K. Malzbender1, P. Barbarino2, P. Barkman Ferrell3, A. Bradshaw4, A.J. Brookes5, C. Díaz6, W.M. van der Flier7,8,9, J. Georges4, O. Hansson10,11, M. Hartmanis12, L. Jönsson13, R. Krishnan34, T. MacLeod3, F. Mangialasche13,14, P. Mecocci13,15, C. Minguillon16,17,18, L. Middleton19,20, S. Pla6, S.P. Sardi34, M. Schöll21,22,23, M. Suárez-Calvet16,17,18,24, W. Weidner2, P.J. Visser7,13,25, H. Zetterberg26,27,28,29,30,31, N. Bose1, A. Solomon13,19,32, M. Kivipelto12,13,14,19,33 on behalf of the AD-RIDDLE Consortium
1. Health and Life Sciences, Gates Ventures, Kirkland, WA, United States; 2. Alzheimer’s Disease International, London, UK; 3. Davos Alzheimer Collaborative, Geneva, Switzerland; 4. Alzheimer Europe, Luxembourg Ville, Luxembourg; 5. Department of Genetics and Genome Biology, University of Leicester, Leicester, United Kingdom; 6. Synapse Research Management Partners SL, Madrid, Spain; 7. Alzheimer Center Amsterdam, Neurology, Vrije Universiteit Amsterdam, Amsterdam UMC location VUmc, Amsterdam, The Netherlands; 8. Amsterdam Neuroscience, Neurodegeneration, Amsterdam, Netherlands; 9. Epidemiology and Data Science, Vrije Universiteit Amsterdam, Amsterdam UMC location VUmc, Amsterdam, The Netherlands; 10. Clinical Memory Research Unit, Department of Clinical Sciences Malmö, Faculty of Medicine, Lund University, Lund, Sweden; 11. Memory Clinic, Skåne University Hospital, Malmö, Sweden; 12. FINGERS Brain Health Institute, Stockholm, Sweden;
13. Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, Stockholm, Sweden; 14. Karolinska University Hospital, Theme Inflammation and Aging, Medical Unit Aging, Stockholm, Sweden; 15. Department of Gerontology and Geriatrics, Department of Medicine and Surgery, University of Perugia, Perugia, Italy; 16. Barcelonaβeta Brain Research Center (BBRC), Pasqual Maragall Foundation, Barcelona, Spain; 17. Hospital del Mar Medical Research Institute (IMIM), Barcelona, Spain; 18. Centro de Investigación Biomédica en Red de Fragilidad y Envejecimiento Saludable (CIBER-FES), Instituto de Salud Carlos III, Madrid, Spain; 19. Ageing Research, School of Public Health, Imperial College London, London, UK; 20. Directorate of Public Health and Primary Care, Imperial College NHS Healthcare Trust, London, UK; 21. Department of Psychiatry and Neurochemistry, Institute of Physiology and Neuroscience, University of Gothenburg, Gothenburg, Sweden; 22. Department of Neurodegenerative Disease, UCL Queen Square Institute of Neurology, University College London, London, UK; 23. Department of Psychiatry, Cognition and Aging Psychiatry, Sahlgrenska University Hospital, Mölndal, Sweden; 24. Servei de Neurologia, Hospital del Mar, Barcelona, Spain; 25. Alzheimer Center Limburg, School for Mental Health and Neuroscience, Maastricht University, Maastricht, Netherlands; 26. Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, The Sahlgrenska Academy at the University of Gothenburg, Mölndal, Sweden; 27. Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden; 28. Department of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK; 29. UK Dementia Research Institute at UCL, London, UK; 30. Hong Kong Center for Neurodegenerative Diseases, Clear Water Bay, Hong Kong, China; 31. Wisconsin Alzheimer’s Disease Research Center, University of Wisconsin School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI, USA; 32. University of Eastern Finland, Institute of Clinical Medicine/Neurology, Kuopio, Finland; 33. University of Eastern Finland, Institute of Public Health and Clinical Nutrition, Kuopio, Finland; 34. Rare and Neurologic Diseases Research, Sanofi Research and Development, Cambridge, MA, United States
Corresponding Author: Miia Kivipelto, MD, PhD, Center for Alzheimer’s Research, Karolinska Universitetssjukhuset, Karolinska Vägen 37 A, QA32, 171 64 Solna, Sweden, Email: miia.kivipelto@ki.se, Phone: +46 73 99 409 22
J Prev Alz Dis 2024;2(11):329-338
Published online January 30, 2024, http://dx.doi.org/10.14283/jpad.2024.32
Abstract
The Real-World Implementation, Deployment, and Validation of Early Detection Tools and Lifestyle Enhancement (AD-RIDDLE) project, recently launched with the support of the EU Innovative Health Initiative (IHI) public-private partnership and UK Research and Innovation (UKRI), aims to develop, test, and deploy a modular toolbox platform that can reduce existing barriers to the timely detection, and therapeutic approaches in Alzheimer’s disease (AD), thus accelerating AD innovation. By focusing on health system and health worker practices, AD-RIDDLE seeks to improve and smooth AD management at and between each key step of the clinical pathway and across the disease continuum, from at-risk asymptomatic stages to early symptomatic ones. This includes innovation and improvement in AD awareness, risk reduction and prevention, detection, diagnosis, and intervention. The 24 partners in the AD-RIDDLE interdisciplinary consortium will develop and test the AD-RIDDLE toolbox platform and its components individually and in combination in six European countries. Expected results from this cross-sectoral research collaboration include tools for earlier detection and accurate diagnosis; validated, novel digital cognitive and blood-based biomarkers; and improved access to individualized preventative interventions (including multimodal interventions and symptomatic/disease-modifying therapies) across diverse populations, within the framework of precision medicine. Overall, AD-RIDDLE toolbox platform will advance management of AD, improving outcomes for patients and their families, and reducing costs.
Key words: Alzheimer’s disease, dementia, biomarkers, precision medicine, implementation, real world data.
Introduction
In an aging global population, the number of people with dementia is forecasted to grow from over 57 million (2019) to 153 million by 2050 (1). The annual global costs of dementia already exceed $1.3 trillion (2)—and health systems worldwide are not prepared for its future impact.
The pathology of AD can start 15–25 years before individuals start showing symptoms, and diagnosis is often substantially delayed (3). Diagnosing AD in the prodromal stages of the disease remains a notable challenge in the primary care setting: as a result, 60-75% of adults with AD never receive a formal diagnosis or are diagnosed at more advanced stages of dementia (4, 5, 6). Yet earlier detection gives patients and healthcare providers more and better options for prevention and treatment, including recently approved disease-modifying therapies (DMT) (7, 8). Data from real-world clinical settings indicate that only a fraction of patients (27% of those with mild cognitive impairment) are potentially eligible for the disease-modifying therapies (DMTs) that currently exist, so it is critical to accurately identify as many of the people who can benefit from those therapies as possible (9). Research indicates that European healthcare systems are currently capacity-constrained and ill-prepared to detect cognitive decline in a timely manner, risking delaying diagnosis and as a result otherwise eligible patients may struggle to access DMTs (10, 11, 12).
