A. Padovani1,2,3, S. Caratozzolo1,2, A. Benussi1,2, A. Galli1,4, L. Rozzini1,2, M. Cosseddu2, R. Turrone2, A. Pilotto1,2,4
1. Neurology Unit, Department of Clinical and Experimental Sciences, University of Brescia, Italy; 2. Neurology Unit, Department of Continuity of Care and Frailty, ASST Spedali Civili Brescia University Hospital, Italy; 3. Brain Health Center, University of Brescia, Italy; 4. Laboratory of Digital Neurology and Biosensors, University of Brescia, Italy
Corresponding Author: Alessandro Padovani, Department of Clinical and Experimental Sciences, Neurology Unit, University of Brescia, Italy, Piazzale Spedali CIvili 1, 25123, Brescia, Italy, alessandro.padovani@unibs.it, Tel: +390303995632
J Prev Alz Dis 2024;2(11):375-381
Published online January 30, 2024, http://dx.doi.org/10.14283/jpad.2023.132
Abstract
BACKGROUND: Depressive symptoms are common in Alzheimer disease (AD) from the prodromal stage. The benefits of antidepressants have been investigated in patients with AD dementia with mixed results.
OBJECTIVES: This study aimed to compare the efficacy of vortioxetine in prodromal and mild-to-moderate AD patients with depression, and to assess the comparative effect on secondary measures, including behavioral disturbances, cognitive function, and activities of daily living.
PARTICIPANTS: All subjects with AD at a single-center dementia center underwent a standard evaluation with mini-mental state examination (MMSE), basic and instrumental activities of daily living (BADL and IADL), geriatric depression scale (GDS), neuropsychiatric inventory (NPI), and clinical evaluation every six months.
MEASUREMENTS: The study specifically assessed patients on vortioxetine with available six-month follow-up data. The changes in GDS, NPI, MMSE, BADL/IADL at six months in the entire AD population and mild-to-moderate AD vs prodromal population were analyzed using repeated measure multivariate analyses. Linear regression analyses were implemented to evaluate baseline demographics and clinical characteristics associated with depressive and cognitive improvements at six months.
RESULTS: Out of 680 AD patients, 115 were treated with vortioxetine, and 89 with six-month follow-up data were included in the analyses. A significant improvement at follow-up was observed for GDS, NPI total and sub score items (mood, anxiety, apathy, sleep disturbances, eating abnormalities). Both mild-to-moderate and prodromal AD showed a positive GDS response, whereas mild-to-moderate AD showed a better improvement on total NPI and apathy/nighttime behaviors subitems compared to prodromal AD. Higher baseline GDS score was the only variable associated with higher responses in linear regression analyses. MMSE showed a significant improvement at six months in the entire cohort, with a greater effect in prodromal vs mild-to-moderate AD. Cognitive improvement (i.e., MMSE changes) was associated with cognitive status at baseline but independent of the antidepressant/behavioral changes (i.e., GDS/NPI).
CONCLUSIONS: Our results suggest that vortioxetine is highly tolerable and clinically effective in both prodromal and mild-to-moderate AD with depression. Patients with mild-to-moderate AD benefited more from a wide range of behavioral disturbances. The study also showed significant improvement in global cognitive measures, especially in prodromal AD subjects. Further studies are needed to investigate the independent beneficial effect of vortioxetine on depression and cognition in AD.
Key words: Alzheimer’s disease, vortioxetine, neuropsychiatric symptoms, antidepressant.
Introduction
Mild cognitive impairment and dementia, including Alzheimer’s disease (AD), are common mental disorders in the elderly, often associated with behavioral and psychological symptoms (1, 2). Depression is frequently seen in AD, but it is often misdiagnosed in clinical practice. It has been estimated that up to 40% of patients in the early stages of AD experience depressive symptoms (3). Studies involving AD patients have identified a cluster of depression-related symptoms, including anxiety, apathy, sleep disturbances, and eating abnormalities (4). Moreover, mood disorders in AD patients may lead to a deterioration of cognitive status, the inability to perform daily activities (5), and associated behavioral disturbances (5–8). This is particularly crucial in the early stages as there is evidence that antidepressants might stabilize or improve cognitive functioning (9–11). However, it remains unclear whether the presence and severity of mood disturbance impact the clinical course of dementing illnesses in the early stages of AD (12, 13).
While the administration of antidepressants is widely endorsed by international guidelines, evidence suggests variations in antidepressant prescriptions across countries (14). Indeed, reports have indicated inappropriate prescription and management according to the clinical setting (15, 16). Unlike studies of depression in older individuals without dementia, clinical investigations on the use of antidepressants in patients with dementia have yielded mixed results, and the efficacy of serotoninergic antidepressants in AD remains uncertain (17–19). Thus, it is still an open question whether the impact of depression in AD depends on cognitive status. Furthermore, it is debatable whether the effectiveness of antidepressants might depend on the severity of dementia and early recognition and treatment of depressive disorders might benefit AD patients (17, 20). To date, few studies have investigated the impact of serotoninergic drugs on mood disturbances, functional status and cognitive performance across different stages of AD in patients with depression (21, 22). Vortioxetine is a multimodal drug approved for major depressive disorder (MDD), functioning through serotonin (5-HT) receptor activity and 5-HT transporter inhibition (23, 24). It has been demonstrated to be beneficial for treating depression in the elderly and improving cognitive function and activities of daily living (ADL) in adults with MDD (25–28). In AD patients, vortioxetine has been found to be safe and tolerable, but its impact on depression and cognition has been inconsistent (29, 30).
