108. Mauschitz MM, Bonnemaijer PWM, Diers K, Rauscher FG, Elze T, Engel C, et al. Systemic and Ocular Determinants of Peripapillary Retinal Nerve Fiber Layer Thickness Measurements in the European Eye Epidemiology (E3) Population. Ophthalmology. 2018;125(10):1526-36. doi: 10.1016/j.ophtha.2018.03.026.
109. Kim HM, Han JW, Park YJ, Bae JB, Woo SJ, Kim KW. Association Between Retinal Layer Thickness and Cognitive Decline in Older Adults. JAMA Ophthalmol. 2022;140(7):683-90. doi: 10.1001/jamaophthalmol.2022.1563.
110. Chan VTT, Sun Z, Tang S, Chen LJ, Wong A, Tham CC, et al. Spectral-Domain OCT Measurements in Alzheimer’s Disease: A Systematic Review and Meta-analysis. Ophthalmology. 2019;126(4):497-510. doi: 10.1016/j.ophtha.2018.08.009.
111. Ge Y-J, Xu W, Ou Y-N, Qu Y, Ma Y-H, Huang Y-Y, et al. Retinal biomarkers in Alzheimer’s disease and mild cognitive impairment: A systematic review and meta-analysis. Ageing Res Rev. 2021;69:101361. doi: 10.1016/j.arr.2021.101361.
112. Hilal S, Cheung CY, Wong TY, Schmetterer L, Chen C. Retinal parameters, cortical cerebral microinfarcts, and their interaction with cognitive impairment. Int J Stroke. 2023;18(1):70-7. doi: 10.1177/17474930221097737.
113. Frost S, Kanagasingam Y, Sohrabi H, Vignarajan J, Bourgeat P, Salvado O, et al. Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl Psychiatry. 2013;3(2):e233. doi: 10.1038/tp.2012.150.
114. Cheung CY-L, Ong YT, Ikram MK, Ong SY, Li X, Hilal S, et al. Microvascular network alterations in the retina of patients with Alzheimer’s disease. Alzheimers Dement. 2014;10(2):135-42. doi: 10.1016/j.jalz.2013.06.009.
115. Jinnouchi H, Kitamura A, Yamagishi K, Kiyama M, Imano H, Okada T, et al. Retinal Vascular Changes and Prospective Risk of Disabling Dementia: the Circulatory Risk in Communities Study (CIRCS). J Atheroscler Thromb. 2017;24(7):687-95. doi: 10.5551/jat.37291.
116. Kotliar K, Ortner M, Conradi A, Hacker P, Hauser C, Günthner R, et al. Altered retinal cerebral vessel oscillation frequencies in Alzheimer’s disease compatible with impaired amyloid clearance. Neurobiol Aging. 2022;120:117-27. doi: 10.1016/j.neurobiolaging.2022.08.012.
117. O’Bryhim BE, Lin JB, Van Stavern GP, Apte RS. OCT Angiography Findings in Preclinical Alzheimer’s Disease: 3-Year Follow-Up. Ophthalmology. 2021;128(10):1489-91. doi: 10.1016/j.ophtha.2021.02.016.
118. Bulut M, Kurtuluş F, Gözkaya O, Erol MK, Cengiz A, Akıdan M, et al. Evaluation of optical coherence tomography angiographic findings in Alzheimer’s type dementia. Br J Ophthalmol. 2018;102(2):233-7. doi: 10.1136/bjophthalmol-2017-310476.
119. Zabel P, Kaluzny JJ, Wilkosc-Debczynska M, Gebska-Toloczko M, Suwala K, Zabel K, et al. Comparison of Retinal Microvasculature in Patients With Alzheimer’s Disease and Primary Open-Angle Glaucoma by Optical Coherence Tomography Angiography. Invest Ophthalmol Vis Sci. 2019;60(10):3447-55. doi: 10.1167/iovs.19-27028.
120. Chua J, Hu Q, Ke M, Tan B, Hong J, Yao X, et al. Retinal microvasculature dysfunction is associated with Alzheimer’s disease and mild cognitive impairment. Alzheimers Res Ther. 2020;12(1):161. doi: 10.1186/s13195-020-00724-0.
121. Robbins CB, Grewal DS, Thompson AC, Powers JH, Soundararajan S, Koo HY, et al. Choroidal Structural Analysis in Alzheimer Disease, Mild Cognitive Impairment, and Cognitively Healthy Controls. Am J Ophthalmol. 2021;223:359-67. doi: 10.1016/j.ajo.2020.09.049.
122. Lee MJ, Deal JA, Ramulu PY, Sharrett AR, Abraham AG. Prevalence of Retinal Signs and Association With Cognitive Status: The ARIC Neurocognitive Study. J Am Geriatr Soc. 2019;67(6):1197-203. doi: 10.1111/jgs.15795.