While prevention, screening, and early detection strategies have been developed and implemented for many other chronic diseases, they have been lacking in the dementia field. Although there are promising findings in these research areas, literature shows that it takes on average 17-20 years for clinical innovations to translate into practice (13). The World Health Organization (WHO) has laid the groundwork to better integrate emerging innovations into clinical practice via the Integrated Care for Older People (ICOPE) guidelines, but substantial work remains to equip health systems to screen for, predict, prevent and treat dementia (14).
Public-private partnerships, such as the EU Innovative Health Initiative (IHI, www.ihi.europa.eu), work across a broad range of sectors and involve all key stakeholders, thus providing optimal ways to address challenges in AD management. The IHI call 3 in 2023 focused on “Screening platform and biomarkers for prediction and prevention of diseases of unmet public health need.” Within this call, the aim of the Real-World Implementation, Deployment, and Validation of Early Detection Tools and Lifestyle Enhancement (AD-RIDDLE) project is to develop, test, and deploy a modular toolbox platform that can eliminate barriers to Alzheimer’s disease (AD) early detection, diagnosis, prevention, and treatment. By focusing on the health system and health worker practices, the AD-RIDDLE consortium (Figure 1) seeks to improve and smooth AD management at and between each key step of the clinical pathway and the disease continuum, from at-risk asymptomatic stages to early symptomatic ones. The public-private partnership assembled to tackle this project will enable linkage of multiple industry sectors to exploit and share data, access to healthcare systems, tools and innovations, and deep domain expertise.
The timely approach of the AD-RIDDLE project
Given the slow, gradual progression of AD neuropathology from an asymptomatic stage or before symptoms are detectable with cognitive tests (preclinical AD) to mild cognitive impairment (prodromal AD) to dementia (the end-stage of AD) there is a window of opportunity for early intervention (15). Individuals could benefit from interventions delaying symptom onset and progression, as well as early AD detection —including via better symptom management, more coordinated care plans, cost savings, and delayed institutionalization (16) before substantial accumulation of neuropathology and the onset of considerable cognitive symptoms.
Risk reduction and early intervention are increasingly possible. AD and related dementias are complex, resulting from interactions between non-modifiable (such as age, sex, and genetics) and modifiable (such as lifestyle, vascular, and metabolic) risk factors (17, 18, 19). AD-RIDDLE Consortium members developed the pioneering Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) multimodal dementia risk detection and prevention model (20, 21), which showed that older people at risk for dementia, even those with genetic susceptibility to the disease, experienced clear cognitive and other health benefits from a multidomain, lifestyle-based intervention combining nutrition advice, exercise, cognitive training, social activities, and management of vascular/metabolic risk (21, 22, 23, 24, 25, 26). Likewise, DMTs and other interventions in the AD field are progressing rapidly: some DMTs, such as the Aβ-targeted drug lecanemab, may soon be available (27, 28). Eligibility for treatment with lecanemab is currently limited to people with prodromal or mild dementia due to AD, and evidence from trials such as TRAILBLAZER-ALZ2 and Clarity AD showed a greater slowing of clinical decline in participants at earlier stages of the disease, underscoring the value of timely detection and diagnosis (29, 30).
The AD-RIDDLE Consortium and aims
Developing and implementing large-scale European strategies for early detection and prevention can enable health systems, providers, caregivers, and patients to take advantage of new advances in AD diagnostics, therapeutics and prevention (18, 31). The 24 partners in the AD-RIDDLE Consortium—a unique, interdisciplinary collaboration of academic researchers, healthcare providers, industry, regulatory bodies, and patient advocacy organizations (Figure 1)—will develop and test the AD-RIDDLE toolbox platform and its components individually and in combination in eight primary care and memory clinics in six European countries (Sweden, Finland, Netherlands, Spain, Italy, and the UK). They will leverage their world-class research, medical practice, and digital and data platform capabilities, as well as significant advances in tools and methods for early AD detection, such as new developments in blood-based biomarkers (BBMs) and digital tools for cognitive assessment, to meet the project’s aims:
1) Increase timeliness and accuracy in early risk detection and AD diagnosis;
2) Increase the availability of validated, novel digital cognitive assessment tools and blood-based biomarkers;
3) Improve the integration of screening, confirmation (CSF/MRI/PET), and prevention to match individuals and therapies using advanced analytics; and
4) Improve access to tailored interventions (including multimodal interventions and symptomatic/disease-modifying therapies) across diverse populations, within the framework of precision medicine.
The AD-RIDDLE project will span five years and include the development of a community outreach toolkit for screening and stakeholder activation, real-world testing of digital cognitive assessments and blood-based biomarkers, development and testing of predictive algorithms for early risk and disease detection, and designing a decision support toolkit for precision prevention therapies. Implementation research will enable an understanding of the barriers and enablers to implement these tools in real-world settings. In parallel, a cross-cutting team will provide guidance and recommendations for the project on aspects related to ethics, health economics, health technology assessment and sustainability. This will include a scoping review of ethical concerns linked to screening approaches for AD/dementia risk, a policy and practice review across European countries, evaluation of data privacy and GDPR compliance of the toolbox platform, and health economic model building and assessment of health technology assessment (HTA) evidence requirements. Finally, all the above activities will be enabled by communication, public involvement, and outreach activities throughout the project duration.
The AD-RIDDLE Toolbox Platform
Innovative elements contributing to the uniqueness of the AD-RIDDLE toolbox platform include its flexible structure and foreseen use. Diagnostic and clinical management pathways differ significantly across countries and within countries, with diverse approaches to cognitive and biomarker testing, follow-up, and reimbursement (32). Thus, taking a one-size-fits-all approach would not be successful. Consequently, the AD-RIDDLE platform will feature a modular toolbox, allowing health systems and healthcare providers to mix, match, and tailor its component tools to their needs. (See Figure 2) Components of the AD-RIDDLE toolbox platform include:
• A modular digital engagement portal with pre-screening tools alongside resources for patients, caregivers, and the general public
• Digital cognitive assessment (DCA) tools
• Blood-based biomarker (BBM) tests
• Protocols for preventive intervention based on iterative research findings
• A platform algorithm that can analyse an array of data and scientific inputs and generate personalized intervention recommendations.