The aim of this retrospective, real-world observational study was to evaluate the clinical efficacy and safety of vortioxetine for AD patients with depressive symptoms, comparing prodromal AD and mild-to-moderate AD. Additionally, we investigated whether the effects of vortioxetine treatment differed between prodromal AD and mild-to-moderate AD in terms of depressive and other behavioral symptoms, as well as cognition and activities of daily living.
Methods
Participants and Procedures
This study comprised a subgroup analysis of a 6-month, retrospective, non-interventional study conducted in routine clinical practice. The study was implemented at the Center for Cognitive Decline and Dementia (CDCD) of the ASST Spedali Civili of Brescia, a general hospital affiliated with the University of Brescia, College of Medicine. Patients were selected from the observational Neuromultibio Study, which aimed to evaluate clinical and biological predictors of outcomes in acute and chronic neurological patients. The study was approved by the Local Ethic Committee (NP 1471, DMA, Brescia, approved in its last version on April 19th, 2022). Patients over 60 years old with mild cognitive impairment or mild AD and depression, evaluated between 01.01.2020 and 01.09.2022, were recruited for the study. All participants provided written informed consent. Participants were classified as having prodromal AD or mild-to-moderate AD according to the presence of biomarkers positivity and the IWG-2 research criteria (31) and National Institute on Aging–Alzheimer’s Association (NIA-AA) 2018 criteria (32). This study included patients who received vortioxetine for the treatment of MDD according to the local approved label, either as first-line treatment for the current depressive episode or after switching from another antidepressant due to an inadequate response or lack of tolerability. Following a standardized dose-escalation regimen, the starting dose of vortioxetine was 5 mg/day for the first two weeks, with an increment of 5 mg/day every two weeks, up to 20 mg/day. The inclusion criteria for the analyses were as follows: patients who (a) met the diagnostic criteria for depression in Alzheimer’s disease (33); (b) scored ≥ 5 on the short form of geriatric depression scale (GDS) (34); (c) scored 0.5-2 on the clinical dementia rating scale (CDR) (35), and (d) had a caregiver. Participants were excluded from the analyses if they (a) had a history of alcohol or substances use within 4 weeks before inclusion; (b) had other major mental disorders; (c) presented comorbid neurological diseases, including cerebrovascular conditions; or (d) had uncontrolled internal medicine conditions.
Clinical evaluation at baseline and 6 moths
The standardized clinical assessment of patients at the CDCD included a general demographic and clinical assessment using the cumulative index rating scale (CIRS) (36) and the clinical frailty scale (CFS) (37). Additionally, a cognitive and behavioral screening was performed every six months, along with a standard clinical neurological/medical evaluation. The assessment comprised the short version of GDS (34), a 15-item self-report scale used to rate depression symptoms in patients with dementia. The higher the score, the more depressive symptoms patients exhibited. Depression and related symptoms were evaluated at baseline and at week 28 using the neuropsychiatric inventory (NPI (38), a commonly used tool for evaluating behavioral symptoms with a caregiver interview. Cognitive function and impact in daily function were evaluated using the CDR (35) and the mini-mental state examination (MMSE) (39). Furthermore, we assessed functional abilities using the basic activities of daily living (BADL) and the instrumental activities of daily living (IADL) (40) every six months. Subgroups of Prodromal vs mild-to-moderate AD were defined based on CDR 0-0.5 and 1-2 scores, respectively. Patients included in the analyses were selected from the target population and only included those who had a clinical follow-up at six months (supplementary figure 1).
Statistical Analysis
Demographics were reported using mean ± standard deviation or number (%), as appropriate. Demographic characteristics of the groups (prodromal vs mild-to-moderate) were compared using t-tests and chi square for continuous and dichotomic variables. The interaction between changes of clinical scores (dependent variable) and the impact of impact of diagnosis (prodromal vs mild-to-moderate AD, independent variable) were evaluated using two-ways repeated measure ANOVA, using baseline age, sex, education, CIRS and frailty as covariates. Clinical scores (dependent variables) included in the analyses were GDS, NPI total and subitems, MMSE, BADL and IADL; the analyses showed the significance from baseline to follow-up measures and the interaction with groups, including the 95% confidence intervals (CI) in the whole sample and clinical subgroups groups. The normality distribution assumption for ANOVA test was separately tested for each dependent variable using Skewness and Kurtosis tests in addition to Q-Q plot by visual inspection. The repeated measure ANOVA models were further tested with groups as independent variable, including as additional covariate the baseline value of the specific target dependent variable (i.e., GDS, NPI, MMSE, dependent variable) to adjust for potential differences in baseline value.
A linear multivariable regression model including the previously defined clinical and demographic characteristics was implemented to evaluate the factors related to changes in GDS and MMSE at six months.
