A. Braun1, M. Höfler2, S. Auer2
1. IMC University of Applied Sciences Krems, Krems, Austria; 2. Department for Dementia Research at the University for Continuing Education Krems, Krems, Austria.
Corresponding Author: Alexander Braun, IMC University of Applied Sciences Krems, Krems, Austria, alexander.braun@fh-krems.ac.at
J Prev Alz Dis 2024;2(11):402-413
Published online January 15, 2024, http://dx.doi.org/10.14283/jpad.2024.12
Abstract
Dementia is from an economic perspective a main challenge for economies worldwide because of increasing costs. Since there is no cure in sight, prevention seems the most promising approach for reducing health care cost due to Dementia. On the contrary, approximately 40% of dementias is attributable to modifiable risk factors and first studies showed that multidomain interventions may be effective for preventing dementia. Considering the increasing economic burden, for many health administrations worldwide, cost-effectiveness plays a mayor role. This scoping review wants to bring evidence to the question if prevention for people at risk may be cost-effective. Therefore, the four databases Medline (via Pubmed), CINHAL (via EBSCO), Business Source Complete (via EBSCO), and the Health Economic Evaluation database (HEED) were used to conduct a scoping review using PICO and a systematic search string. 3,629 studies were identified and seven met all inclusion criteria. The included studies showed clear cost-effectiveness for most multidomain interventions. The gained QALYs at mean were 0.08 (SD=0.08) and the intervention average costs 472.20 EUR per Person (SD=74.06 EUR). The Incremental Cost-Effectiveness Ratios varied between –80,427.97 and 104,189.82 EUR per QALY. The three core results are (i) prevention programs focusing on people at risk may be cost-effective and cost-efficient, (ii) multimodal prevention reveal cost saving potential, when the people at risk are defined well, (iii prevention in middle-aged cohorts may be also cost-effective if life-style related risk factors are addressed.
Key words: Cost and cost analysis, dementia, prevention, health economics.
Introduction
Dementia is a global challenge and an economic burden (1–4). It is an inconvertible fact that the number of people with dementia is increasing rapidly in aging societies and the numbers are projected to be doubling every twenty years (4). So, societies must develop strategies to change this trend. However, recent scientific achievements suggest a change in the paradigm of the understanding of dementia (5) and risk reduction strategies can be concluded from the evidence (6, 7). The WHO urges countries around the world to develop risk reduction strategies for dementia (8, 9). The WHO pushes also ongoing research for optimizing brain health and specialized prevention for brain diseases. Especially economic research to inform health policy decisions and payers are prioritized by the WHO (10). Fortunately, there is also growing evidence that 40% of dementias could be explained by lifestyle-related risk factors, thus, are considered as preventable and reduction of risks seems now feasible (8, 9, 11–13). For this reason, we suggest the best effects to cope with these issues are threefold. Good prevention practice could be addressing: (i) reduction of risk factors of dementia, (ii) early detection of pre-clinical dementia, and (iii) social support of people with dementia (12, 14–19). The WHO developed – based on existing literature – a guideline to reduce the modifiable risk factors and the LANCET-Commission on Dementia prevention stated that there is evidence that the risk for developing dementia could be reduced by lifestyle intervention strategies such as physical activity, adhere to a healthy diet, engage in regular cognitively stimulating activities (8, 12). In addition, risk can be reduced by evidence-supported lifestyle factors in middle age such as treatment of hearing-loss, treating high blood pressure and overweight, and in higher age avoid smoking, treatment of depression and diabetes, increase physical activity and avoid social isolation (8, 12).
Dubois et al. (2010) define a target group for prevention of dementia as ‘people at risk’. This definition means that people may have some substantial risk for developing dementia but are “clinically asymptomatic individuals with biological evidence of Alzheimer’s pathology” (Dubois et al., 2010: p. 5). So far, the health economic literature has focused solely on the risk-factors (21) or clinical disease stage (22–24). Thus, we identified a research gap in the literature of Cost-Effectiveness of primary prevention for people at the at-risk-state (25, 26). This research gap raised main methodological questions for health economic evaluations and has its origins in the methodology of indirect costs of dementia (26, 27). From an economic perspective it became evident that the costs in advanced dementia stages are the cost-drivers and a delay of dementia may be a good measure for reducing health care costs (22, 28–30). Some key insights from health economics are that advanced stages are more costly than early stages and the progression of existing dementia explain more cost increase than the increase in absolute numbers (14, 22, 30, 31). The investment in primary prevention interventions could possibly prevent persons affected by dementia to care-intensive stages and thus substantially lower cost of care (32). This is verified by Hayek et al. (2019) who showed that an early detection of dementia has evident impact on clinical and social support but from an economic perspective, the early detection strategy dismantle hidden costs but have just minor impact on health care costs in general because it does not prevent them (16, 33, 34). This issue was also mentioned as research gap in the guideline of the National Institute for Health and Care Excellence (NICE) in the United Kingdom (35).