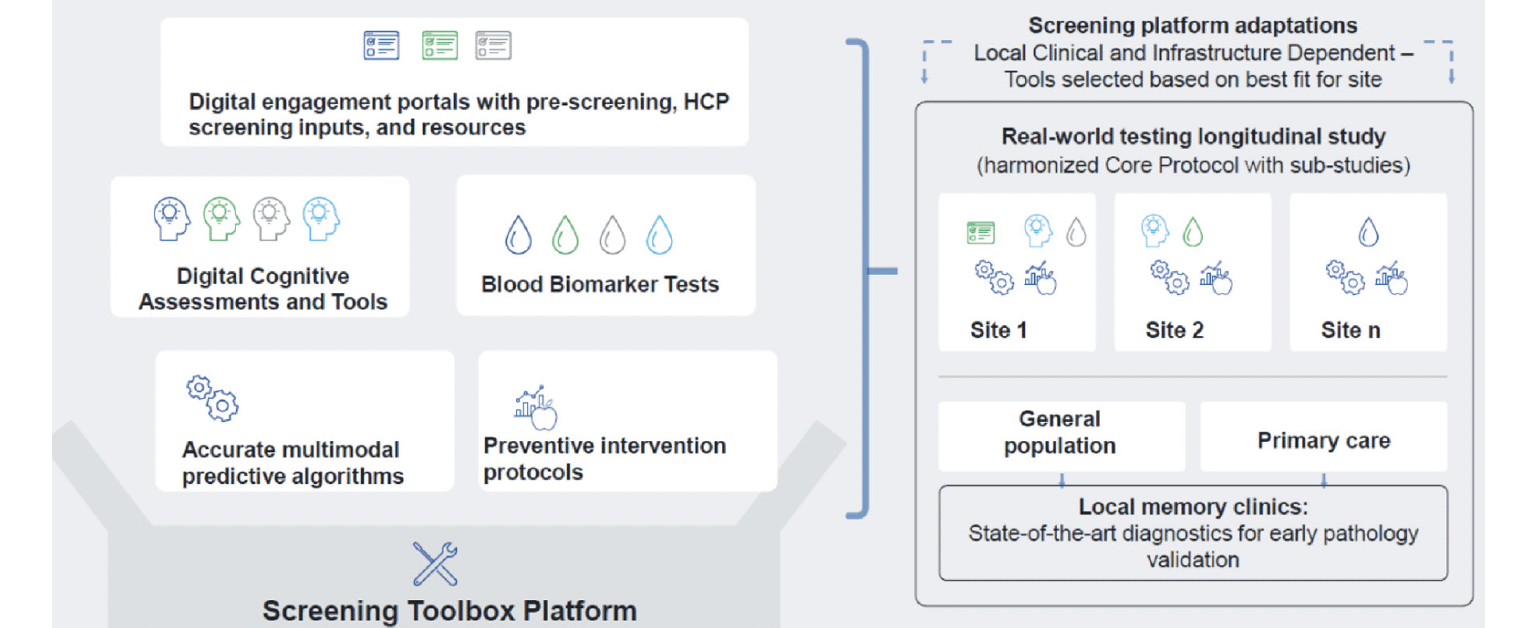
Figure 2. The AD-RIDDLE Toolbox Platform – a modular structure allowing each clinical site to choose and combine the most suitable tools for screening purposes
Abbreviations: HCP, healthcare professionals
Timely diagnosis of AD and dementia requires an orchestrated multi-step process in primary care settings and beyond, involving multiple healthcare professionals: physicians—including neurologists, geriatricians, and/or psychiatrists—as well as nurses, neuropsychologists, speech therapists, and MRI and/or PET neuroimaging specialists. Variations in professionals involved partly depend on which specialty (Geriatrics, Neurology, or Psychiatry) is assigned primary diagnostic and therapeutic responsibility in a specific country or region. This variation of tasks and responsibilities in different contexts is one reason why the AD-RIDDLE toolbox platform is designed to be modular and flexible, with multiple entry points and means of engagement for patients, caregivers, and healthcare providers. To test the platform in the real world, eight clinical sites across six European countries participating in AD-RIDDLE have the capacity and experience to cover the entire screening and prevention trajectory, from the general population to primary care to memory clinics. Each study site will have the flexibility to assemble a set of tools adapted to the local healthcare setting, and tailored to meet the needs of the patients and caregivers it serves, addressing the psychosocial and biographical components of cognitive impairment. For example, patients diagnosed with AD could be allocated to personalised, pharmacological or non-pharmacological treatments, including disease-modifying therapies for patients (e.g. anti-amyloid drugs for those with confirmed amyloid pathology), interventions targeting modifiable risk factors (e.g. FINGER multidomain intervention) or combination therapies. Older adults at risk of AD/dementia will have timely access to personalised, preventive therapies based on lifestyle interventions, with the toolbox platform enabling early detection and monitoring of people who could benefit from self-guided interventions, and those who need support from healthcare professionals to optimise the therapeutic response.
Another key feature of the AD-RIDDLE project is the implementation research module, which aims to identify generalizable learnings that can be applied across diverse clinical settings to increase the adoption of key components of the modular toolbox platform (e.g., DCA, BBM, fluid biomarkers). The implementation evaluation will be guided by a mixed-methods protocol developed with clinical setting leaders, taking into account the unique requirements and current practices of each setting, guided by the revised Consolidated Framework for Implementation Research (CFIR; 33). The evaluation will document data about the implementation process and outcomes including:
• The challenges of incorporating toolkit components into existing care pathways both within and across sites
• Barriers and facilitators to sustained practice change within and across sites
• The role of site and setting factors on implementation successes and challenges
Communications in clinical settings regarding risk assessment, diagnostic procedures, and decisions about engaging in preventive or therapeutic interventions (including both pharmacological and non-pharmacological) will also be assessed. As a result, AD-RIDDLE aims to identify best practices to enable the improvement of health literacy and shared decision-making of patients and care partners, as well as efficient allocation and improved adherence to preventive or therapeutic treatments. Furthermore, participating sites will engage with their patients to ensure that digital literacy or connectivity does not become a barrier to accessing needed care.
AD-RIDDLE envisions the continuum of AD screening, diagnosis, and treatment as a pipeline, and right now, that pipeline is complex. The project aims to take apart the pipes, identify the obstacles and their location, and put them back together again so that patient and provider can flow more smoothly, making a difficult process more efficient, effective, and patient-focused. Drugs and other therapies are only useful if they reach the patients who need them—and they can only reach the patients who need them if people with preclinical or prodromal AD receive a timely, accurate diagnosis based on pathology and associated clinical care.
Effectively utilizing novel screening tools, diagnostics, and treatments for Alzheimer’s disease is as new to doctors and other healthcare providers as it is to patients. Physicians are typically incentivized to work in their practice, not on it. AD-RIDDLE focuses on both, collecting data at each step so health systems can do more and better for an aging global population.