A significance level of p=0.05 was deemed significant for the analyses. Data analyses were carried out using SPSS software (version 22.0) and R (version 4.0.0; R Core Team, Wien-Austria).
Results
Subjects selection and baseline characteristics
Out of 680 AD patients who underwent assessment at the CDCD with 6 months of available follow-up data, 115 met the inclusion/exclusion criteria at baseline. Twenty-six patients were excluded due to treatment discontinuation (n=12), hospitalization for reasons unrelated to AD/depressive symptoms (n=2), low treatment compliance (n=6) and lost at follow-up (n=6) (Supplementary figure 1). Finally, 89 AD patients with depression entered the final analyses, including 40 with prodromal AD and 49 with mild-to-moderate AD patients. Within the mild-to-moderate AD group, 41 patients were taking donepezil (5 mg/day, n=17; 10 mg/day, n=24) at enrollment.
General demographics and baseline clinical characteristics of the two groups are shown in Table 1. No significant differences were observed between prodromal AD and mild-to-moderate AD patients regarding demographic characteristics, but they differed in terms of multimorbidity, frailty, cognition, and daily living activities (Table 1). Both AD groups exhibited comparable baseline severity of depression as measured by GDS and NPI depression subscale, although mild-to-moderate AD patients showed more severe anxiety, apathy, and sleep disturbances (Table 1).
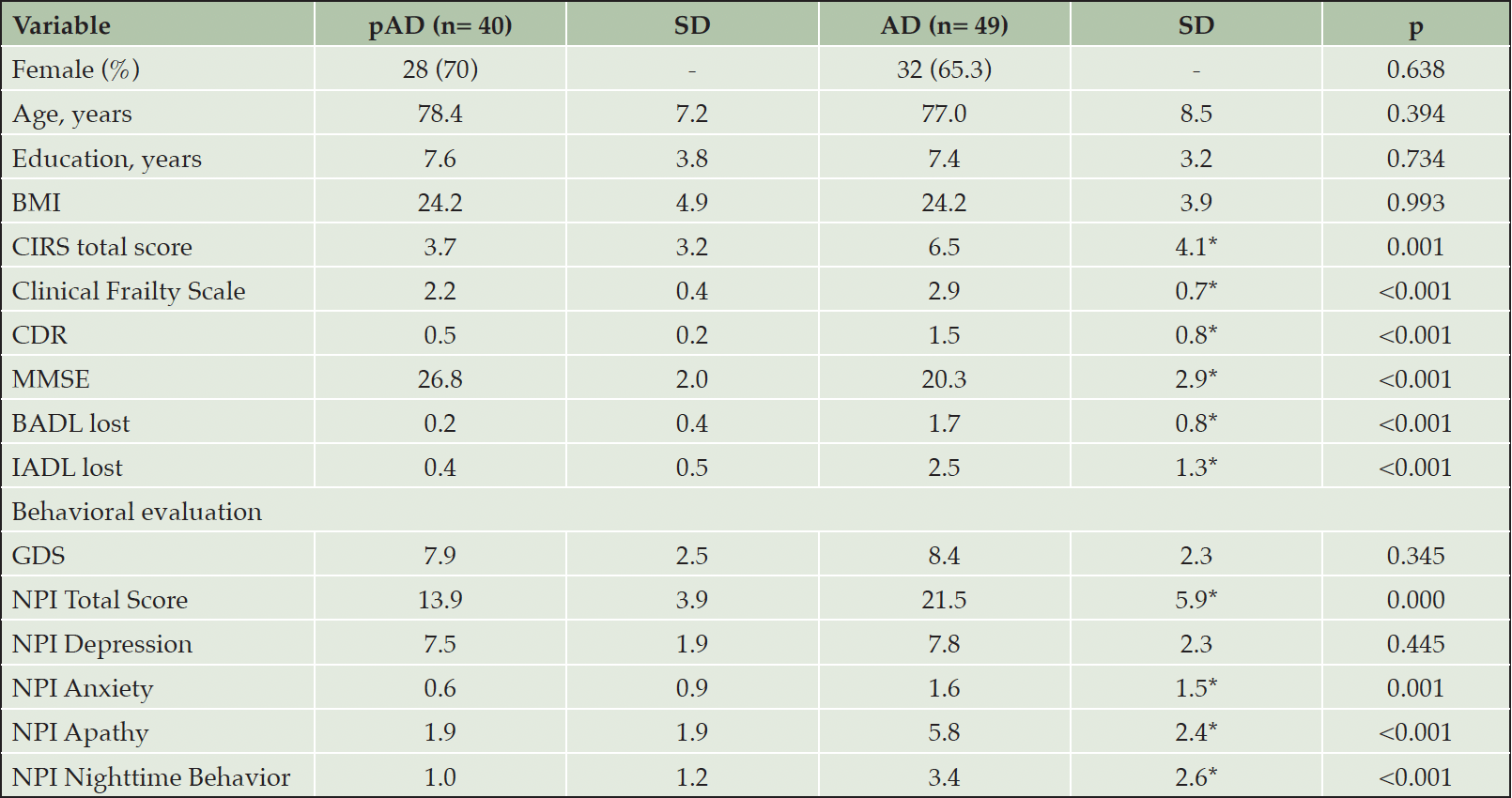
Table 1. Demographics and clinical characteristics of prodromal and mild-to-moderate AD included in the final analyses
Abbreviations: AD, subjects with mild Alzheimer’s disease; BADL, basic activities of daily living; BMI, body mass index; CDR, Clinical Dementia Rating scale; CIRS, cumulative Illness rating scale; GDS, geriatric depression scale; IADL, Instrumental Basic Activities of Daily Living; MMSE, Mini Mental State Examination; NPI, Neuropsychiatric Inventory; pAD, prodromal Alzheimer’s disease patients; SD, standard deviation.