123. van de Kreeke JA, Nguyen H-T, Konijnenberg E, Tomassen J, den Braber A, Ten Kate M, et al. Optical coherence tomography angiography in preclinical Alzheimer’s disease. Br J Ophthalmol. 2020;104(2):157-61. doi: 10.1136/bjophthalmol-2019-314127.
124. Abraham AG, Guo X, Arsiwala LT, Dong Y, Sharrett AR, Huang D, et al. Cognitive decline in older adults: What can we learn from optical coherence tomography (OCT)-based retinal vascular imaging? J Am Geriatr Soc. 2021;69(9):2524-35. doi: 10.1111/jgs.17272.
125. Katsimpris A, Karamaounas A, Sideri AM, Katsimpris J, Georgalas I, Petrou P. Optical coherence tomography angiography in Alzheimer’s disease: a systematic review and meta-analysis. Eye (Lond). 2022;36(7):1419-26. doi: 10.1038/s41433-021-01648-1.
126. Costanzo E, Lengyel I, Parravano M, Biagini I, Veldsman M, Badhwar A, et al. Ocular Biomarkers for Alzheimer Disease Dementia: An Umbrella Review of Systematic Reviews and Meta-analyses. JAMA Ophthalmol. 2023;141(1):84-91. doi: 10.1001/jamaophthalmol.2022.4845.
127. Goedert M. NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science. 2015;349(6248):1255555. doi: 10.1126/science.1255555.
128. Avila J, Pallas N, Bolós M, Sayas CL, Hernandez F. Intracellular and extracellular microtubule associated protein tau as a therapeutic target in Alzheimer disease and other tauopathies. Expert Opin Ther Targets. 2016;20(6):653-61. doi: 10.1517/14728222.2016.1131269.
129. Koronyo Y, Biggs D, Barron E, Boyer DS, Pearlman JA, Au WJ, et al. Retinal amyloid pathology and proof-of-concept imaging trial in Alzheimer’s disease. JCI Insight. 2017;2(16). doi: 10.1172/jci.insight.93621.
130. den Haan J, Morrema THJ, Verbraak FD, de Boer JF, Scheltens P, Rozemuller AJ, et al. Amyloid-beta and phosphorylated tau in post-mortem Alzheimer’s disease retinas. Acta Neuropathol Commun. 2018;6(1):147. doi: 10.1186/s40478-018-0650-x.
131. Shi H, Koronyo Y, Rentsendorj A, Regis GC, Sheyn J, Fuchs D-T, et al. Identification of early pericyte loss and vascular amyloidosis in Alzheimer’s disease retina. Acta Neuropathol. 2020;139(5):813-36. doi: 10.1007/s00401-020-02134-w.
132. Ho C-Y, Troncoso JC, Knox D, Stark W, Eberhart CG. Beta-amyloid, phospho-tau and alpha-synuclein deposits similar to those in the brain are not identified in the eyes of Alzheimer’s and Parkinson’s disease patients. Brain Pathol. 2014;24(1):25-32. doi: 10.1111/bpa.12070.
133. Grimaldi A, Brighi C, Peruzzi G, Ragozzino D, Bonanni V, Limatola C, et al. Inflammation, neurodegeneration and protein aggregation in the retina as ocular biomarkers for Alzheimer’s disease in the 3xTg-AD mouse model. Cell Death Dis. 2018;9(6):685. doi: 10.1038/s41419-018-0740-5.
134. Rodrigues-Neves AC, Carecho R, Correia SC, Carvalho C, Campos EJ, Baptista FI, et al. Retina and Brain Display Early and Differential Molecular and Cellular Changes in the 3xTg-AD Mouse Model of Alzheimer’s Disease. Mol Neurobiol. 2021;58(7):3043-60. doi: 10.1007/s12035-021-02316-x.
135. Marquez A, Guernsey LS, Frizzi KE, Cundiff M, Constantino I, Muttalib N, et al. Tau associated peripheral and central neurodegeneration: Identification of an early imaging marker for tauopathy. Neurobiol Dis. 2021;151:105273. doi: 10.1016/j.nbd.2021.105273.
136. Wang L, Mao X. Role of Retinal Amyloid-β in Neurodegenerative Diseases: Overlapping Mechanisms and Emerging Clinical Applications. Int J Mol Sci. 2021;22(5). doi: 10.3390/ijms22052360.
137. Pelak VS. Ocular motility of aging and dementia. Curr Neurol Neurosci Rep. 2010;10(6):440-7. doi: 10.1007/s11910-010-0137-z.
138. Anderson TJ, MacAskill MR. Eye movements in patients with neurodegenerative disorders. Nat Rev Neurol. 2013;9(2):74-85. doi: 10.1038/nrneurol.2012.273.