Methodology
Building on existing technology, tools, and biomarkers, many developed by AD-RIDDLE Consortium partners through earlier EU-funded projects, development of the AD-RIDDLE toolbox platform will be based on the crucial need to provide modular solutions that can be flexibly combined and matched in different health systems and settings across multiple countries.
The AD-RIDDLE toolbox components are:
1) Digital engagement portals with resources to enable patient pre-screening and screening
To raise awareness and enable screening in the general population, the AD-RIDDLE partners will develop a community entry point toolkit, or “front door.” This will take the form of a digital engagement portal, developed initially as a test wireframe, and iteratively refined in consultation with healthcare professionals, patients, caregivers and older adults during the project into a fully functional product that is accessible and useable for people across a wide digital literacy spectrum. Key components of this portal are expected to include: a public-facing website online cognitive pre-screening tools, chatbots, or other interface for public engagement, a contact management system to enable self-guided assessment and follow-up communications, patient and family/care partner resources, and general healthcare provider support tools to enable screening and activation. The digital engagement portal will be launched alongside a public awareness campaign, co-designed with patients, caregivers and older adults in order to cross the digital divide, leveraging existing networks to reach diverse audiences.
The goal is to provide a seamless entry point that will guide diverse users through the platform. Developers will capitalize on the experience and assets of consortium members related to dementia-inclusive messaging to promote positive engagement with brain health and reduce the stigma around cognitive impairment and dementia.
2) Digital cognitive assessments and tools
Compared with standard paper-and-pencil testing, digital tools for early AD detection and prevention have great potential due to the possibility for higher sensitivity to early disease and better ecological validity (34, 35). By removing the need for individuals to travel for their assessment(s), they capture sensitive, specific, and valuable cognitive measures on a large scale with ease while offering greater cost-effectiveness, accessibility, and data aggregation (such as, in some settings, allowing for task-shifting to decrease the utilization of more expensive health resources) (36). The AD-RIDDLE platform will offer a variety of digital cognitive assessment (DCA) tools via computer, smartphone, and/or tablet for predicting AD risk in cognitively healthy individuals and monitoring the real-world impact of prevention and treatment. These DCAs will include the eCOG and cNeuro cDSI from Combinostics, the Amsterdam IADL Questionnaire, Neotiv’s digital cognitive assessment battery and the NeotivTrials Web Portal, CANTAB and NeuroVocalix from Cambridge Cognition, among others – offering a variety of CE-marked tools validated across multiple languages and settings.
3) Blood-based biomarkers (BBMs) relevant for AD
Among the highest priorities in AD biomarker research is evaluating high-performing BBMs in diverse real-world populations in both memory and primary care clinics. To determine clinical robustness, these biomarkers should be studied prospectively; yet to date no studies have been published in which high-performing AD BBMs were prospectively and rigorously validated in patients with memory impairment in memory clinics, and certainly not in primary care (37, 38).
Based on the healthcare infrastructure in the selected European countries involved in AD-RIDDLE, research biomarker protocols and BBMs already approved for use in clinical practice, AD-RIDDLE has a unique opportunity to study the clinical robustness and accuracy of plasma AD biomarkers prospectively in broad, diverse, and unselected populations that are generalizable to other real-world settings.
AD-RIDDLE Consortium members have shown that AD BBMs, together with brief cognitive tests, can accurately predict future development of dementia in specialist memory clinic patients with mild memory complaints (39). Others have recently achieved major breakthroughs in accurate AD BBMs, including Aβ42/40 ratio, phosphorylated (p)-tau217, p-Tau231, p-Tau181, neurofilament light chain (NFL, neurodegeneration marker), and glial fibrillary acidic protein (GFAP, early marker of Aβ-related reactive astrogliosis) (40, 37). However, such novel biomarkers must now be thoroughly and prospectively validated in clinical practice before they can be implemented in specialist or primary care clinics worldwide. The project will also test exploratory fluid biomarkers based on ongoing work by Consortium partners.
4) Predictive algorithms for early risk and disease detection; and 5) Decision support toolkit for precision prevention therapies
There has been extensive research on predictive algorithms for AD/dementia, considering various combinations of genetic and environmental factors, medical history, cognitive performance, and/or biomarkers (including blood, CSF, and imaging) (41). However, the use of predictive algorithms in clinical practice is very limited, due to the lack of widely accessible digital tools to implement them and limited evidence on their real-world accuracy in general populations at primary care or memory clinics. The current disconnect between risk/disease prediction and dementia prevention creates difficulties for clinicians when early detection of people at-risk or in early AD stages is not linked to clear intervention-related decision-making.
The AD-RIDDLE project is unique in its combination of several large datasets, including both observational cohorts and interventional randomized clinical trials (RCTs), for AD/dementia prevention. The datasets are particularly suited for prediction and prevention work because they cover the full spectrum of stages—from brain-at-risk through preclinical and prodromal AD to dementia—and can seamlessly connect risk prediction to real-world preventive interventions. Consortium members maintain these datasets and make them readily accessible for analysis, leveraging the EU-funded European Platform for Neurodegenerative Diseases (EPND) (42). The development and validation of the algorithm will also be based on new real-world data collected during this project.
Real-world testing of the toolbox platform, digital cognitive assessments, and BBMs
A three-year–long real-world testing study, co-designed with Consortium partners, will be conducted at eight sites in six European countries. The partners will recruit participants from general populations, primary care or memory clinic settings, targeting individuals across the entire continuum from brain-at-risk to preclinical and prodromal AD. The Davos Alzheimer’s Collaborative, a new global health initiative launched in 2021 by the World Economic Forum, will provide the Implementation Science support to collect the evidence basis of health system barriers and facilitators using rigorous evidence-based methods to support future clinical and policy changes (43).
Consortium partners will first aim to understand the standard of care and current best practices within each clinical setting, and subsequently develop a Core Protocol to coordinate sub-studies investigating how different primary care and/or memory clinic settings in different countries can adapt and use the AD-RIDDLE toolbox platform. This approach aligns with the modular toolbox platform design and will allow adaptation to match a variety of clinical site needs.
Potential obstacles
The main potential obstacle for the AD-RIDDLE project is that implementation may be delayed at the health system or site level. With sufficient lead time for local stakeholder engagement, however, clinical routines can be adapted, management and technical challenges can be overcome, and health care providers can be trained. The clinical partners will provide evidence of the added value and efficiency of AD-RIDDLE tools, paving the way for implementation outside the consortium.