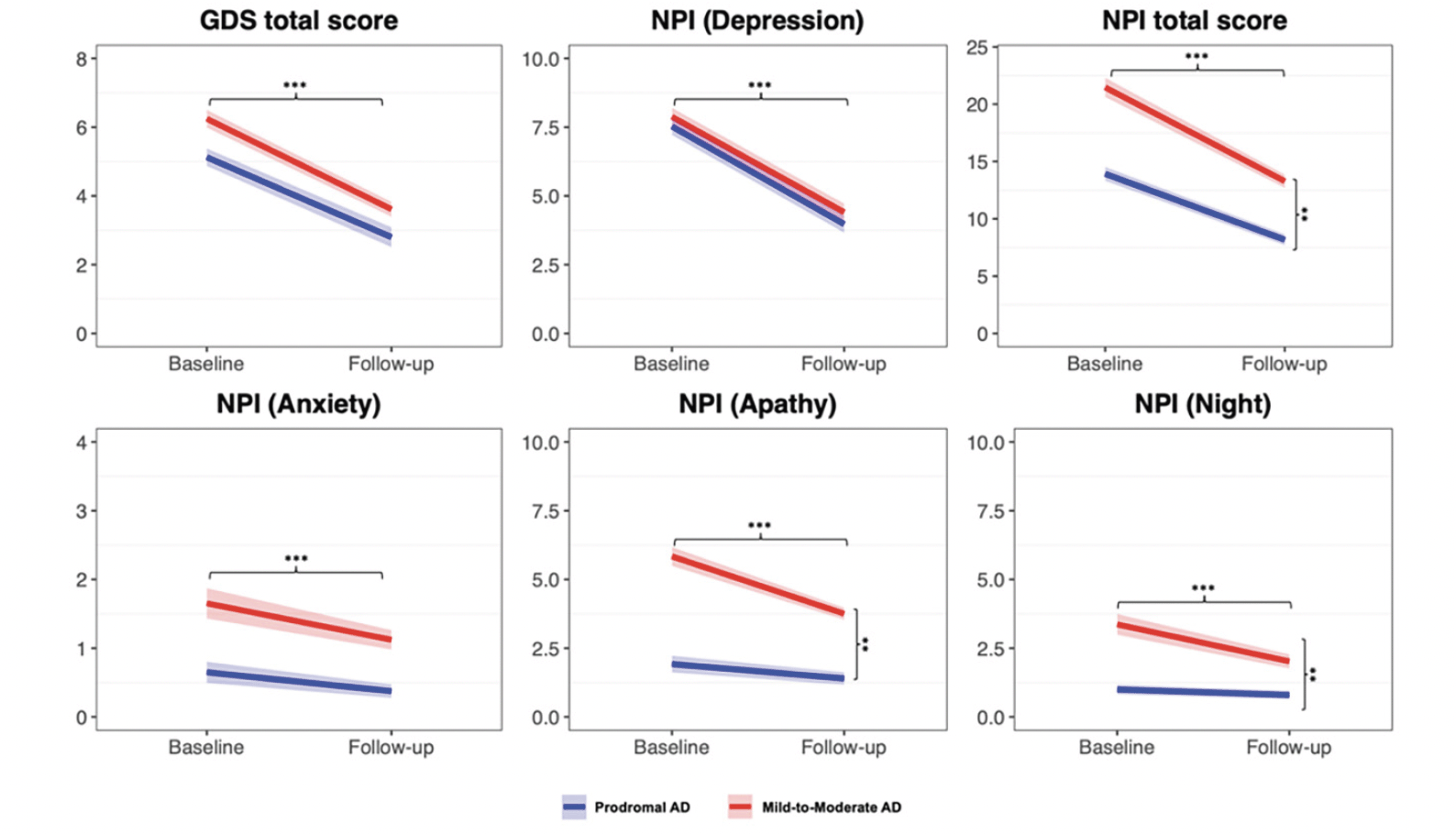
***= Significant improvement from baseline score in the whole sample **= treatment x group effect with differences between mild and prodromal AD; Abbreviations: AD, subjects with mild Alzheimer’s disease; GDS, geriatric depression scale; NPI, Neuropsychiatric Inventory.