139. Van Stavern GP, Bei L, Shui Y-B, Huecker J, Gordon M. Pupillary light reaction in preclinical Alzheimer’s disease subjects compared with normal ageing controls. Br J Ophthalmol. 2019;103(7):971-5. doi: 10.1136/bjophthalmol-2018-312425.
140. Braak H, Del Tredici K. Where, when, and in what form does sporadic Alzheimer’s disease begin? Curr Opin Neurol. 2012;25(6):708-14. doi: 10.1097/WCO.0b013e32835a3432.
141. Stefánsson E, Olafsdottir OB, Eliasdottir TS, Vehmeijer W, Einarsdottir AB, Bek T, et al. Retinal oximetry: Metabolic imaging for diseases of the retina and brain. Prog Retin Eye Res. 2019;70. doi: 10.1016/j.preteyeres.2019.04.001.
142. Einarsdottir AB, Hardarson SH, Kristjansdottir JV, Bragason DT, Snaedal J, Stefánsson E. Retinal oximetry imaging in Alzheimer’s disease. J Alzheimers Dis. 2016;49(1):79-83. doi: 10.3233/JAD-150457.
143. Yap TE, Donna P, Almonte MT, Cordeiro MF. Real-Time Imaging of Retinal Ganglion Cell Apoptosis. Cells. 2018;7(6). doi: 10.3390/cells7060060.
144. Cordeiro MF, Hill D, Patel R, Corazza P, Maddison J, Younis S. Detecting retinal cell stress and apoptosis with DARC: Progression from lab to clinic. Prog Retin Eye Res. 2022;86:100976. doi: 10.1016/j.preteyeres.2021.100976.
145. Corral-Domenge C, de la Villa P, Mansilla A, Germain F. Tools and Biomarkers for the Study of Retinal Ganglion Cell Degeneration. Int J Mol Sci. 2022;23(8). doi: 10.3390/ijms23084287.
146. Van Stavern GP. Current opinion neurology: visual pathway biomarkers in Alzheimer’s disease. Curr Opin Neurol. 2020;33(1):79-86. doi: 10.1097/WCO.0000000000000788.
147. Król-Grzymała A, Sienkiewicz-Szłapka E, Fiedorowicz E, Rozmus D, Cieślińska A, Grzybowski A. Tear Biomarkers in Alzheimer’s and Parkinson’s Diseases, and Multiple Sclerosis: Implications for Diagnosis (Systematic Review). Int J Mol Sci. 2022;23(17). doi: 10.3390/ijms231710123.
148. Nandi SK, Singh D, Upadhay J, Gupta N, Dhiman N, Mittal SK, et al. Identification of tear-based protein and non-protein biomarkers: Its application in diagnosis of human diseases using biosensors. Int J Biol Macromol. 2021;193(Pt A):838-46. doi: 10.1016/j.ijbiomac.2021.10.198.
149. Ponirakis G, Al Hamad H, Sankaranarayanan A, Khan A, Chandran M, Ramadan M, et al. Association of corneal nerve fiber measures with cognitive function in dementia. Ann Clin Transl Neurol. 2019;6(4):689-97. doi: 10.1002/acn3.746.
150. More SS, Beach JM, McClelland C, Mokhtarzadeh A, Vince R. In Vivo Assessment of Retinal Biomarkers by Hyperspectral Imaging: Early Detection of Alzheimer’s Disease. ACS Chem Neurosci. 2019;10(11):4492-501. doi: 10.1021/acschemneuro.9b00331.
151. Hadoux X, Hui F, Lim JKH, Masters CL, Pébay A, Chevalier S, et al. Non-invasive in vivo hyperspectral imaging of the retina for potential biomarker use in Alzheimer’s disease. Nat Commun. 2019;10(1):4227. doi: 10.1038/s41467-019-12242-1.
152. Wisely CE, Wang D, Henao R, Grewal DS, Thompson AC, Robbins CB, et al. Convolutional neural network to identify symptomatic Alzheimer’s disease using multimodal retinal imaging. Br J Ophthalmol. 2022;106(3):388-95. doi: 10.1136/bjophthalmol-2020-317659.
153. Cheung CY, Ran AR, Wang S, Chan VTT, Sham K, Hilal S, et al. A deep learning model for detection of Alzheimer’s disease based on retinal photographs: a retrospective, multicentre case-control study. Lancet Digit Health. 2022;4(11):e806-e15. doi: 10.1016/S2589-7500(22)00169-8.
154. Patil AD, Biousse V, Newman NJ. Artificial intelligence in ophthalmology: an insight into neurodegenerative disease. Curr Opin Ophthalmol. 2022;33(5):432-9. doi: 10.1097/ICU.0000000000000877.
© The Authors 2023