Early detection and tailored interventions require appropriate communication with key stakeholders, including patients, caregivers, and the public to ensure that messaging uses appropriate language, is supplied in the appropriate form (digital and/or paper), and responds to the needs of specific communities, including hard-to-reach populations. Likewise, clinicians will need clear guidance to manage reactions to biomarker disclosures, engage patients in decisions regarding the assessment of risk and prevention potential, and identify preventative and therapeutic treatments. AD-RIDDLE will address these challenges by building on the knowledge and expertise of consortium partners, and by embedding end user involvement at all stages of the project, from public involvement consultations with patients, caregivers and older adults, to focus groups and workshops with healthcare professionals. Meaningful involvement of patients, caregivers and clinicians will enhance the useability, accessibility and value of the AD-RIDDLE toolbox platform, as well as its expected impacts at both individual and societal levels.
Expected Impact of AD-RIDDLE
AD-RIDDLE began in January 2024 and will last 5 years. It aims to address many of the challenges AD and its related costs place on communities, health systems, healthcare professionals, patients, and caregivers. Expected outcomes for target groups include:
• Older adults (60+ years) and patients with preclinical or prodromal AD will benefit from access to timely, personalized, evidence-based preventive therapies.
• Healthcare professionals will benefit from validated tools that will allow them to identify at-risk patients and select appropriate preventive action.
• Researchers will benefit from validated biomarkers to accelerate the development of precision therapies to prevent or delay AD dementia.
• Health systems will benefit from evidence that helps to identify the right patients, at the right time, for the right interventions to improve treatment outcomes and decrease resource utilization.
• Patients and caregivers will benefit from improved, timely detection and diagnosis. There are also societal benefits to a timely diagnosis: for instance, enabling patient and family education and counselling, and/or allowing for the early introduction of strategies and tools to maximize independence (44).
• Health technology developers will benefit from understanding barriers to adopting and operationalizing validated tools to improve commercialization efforts.
A specific area of focus to ensure sustainability will be collaborative work with partners from the private sector to ensure exploitation readiness of the tools within the AD-RIDDLE toolbox platform and to support regulatory validation where necessary. Insights from companies with certified tools will be leveraged for tools that are not yet available for clinical use. We will also proactively engage with healthcare systems from the early stages of the project, to understand market needs, barriers, and enablers for adoption, and to advocate for continued use of AD-RIDDLE tools.
The Scientific impact of AD-RIDDLE will include improved methodologies for early detection and more precise clinical characterization of AD, helping to accelerate clinical research and the development of new preventative therapies based on pharmacological and non-pharmacological interventions.
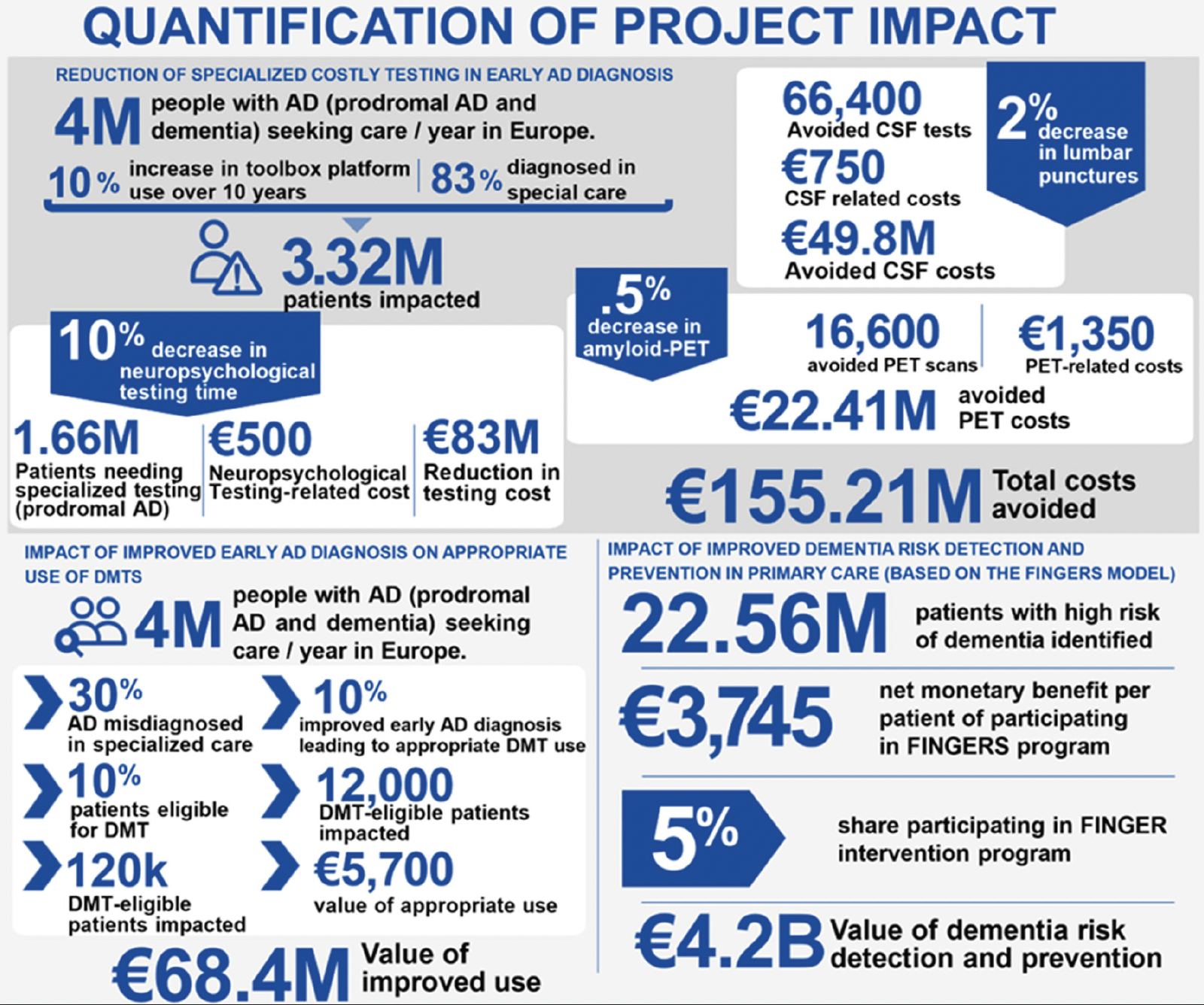
The economic impact would entail cost savings resulting from reduced medical costs and reduced costs of social and informal care related to AD and dementia. The quantification of impact is based on available data on the occurrence of AD pathology in older adults in Europe (preclinical and prodromal AD) and current procedures and costs for diagnosis, which rely on costly tools available only in specialist settings. Impact assessment is also based on a cost-effectiveness analysis from FINGER, which showed that saving $2000 USD per individual by reducing dementia risk can have a huge societal impact given the size of the target population (45, 46, 2) (Figure 3, see also supplementary table 1 for details on quantification of project impact, including individual and aggregated cost estimates, and full references). Furthermore, recent data from the European Brain Council indicate that early diagnosis by BBMs and cognitive testing can save money by reducing medical costs and the burden of social or informal care (47). The findings of AD-RIDDLE, and the expected uptake of the toolbox components across Europe, are forecasted to optimize the use and thus costs of time-consuming and burdensome diagnostics (CSF, PET-scans), and increase the timely access to therapeutic approaches, including pharmacological and non-pharmacological interventions. Beyond the economic benefit, this can also impact citizens (both those across the AD continuum and their relatives/carers) by potentially offering optimized and thus less stressful diagnostic, preventative and therapeutic pathways, with disease detection at earlier stages, and opportunities to postpone cognitive decline and disability.