Clinical follow-up and response to treatment
At follow-up, we observed significant changes in depressive symptoms, evaluated with both GDS and NPI depression subitem. Specifically, sixty-five (62%) patients exhibited an antidepressant effect, according to GDS improvement of ≥ 2 points from baseline, and showed significant improvement in NPI depression subitem and CDR scores. Regarding adverse events, the most frequently reported were nausea (4.5%), diarrhea (3,4%), and dizziness (2.2%). No deaths or serious adverse events occurred during the study. In the entire group, the non-parametric ANCOVA model adjusted for age and sex showed a significant decrease in GDS (F=69.923; p<0.001), NPI total (F=76.329; p<0.001), NPI depression (F=140.258; p<0.001), NPI apathy (F=9.164; p=0.002), and NPI sleep (F= 3.728; p=0.050) (Figure 1). No differences were observed between prodromal and mild-to-moderate AD in terms of GDS and NPI depression response (Figure 1, Supplementary Table 1). Conversely, mild-to-moderate AD patients exhibited a greater improvement compared to prodromal AD in NPI total score (F= 7.876; p=0.006), NPI apathy (F= 17.966; p<0.001), and NPI sleep (F= 13.676; p<0.001). The same differences were observed after adjusting for baseline GDS and NPI scores severity in the subgroups.
In a multivariate model testing the association demographic (age, gender, education) and clinically related factors at baseline (GDS, NPI, CDR, MMSE, CIRS) with change of GDS score, baseline GDS (B=0.63; t=8.2, p<0.001) was the only significant variable associated with higher GDS response at 6-months (Supplementary Table 2).
When adjusting for age, sex and CIRS, a trend of improvement in MMSE was observed (F=3.473; p=0.065). No differences in CDR scores were observed at 6 months in the entire group. In a multivariate model testing the association of demographic (age, gender, education) and clinically related factors at baseline (GDS, NPI, CDR, MMSE, CIRS) with changes in MMSE score, only higher baseline MMSE scores were associated with better improvement in MMSE change. Depression severity at baseline and depression improvement were not associated with either cognitive improvement or improvement in daily living activities at 6 months, as revealed by partial correlation analyses and linear regression analyses.
Discussion
This retrospective observational study presents the effects of vortioxetine on depressive symptoms, behavioral disturbances, cognitive performance, and daily living activities in prodromal and mild-to-moderate AD patients with depression. To our knowledge, this is the first study that compares the impacts of vortioxetine on depression and cognition across various severity stages of AD. The main analysis of this study showed that prodromal and mild-to-moderate biomarker-confirmed AD patients exhibited significant improvement in depressive symptoms at 6 months. The response rate was observed in approximately two-third of the patients and was predicted by baseline severity of depression, controlling for demographic and clinical factors, including frailty and multimorbidity. The mood outcomes results were consistent in prodromal and mild-to-moderate AD, showing a significant reduction from baseline to endpoint in both GDS and NPI depression subitems, as well as in NPI total scores. Both groups also showed significant improvement on MMSE. Compared to prodromal AD, mild-to-moderate AD showed significantly greater reduction from baseline to endpoint in NPI total score. However, a group difference favoring prodromal AD was observed for cognitive function. The improvement in cognitive performance was independent of the antidepressant effect but was associated with baseline MMSE scores.
Safety data from this study supports the known favorable tolerability profile of vortioxetine administered for 6 months in the AD patient population. The reported incidence of adverse events was low, which aligns with the adopted dose escalation approach. The most prevalent adverse events were nausea and diarrhea, which occurred in less than 5% of patients. These events were mild, transient, and did not lead to withdrawal.
The results contrast several studies intended to evaluate the effect of SSRI in dementia but align with more recent data on vortioxetine (29, 41, 42). Despite the common use of SSRIs and SNRIs to treat depression in the elderly and dementia, (19, 43), they lack consistent evidence for a reliable antidepressant benefit in AD (19, 44, 45). Vortioxetine is an antidepressant with a multimodal mechanism of action related to direct modulation of 5-HT receptor activity and inhibition of the 5-HT transporter (46), leading to modulation of neurotransmission in several systems crucial for mood and cognitive function (47). In fact, such modulation is likely responsible for the antidepressant and behavioral effects as well as improvements in cognitive function both in depressed patients and in patients with Alzheimer’s disease and depressive symptoms (29, 42, 48). Consistent with these studies, we observed a significant effect of vortioxetine 20 mg/day treatment on depression, measured by GDS and NPI depression sub-item in both prodromal and mild-to-moderate AD patients. There was no significant group interaction for the antidepressant efficacy of vortioxetine, but the effect was primarily dependent on depression severity at baseline, controlling for demographic and clinical factors in multivariate linear regression analyses. A significant effect was also observed on mood-related symptoms, including anxiety, apathy, agitation, and sleep disorders, confirming previous findings (49). Both prodromal and mild-to-moderate AD groups demonstrated a significant cognitive benefit as measured by MMSE. The cognitive effect was found to be more pronounced in prodromal AD and was predicted by baseline cognitive status, but there was no relationship with the antidepressant effect or with behavioral changes. These data strongly support previous findings on MDD which have demonstrated that vortioxetine has positive effects on cognitive performance (26, 28, 42), and that the cognitive effect of vortioxetine is largely independent of its effect on depressive symptoms (29, 42). Few studies have investigated the effect of vortioxetine in AD patients, reporting inconsistent results. Cumbo and colleagues (29), in a 12-month open label observational study on 108 patients receiving either vortioxetine or other common antidepressant including paroxetine, observed a significant improvement versus control for vortioxetine on most of the cognitive tests, and showed significant baseline-to-endpoint reduction in both Hamilton depression rating scale and Cornell total scores. More recently, Cumbo and colleagues (42) in a 6-month prospective multicenter cohort study of 207 outpatients with MDD and a pre-baseline diagnosis of AD, found that after 24 weeks of vortioxetine 5 mg/day treatment, overall clinical improvement was observed in more than two-thirds and remission from depressive symptoms in more than half. Conversely, in a short-term, randomized, double-blind, placebo-controlled study of vortioxetine in patients with AD experiencing severe depression, no statistically significant differences between groups were seen in depressive symptoms, cognitive function, or overall patient functioning after 12 weeks of treatment (30). However, the study was limited by several factors, including the high dropout rate, a short period of observation, and a low mean dosage administered. It should be noted that participants in this study had more severe dementia, likely limiting the impact of the antidepressant. Indeed, Landes and colleagues (50) showed severe depressive symptoms in patients with advanced neurodegeneration, suggesting that the antidepressant effect is negligible when neurodegeneration is advanced.