Abbreviations: AD, Alzheimer’s disease; CSF, cerebrospinal fluid; DMT, disease-modifying therapy; PET, positron emission tomography. See also Supplementary Table 1 for additional details.
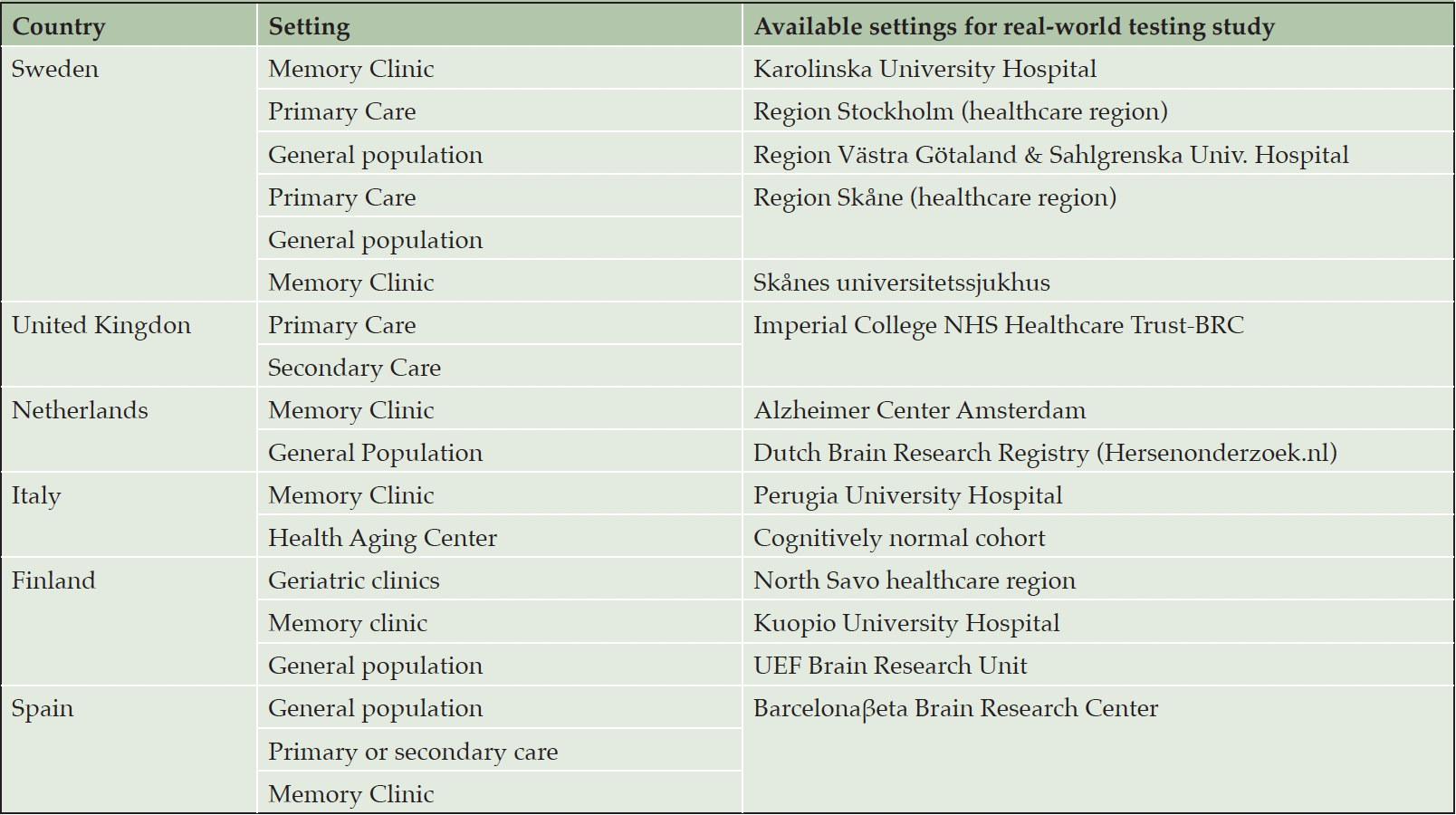
Table 1. Available settings for AD-RIDDLE’s real world testing study span a range of European countries and healthcare settings, offering a diversity of current standards of care, local restrictions, and workflows
Overall, the social impact related to the development of tools enhancing access to early diagnosis and preventive therapies will improve the lives of people with AD and reduce the individual and social burden related to the disease, enabling the promotion of sustainable societies, and curbing the strain of aging populations on health systems.
Acknowledgments: This project is supported by the Innovative Health Initiative Joint Undertaking (IHI JU) under grant agreement No. 101132933. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or Innovative Health Initiative JU. Neither the European Union nor the granting authority can be held responsible for them. The JU receives support from the European Union’s Horizon Europe research and innovation programme and COCIR, EFPIA, EuropaBio, MedTechEurope, Vaccines Europe, Davos Alzheimer’s Collaborative, Combinostics OY, Cambridge Cognition Ltd., C2N Diagnostics LLC, and Neotiv GmbH. The participation of Cambridge Cognition Ltd. (a hybrid partner), University of Leicester, Imperial College of Science Technology and Medicine and the National Institute for Health and Care Excellence will be funded by UK Research and Innovation (UKRI) under the UK government’s Horizon Europe funding guarantee.
AD-RIDDLE Consortium Members: • Region Stockholm (Karolinska University Hospital); • Karolinska Institutet; • FINGERS Brain Health Institute; • Göteborgs Universitet; • Lund University; • University of Eastern Finland;
• Stichting Amsterdam UMC; • Universiteit Maastricht; • Fundacio Barcelonabeta Brain Research Center; • SYNAPSE Research Management Partners; • Alzheimer Europe; • Alzheimer’s Disease International; • Universitá Degli Studi Di Perugia; • Gates Ventures; • Davos Alzheimer’s Collaborative; • Combinostics; • Fujirebio; • Sanofi • C2N Diagnostics; • Neotiv; • Cambridge Cognition; • University of Leicester; •Imperial College of Science Technology and Medicine; • NICE | National Institute for Health and Care Excellence.