We acknowledge that this study had several limitations. First, this was an observational, retrospective, 6 months, single- center study conducted on a sample of AD patients with depression on vortioxetine, contrasting prodromal AD and mild-to-moderate AD. While prodromal AD and mild-to-moderate AD did not differ in terms of demographic characteristics, there were clinical differences between the two groups, including multimorbidity, behavioral symptoms, and treatments such as anticholinesterases. These differences might have differentially influenced treatment outcomes in each group, although the antidepressant effect was not influenced by frailty and comorbidities scores. Since this study is based on observational data, there is no untreated control group that may have facilitated assessment of the clinical relevance observed during vortioxetine treatment. It is possible that non-treatment-specific factors related to study participation, such as increased interaction with clinicians, frequent assessment, or practice effects, could have contributed to the findings (51). The study did not include more severe AD patients, making it difficult to exclude the possibility that vortioxetine might have a lower impact on depression and cognition in advanced stages. Nevertheless, the significant improvements observed are consistent with and appear to support the direct effect of vortioxetine on depression and cognition, as demonstrated in randomized controlled trials conducted with MDD patients (26–28). The main strengths of the current analysis include its conduct in a cohort of AD patients with depression across severity stages, confirmed by biomarker evidence, adopting a dose-escalation regimen up to 20 mg/day dosage. A further strength is the standard 6-month clinical protocol evaluation, which offers significant advantages over cross-sectional and prospective studies with shorter follow-ups in terms of evaluating the effect of treatment on symptoms of depression, and functional and cognitive status using longitudinal real-life data. The study enrolled prodromal AD and mild-to-moderate AD to investigate potential differential clinical effects of treatment according to AD stages. Finally, the study included both experimenter-based and caregiver-based instruments to assess and compare treatment responses.
In summary, the results of this analysis demonstrate the effectiveness and tolerability of vortioxetine for the treatment of MDD in patients with AD. Clinically meaningful improvements in depressive symptoms, behavioral symptoms, and cognitive symptoms, as well as measures of daily living activities, were observed over the 6 months of vortioxetine 20 mg/day treatment. Vortioxetine was well tolerated in this real-world patient cohort, most of whom were receiving concomitant medication. Additional prospective, controlled, long-term studies are required to further explore and quantify the effects of vortioxetine treatment on depression, Behaviour and Psychological Symptoms of Dementia (BPSDs), cognition, and function in subjects across the spectrum of AD.
Funding: None. Open access funding provided by Università degli Studi di Brescia within the CRUI-CARE Agreement.
Acknowledgements: The authors would like to express thanks to the investigators and their teams and the patients who took part in this study.
Conflict of interest: Alessandro Padovani received grant support from Ministry of Health (MINSAL) and Ministry of Education, Research and University (MIUR), IMI H2020 initiative (IMI2-2018-15-06). Salvatore Caratozzolo reports no competing interest. Alberto Benussi received grant support from Fondazione Cariplo (grant n° 2021-1516) and by the Fondation pour la Recherche sur Alzheimer. Alice Galli reports no conflict of interest. Luca Rozzini reports no conflict of interest. Maura Cosseddu reports no competing interest. Rosanna Turrone reports no competing interest. Andrea Pilotto received grant support from IMI H2020 initiative (IMI2-2018-15-06), Ministry of Health (MINSAL) Ministry of Education, Research and University (MIUR).
Ethical standards: NP 1471, DMA, Brescia, approved in its last version on April 19th, 2022). Patients over 60 years old with mild cognitive impairment or mild AD and depression, evaluated between 01.01.2020 and 01.09.2022, were recruited for the study. All participants provided written informed consent.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Spalletta G, Musicco M, Padovani A, et al. Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer disease. The American Journal of Geriatric Psychiatry. 2010;18(11):1026-1035.
2. Richard E, Reitz C, Honig LH, et al. Late-life depression, mild cognitive impairment, and dementia. JAMA Neurol. 2013;70(3):383-389.
3. Ismail Z, Elbayoumi H, Fischer CE, et al. P2-087: A systematic review and meta-analysis for the prevalence of depression in mild cognitive impairment. Alzheimer’s & Dementia. 2015;11(7S_Part_11):P516-P517.
4. Siafarikas N, Selbaek G, Fladby T, Benth JŠ, Auning E, Aarsland D. Frequency and subgroups of neuropsychiatric symptoms in mild cognitive impairment and different stages of dementia in Alzheimer’s disease. Int Psychogeriatr. 2018;30(1):103-113.
5. Lyketsos CG, Steele C, Baker L, et al. Major and minor depression in Alzheimer’s disease: prevalence and impact. Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9(4):556-561.
6. Cannon-Spoor HE, Levy JA, Zubenko GS, et al. Effects of previous major depressive illness on cognition in Alzheimer disease patients. The American journal of geriatric psychiatry. 2005;13(4):312-318.
7. Starkstein SE, Mizrahi R, Power BD. Depression in Alzheimer’s disease: phenomenology, clinical correlates and treatment. International Review of Psychiatry. 2008;20(4):382-388.
8. Vicini Chilovi B, Conti M, Zanetti M, Mazzù I, Rozzini L, Padovani A. Differential impact of apathy and depression in the development of dementia in mild cognitive impairment patients. Dement Geriatr Cogn Disord. 2009;27(4):390-398.
9. Gallassi R, Di Sarro R, Morreale A, Amore M. Memory impairment in patients with late-onset major depression: the effect of antidepressant therapy. J Affect Disord. 2006;91(2-3):243-250.
10. Mowla A, Mosavinasab M, Pani A. Does fluoxetine have any effect on the cognition of patients with mild cognitive impairment?: a double-blind, placebo-controlled, clinical trial. J Clin Psychopharmacol. 2007;27(1):67-70.
11. Mowla A, Mosavinasab M, Haghshenas H, Haghighi AB. Does serotonin augmentation have any effect on cognition and activities of daily living in Alzheimer’s dementia?: a double-blind, placebo-controlled clinical trial. J Clin Psychopharmacol. 2007;27(5):484-487.
12. Brendel M, Sauerbeck J, Greven S, et al. Serotonin selective reuptake inhibitor treatment improves cognition and grey matter atrophy but not amyloid burden during two-year follow-up in mild cognitive impairment and Alzheimer’s disease patients with depressive symptoms. Journal of Alzheimer’s disease. 2018;65(3):793-806.
13. Enache D, Fereshtehnejad S, Kåreholt I, et al. Antidepressants and mortality risk in a dementia cohort: data from SveDem, the Swedish Dementia Registry. Acta Psychiatr Scand. 2016;134(5):430-440.
14. Giebel CM, Sutcliffe C, Renom-Guiteras A, et al. Depressive symptomatology in severe dementia in a European sample: prevalence, associated factors and prescription rate of antidepressants. Int Psychogeriatr. 2015;27(4):657-667.
15. Puranen A, Taipale H, Koponen M, et al. Incidence of antidepressant use in community-dwelling persons with and without Alzheimer’s disease: 13-year follow-up. Int J Geriatr Psychiatry. 2017;32(1):94-101.
16. Bhattacharjee S, Lee JK, Patanwala AE, et al. Extent and predictors of potentially inappropriate antidepressant use among older adults with dementia and major depressive disorder. The American Journal of Geriatric Psychiatry. 2019;27(8):794-805
17. Sabates J, Chiu WH, Loi S et al.The Associations Between Neuropsychiatric Symptoms and Cognition in People with Dementia: A Systematic Review and Meta-Analysis. Neuropsychol Rev. 2023 Jul 21. doi: 10.1007/s11065-023-09608-0.
18. Ebrahim IM, Ghahremani M, Camicioli R et al.. Effects of race, baseline cognition, and APOE on the association of affective dysregulation with incident dementia: A longitudinal study of dementia-free older adults. J Affect Disord. 2023 Jul 1;332:9-18.
19. Banerjee S, Hellier J, Dewey M, et al. Sertraline or mirtazapine for depression in dementia (HTA-SADD): a randomised, multicentre, double-blind, placebo-controlled trial. The Lancet. 2011;378(9789):403-411.
20. Cooper C, Katona C, Lyketsos K, et al. A systematic review of treatments for refractory depression in older people. American Journal of psychiatry. 2011;168(7):681-688.
21. Khoury R, Grossberg GT. Impact of antidepressant use on the trajectory of Alzheimer’s disease: evidence, mechanisms, and therapeutic implications. CNS Drugs. 2019;33(1):17-29.
22. Munro CA, Brandt J, Sheppard JME, et al. Cognitive response to pharmacological treatment for depression in Alzheimer disease: secondary outcomes from the depression in Alzheimer’s disease study (DIADS). The American journal of geriatric psychiatry. 2004;12(5):491-498.
23. Taylor WD, Boyd BD, Elson D, et al. Preliminary evidence that cortical amyloid burden predicts poor response to antidepressant medication treatment in cognitively intact individuals with late-life depression. The American Journal of Geriatric Psychiatry. 2021;29(5):448-457.