Conflict of Interest: Dr. Zetterberg has served at scientific advisory boards and/or as a consultant for AbbVie, Alector, Annexon, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. Dr. Hansson has acquired research support (for the institution) from ADx, AVID Radiopharmaceuticals, Biogen, Eli Lilly, Eisai, Fujirebio, GE Healthcare, Pfizer, and Roche. In the past 2 years, he has received consultancy/speaker fees from Amylyx, Alzpath, Biogen, Cerveau, Fujirebio, Genentech, Novartis, Roche, and Siemens. Dr. Minguillon reported receiving grants from JPND EU-Fingers and from Alzheimer’s Drug Discovery Foundation outside the submitted work. Dr. Middleton reports grants from Janssen, Merck, Takeda, Gates Ventures, Eisai and NIHR, outside the submitted work. Dr Schöll has served on advisory boards for Roche and Novo Nordisk (outside scope of submitted work). Dr. Ferrell has provided consulting services to Gates Ventures, Morningside Technical Advisory, and the ADDF Diagnostics Accelerator, and is a former employee and current shareholder of Eli Lilly & Company. Dr. van der Flier’s research programs have been funded by ZonMW, NWO, EU-JPND, EU-IHI, Alzheimer Nederland, Hersenstichting CardioVascular Onderzoek Nederland, Health~Holland, Topsector Life Sciences & Health, stichting Dioraphte, Gieskes-Strijbis fonds, stichting Equilibrio, Edwin Bouw fonds, Pasman stichting, stichting Alzheimer & Neuropsychiatrie Foundation, Philips, Biogen MA Inc, Novartis-NL, Life-MI, AVID, Roche BV, Fujifilm, Eisai, Combinostics. Dr. van der Flier holds the Pasman chair. Dr. van der Flier is recipient of ABOARD, which is a public-private partnership receiving funding from ZonMW (#73305095007) and Health~Holland, Topsector Life Sciences & Health (PPP-allowance; #LSHM20106). Dr. van der Flier has been an invited speaker at Biogen MA Inc, Danone, Eisai, WebMD Neurology (Medscape), NovoNordisk, Springer Healthcare, European Brain Council. WF is consultant to Oxford Health Policy Forum CIC, Roche, Biogen MA Inc, and Eisai. Dr. van der Flier is member of steering cie of NovoNordisk evoke/evoke+. Dr. van der Flier participated in advisory boards of Biogen MA Inc, Roche, and Eli Lilly. All funding is paid to her institution. Dr. van der Flier is member of the steering committee of PAVE and Think Brain Health, and was associate editor of Alzheimer, Research & Therapy in 2020/2021, as well as associate editor at Brain. Dr. Krishnan and Dr. Sardi are employees and shareholders of Sanofi. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical standards: The AD-RIDDLE project will partner with observational cohort studies and clinical trial cohorts that have obtained ethical approval at their home institution (see Table 1 above). For new studies carried out under this initiative, additional ethical approvals by research ethics committees in each country involved will be sought and informed consent will be obtained from participants prior to inclusion. Data accessed for this project will be pseudonymized (personal identifiers replaced with a code unique to the patient), and identifiable information will have been removed so that individual patient identities cannot be determined. Results will only be disclosed at the group level so that information on individual patients cannot be discerned.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105-e125. doi:10.1016/S2468-2667(21)00249-8.
2. Wimo et al. Dementia prevention: The potential long-term cost-effectiveness of the FINGER prevention program. Alzheimers Dement. 2022 Epub Jul 16. doi:10.1002/alz.12698
3. Kvello-Alme M, Bråthen G, White LR, Sando SB. Time to Diagnosis in Young Onset Alzheimer’s Disease: A Population-Based Study from Central Norway. J Alzheimers Dis. 2021;82(3):965-974. doi:10.3233/JAD-210090. PMID: 34120901; PMCID: PMC8461696.
4. Lang L, Clifford A, Wei L, et al. Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta-analysis. BMJ Open. 2017;7(2):e011146. doi:10.1136/bmjopen-2016-011146
5. Gauthier S, Rosa-Neto P, Morais JA, & Webster C.2021. World Alzheimer Report 2021: Journey through the diagnosis of dementia. London, England: Alzheimer’s Disease International.
6. Gauthier et al. World Alzheimer Report 2022: Life after diagnosis: Navigating treatment, care and support. London, England: Alzheimer’s Disease International, 2022.
7. Harris E. Alzheimer Drug Lecanemab Gains Traditional FDA Approval. JAMA. 2023;330(6):495. doi:10.1001/jama.2023.12548
8. Rabinovici GD. Controversy and Progress in Alzheimer’s Disease – FDA Approval of Aducanumab. N Engl J Med. July 28, 2021. doi:10.1056/NEJMp2111320
9. Rosenberg et al. β-Amyloid, Tau, Neurodegeneration Classification and Eligibility for Anti-amyloid Treatment in a Memory Clinic Population. Neurology. 2022;99(19):e2102-e2113. doi:10.1212/WNL.0000000000201043
10. Mattke, S., Gustavsson, A., Jacobs, L. et al. Estimates of Current Capacity for Diagnosing Alzheimer’s Disease in Sweden and the Need to Expand Specialist Numbers. J Prev Alzheimers Dis (2023). doi:https://doi.org/10.14283/jpad.2023.94
11. Mattke S, Tang Y, Hanson M. Expected wait times for access to a disease-modifying Alzheimer’s treatment in England: A modelling study. Journal of Health Services Research & Policy. 2023;0(0). doi:10.1002/alz.12470
12. Mattke S, Wang M, Ullrich A. How prepared are the EU-5 countries’ health systems to deliver a disease-modifying treatment for Alzheimer’s? Alzheimers Dement. 2020;16(S10). doi:10.1002/alz.037257
13. Bauer, Mark S. & Kirchner, JoAnn (2020). Implementation Science: What is it and why should I care? Psychiatry Research. Volume 283, 2020. doi:10.1016/j.psychres.2019.04.025
14. Integrated care for older people (ICOPE): Guidance for person-centred assessment and pathways in primary care. Geneva: World Health Organization; 2019 (WHO/FWC/ALC/19.1). Licence: CC BY-NC-SA 3.0 IGO.
15. Vermunt et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 2019;15(7):888-898. doi:10.1016/j.jalz.2019.04.001
16. Dubois B, Padovani A, Scheltens P, Rossi A, D’ll’Agnello G. Timely Diagnosis for Alzheimer’s Disease: A Literature Review on Benefits and Challenges. J Alzheimers Dis. 2016;49(3):617-31. doi:10.3233/JAD-150692. PMID: 26484931; PMCID: PMC4927869.