24. Bang-Andersen B, Ruhland T, Jørgensen M, et al. Discovery of 1-[2-(2, 4-dimethylphenylsulfanyl) phenyl] piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem. 2011;54(9):3206-3221.
25. Mørk A, Pehrson A, Brennum LT, et al. Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. Journal of Pharmacology and Experimental Therapeutics. 2012;340(3):666-675.
26. Katona C, Hansen T, Olsen CK. A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol. 2012;27(4):215-223.
27. Montgomery SA, Nielsen RZ, Poulsen LH, Häggström L. A randomised, double-blind study in adults with major depressive disorder with an inadequate response to a single course of selective serotonin reuptake inhibitor or serotonin–noradrenaline reuptake inhibitor treatment switched to vortioxetine or agomelatine. Human Psychopharmacology: Clinical and Experimental. 2014;29(5):470-482.
28. McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. International Journal of Neuropsychopharmacology. 2014;17(10):1557-1567.
29. Cumbo E, Cumbo S, Torregrossa S, Migliore D. Treatment effects of vortioxetine on cognitive functions in mild Alzheimer’s disease patients with depressive symptoms: a 12 month, open-label, observational study. J Prev Alzheimers Dis. 2019;6:192-197.
30. Jeong HW, Yoon KH, Lee CH, Moon YS, Kim DH. Vortioxetine treatment for depression in Alzheimer’s disease: a randomized, double-blind, placebo-controlled study. Published online 2022.
31. Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13(6):614-629.
32. Jack Jr CR, Bennett DA, Blennow K, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;14(4):535-562.
33. Teng E, Ringman JM, Ross LK, et al. Alzheimer’s Disease Research Centers of California-Depression in Alzheimer’s Disease Investigators. Diagnosing depression in Alzheimer disease with the national institute of mental health provisional criteria. Am J Geriatr Psychiatry. 2008;16(6):469-477.
34. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clinical Gerontologist: The Journal of Aging and Mental Health. Published online 1986.
35. Hughes CP, Berg L, Danziger W, Coben LA, Martin RL. A New Clinical Scale for the Staging of Dementia. The British Journal of Psychiatry. 1982;140(6):566-572. doi:DOI: 10.1192/bjp.140.6.566
36. Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the Cumulative Illness Rating Scale in a geriatric residential population. J Am Geriatr Soc. 1995;43(2):130-137.
37. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Cmaj. 2005;173(5):489-495.
38. Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5 Suppl 6):10S-16S.
39. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi:https://doi.org/10.1016/0022-3956(75)90026-6
40. Lawton MP, Brody EM. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living1. Gerontologist. 1969;9(3_Part_1):179-186. doi:10.1093/geront/9.3_Part_1.179
41. Tan SN, Tan C. Vortioxetine improves cognition in mild cognitive impairment. Int Clin Psychopharmacol. 2021;36(6):279.
42. Cumbo E, Adair M, Åstrom DO, Christensen MC. Effectiveness of vortioxetine in patients with major depressive disorder and comorbid Alzheimer’s disease in routine clinical practice: An analysis of a post-marketing surveillance study in South Korea. Front Aging Neurosci. 2022;14.
43. Dudas R, Malouf R, McCleery J, Dening T. Antidepressants for treating depression in dementia. Cochrane Database of Systematic Reviews. 2018;(8).
44. Orgeta V, Tabet N, Nilforooshan R, Howard R. Efficacy of antidepressants for depression in Alzheimer’s disease: systematic review and meta-analysis. Journal of Alzheimer’s Disease. 2017;58(3):725-733.
45. He Y, Li H, Huang J, et al. Efficacy of antidepressant drugs in the treatment of depression in Alzheimer disease patients: A systematic review and network meta-analysis. Journal of Psychopharmacology. 2021;35(8):901-909.
46. Stahl SM. Modes and nodes explain the mechanism of action of vortioxetine, a multimodal agent (MMA): modifying serotonin’s downstream effects on glutamate and GABA (gamma amino butyric acid) release. CNS Spectr. 2015;20(4):331-336.
47. Dale E, Zhang H, Leiser SC, et al. Vortioxetine disinhibits pyramidal cell function and enhances synaptic plasticity in the rat hippocampus. Journal of psychopharmacology. 2014;28(10):891-902.
48. Bishop MM, Fixen DR, Linnebur SA, Pearson SM. Cognitive effects of vortioxetine in older adults: a systematic review. Ther Adv Psychopharmacol. 2021;11:20451253211026796.
49. Crocco EA, Jaramillo S, Cruz-Ortiz C, Camfield K. Pharmacological management of anxiety disorders in the elderly. Curr Treat Options Psychiatry. 2017;4:33-46.
50. Landes AM, Sperry SD, Strauss ME. Prevalence of apathy, dysphoria, and depression in relation to dementia severity in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2005;17(3):342-349.
51. Sanderson C, Hardy J, Spruyt O, Currow DC. Placebo and nocebo effects in randomized controlled trials: the implications for research and practice. J Pain Symptom Manage. 2013;46(5):722-730.
© The Authors 2024