17. Kivipelto et al. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14(11):653-666. doi:10.1038/s41582-018-0070-3
18. Livingston et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-446. doi:10.1016/S0140-6736(20)30367-6
19. Long, S., Benoist, C., Weidner, W. 2023. World Alzheimer Report 2023: Reducing dementia risk: never too early, never too late. London, England: Alzheimer’s Disease International.
20. Kivipelto et al. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimers Dement. 2013;9(6):657-65. doi:10.1016/j.jalz.2012.09.012
21. Ngandu et al. Recruitment and baseline characteristics of participants in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER)-a randomized controlled lifestyle trial. Int J Environ Res Public Health. 2014;11(9):9345-60. doi: https://doi.org/10.3390/ijerph110909345
22. Strandberg et al. Health-related quality of life in a multidomain intervention trial to prevent cognitive decline (FINGER), European Geriatric Medicine. 2017;8(2):164-167. doi:https://doi.org/10.1016/j.eurger.2016.12.005.
23. Marengoni et al. The Effect of a 2-Year Intervention Consisting of Diet, Physical Exercise, Cognitive Training, and Monitoring of Vascular Risk on Chronic Morbidity-the FINGER Randomized Controlled Trial. J Am Med Dir Assoc. 2018;19(4):355-360.e1. doi:10.1016/j.jamda.2017.09.020
24. Solomon et al. Effect of the Apolipoprotein E Genotype on Cognitive Change During a Multidomain Lifestyle Intervention: A Subgroup Analysis of a Randomized Clinical Trial. JAMA Neurol. 2018;75(4):462-470. doi:10.1001/jamaneurol.2017.4365
25. Kulmala et al. The Effect of Multidomain Lifestyle Intervention on Daily Functioning in Older People. J Am Geriatr Soc. 2019;67(6):1138-1144. doi:10.1111/jgs.15837
26. Lehtisalo et al., Effect of a multi-domain lifestyle intervention on cardiovascular risk in older people: the FINGER trial. Eur Heart J. 2022;43(21):2054-2061. doi:10.1093/eurheartj/ehab922
27. Larkin HD. Lecanemab Gains FDA Approval for Early Alzheimer Disease. JAMA. 2023;329(5):363. doi:10.1001/jama.2022.24490
28. Van Dyck et al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
29. Sims JR, Zimmer JA, Evans CD, et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA. 2023;330(6):512–527. doi:10.1001/jama.2023.13239
30. Sperling R, Li D, Dhadda S, et al. Lecanemab for the Treatment of Early Alzheimer’s Disease: The Extension of Efficacy Results from Clarity AD. J Prev Alzheimers Dis. 2023;10(S1):S56-S240. doi:10.14283/jpad.2022.130
31. Frisoni et al. Dementia prevention in memory clinics: recommendations from the European task force for brain health services. Lancet Reg Health Eur. 2023;26:100576. doi:https://doi.org/10.1016/j.lanepe.2022.100576
32. Roth S, Burnie N, Suridjan I, Yan JT, Carboni M. Current Diagnostic Pathways for Alzheimer’s Disease: A Cross-Sectional Real-World Study Across Six Countries. J Alzheimers Dis Rep. 2023 Jun 29;7(1):659-674. doi:10.3233/ADR230007. PMID: 37483324; PMCID: PMC10357118. doi:10.3233/ADR230007
33. Damschroder et al (2022). The updated Consolidated Framework for Implementation Research based on user feedback. Implementation science, 17(1), 75. doi:https://doi.org/10.1186/s13012-022-01245-0
34. Jutten et al. Why a clinical trial is as good as its outcome measure: A framework for the selection and use of cognitive outcome measures for clinical trials of Alzheimer’s disease. Alzheimers Dement. 2023;19(2):708-720. doi:10.1002/alz.12773
35. Öhman et al. Current advances in digital cognitive assessment for preclinical Alzheimer’s disease. Alzheimers Dement (Amst). 2021;13(1):e12217. doi:10.1002/dad2.12217
36. Ross E, Levy C, Ty D, Altimus C, Super N. Roadmap for Investment in Dementia Care. Milken Institute; 2022.
37. Hansson et al. The Alzheimer’s Association appropriate use recommendations for blood biomarkers in Alzheimer’s disease. Alzheimers Dement. 2022;18(12):2669-2686. doi:10.1002/alz.12756
38. Hampel H, Hu Y, Cummings J, et al. Blood-based biomarkers for Alzheimer’s disease: Current state and future use in a transformed global healthcare landscape. Neuron. 2023;111(18):2781-2799. doi:10.1016/j.neuron.2023.05.017
39. Palmqvist et al. Prediction of future Alzheimer’s disease dementia using plasma phosopho-tau combined with other accessible measures. Nat Med. 2021;27(6):1034-1042. doi:10.1038/s41591-021-01348-z
40. Hansson O. Biomarkers for neurodegenerative diseases. Nat Med. 2021;27(6):954-963. doi:10.1038/s41591-021-01382-x
41. Hou et al. Models for predicting risk of dementia: a systematic review. J Neurol Neurosurg Psychiatry, 2019. 90(4):373-379. doi:10.1136/jnnp-2018-318212
42. Bose N, Brookes AJ, Scordis P, Visser PJ. Data and sample sharing as an enabler for large-scale biomarker research and development: The EPND perspective. Front Neurol. 2022;13:1031091. doi:10.3389/fneur.2022.1031091
43. Ball DE, Mattke S, Frank L, Murray JF, Noritake R, MacLeod T, Benham-Hermetz S, Kurzman A, Ferrell P. A framework for addressing Alzheimer’s disease: Without a frame, the work has no aim. Alzheimers Dement. 2023 Apr;19(4):1568-1578. doi:10.1002/alz.12869. Epub 2022 Dec 8. PMID: 36478657.
44. Liss JL, Seleri Assunção S, Cummings J, et al. Practical recommendations for timely, accurate diagnosis of symptomatic Alzheimer’s disease (MCI and dementia) in primary care: a review and synthesis. J Intern Med. 2021;290(2):310-334. doi:10.1111/joim.13244
45. World Health Organization. Global status report on the public health response to dementia. World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. https://www.who.int/publications/i/item/9789240033245 Accessed 14 January 2024
46. Ngandu et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255-63. doi:10.1016/S0140-6736(15)60461-5
47. European Brain Council and the European Federation of Pharmaceutical Industries and Associations (EFPIA), 2023. RETHINKING Alzheimer’s disease White Paper. https://www.braincouncil.eu/ebc-efpia-launch-rethinking-alzheimers-disease-white-paper/
© The Authors 2024