Further, the growing evidence on possible effective prevention strategies also raised the question of whether primary prevention strategies could be cost-saving for public health authorities or if investment into prevention of dementia could be cost-effective (12, 25, 36–38). For instance, the ‘Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability’ (FINGER)-study, a randomized controlled trial based on multimodal nonpharmacological lifestyle interventions, showed effectiveness for preventing dementia due to multimodal interventions and alongside health economic studies on cost-effectiveness of prevention of dementia have been published and indicated a cost-saving potential for prevention strategies (7, 39–41). Walsh et al. (2022) showed also with a high-quality systematic review of evidence, that the reduction of the defined risk factors by Livingston et al. (2020) and the WHO (2019) is highly cost-effective and in some cases also cost-saving. But no health economic evidence-synthesis was conducted using a clearly defined ‘people at risk’ population. Thus, this scoping review intends to summarize and provide evidence on the investment in ‘Brain health risk reduction programs’ with the final goal to prevent very costly care for advanced dementia stages (42). Following these insights, we want to answer the research question: “How is the evidence of cost-effectiveness for primary prevention of people at risk for dementia?”
Methods
This review was conducted based on the recommendations for scoping reviews by the Joanne Briggs Institute (43, 44). This scoping review followed in reporting the Extension for Scoping Reviews of the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analysis’ (PRISMA-ScR) (45). In addition to validating the coding for the abstract and full text screening inclusion and exclusion criteria (Appendix 1) were developed by all three authors. Although, the economic evaluation of preventive measures for dementia has just been evolved in recent years, and, thus, the number of publications might be limited, a scoping review is able to provide a comprehensive and systematic overview of evidence so far also by including also grey literature and pre-prints.
Search strategy and evaluation process
Four electronic databases from medicine, health and nursing sciences, and health economics were searched for studies that are eligible for review. These databases were Medline (via Pubmed), CINHAL (via EBSCO), Business Source Complete (via EBSCO), and the Health Economic Evaluation database (via CRD). After the inclusion process, a forward citation tracking for key articles and snowballing from the reference lists were applied. Searches were undertaken on 09th February 2023 and searched all published papers until this date. After this process hand searches were applied in medRxiv, google scholar, CADTH Grey matters, CRD database and the International HTA database for identify grey literature for HTA-agencies and pre-prints (in August 2023). All three reviewers made an independent screening process, and each abstract was screened by at least two reviewers, who evaluated them by the defined inclusion- or exclusion-criteria. Any disagreements between these two reviewers were solved by the decision of the third reviewer. The AbstrackR literature evaluation, a software for semi-automatic citation screening was used (46).
Inclusion criteria
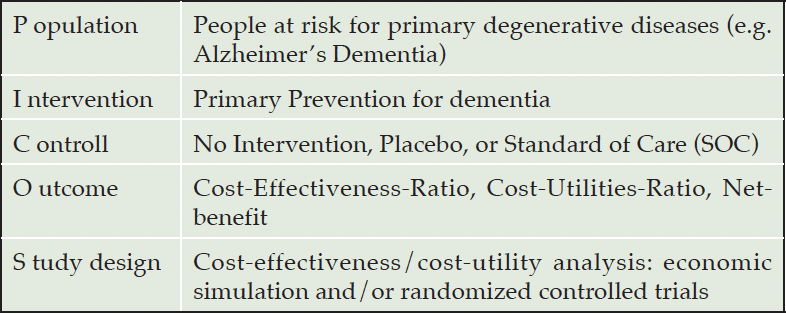
Following this methodology, all three authors developed a search strategy based on the ‘Population, Intervention, Control, Outcomes’(PICO)-scheme (Table 1). Adapted from Solomon, Ngandu & Kivipelto (2022) we defined the ‘Population’ as people at risk for dementia, hence, we excluded studies focusing on (pre)symptomatic forms (which includes all preclinical forms before the prodromal state). Further, we searched for Cost-Effectiveness Analysis (CEA) or Cost-Utility Analysis (CUA) that evaluated primary prevention for dementia. The search string was used Boolean operators and the search strategy and keywords are listed in Appendix 1. This scoping review used inclusion criteria for the employed study design. Included studies would either a Cost-Effectiveness-Analysis (CEA), its special form of Cost-Utility-Analysis (CUA) by using data from alongside RCTs or health economic model/simulation techniques. Studies published in English, German, or Spanish were included in this review. The inclusion and exclusion criteria are listed in more detail in appendix 2.
Data extraction
Data were extracted by using a pre-developed template sheet. This template included the first authors name, publication year, country, study design and used model, sample size, data sources, and program focus. For the cost-effectiveness evaluation the costing methodology, costs (direct, indirect, and intangible costs), discount rate, Incremental Cost-Effectiveness Ratio (ICER)/Incremental Net-benefit (INB) and their uncertainty measures via sensitivity analysis were used. The data extractions were performed by one experienced health economist and were cross-checked by another reviewer. All currencies were converted to 2022 EURO using CCEMG–EPPI-Centre Cost Converter (v.1.6 last update: 29 April 2019), applying the latest available purchase power parities from the International Monetary Fund (IMF).
Economic Primary Outcomes
Because the health economic evaluation methodology uses different primary outcomes for decision making, it becomes crucial to define the three different primary outcomes and definitions that are considered in this scoping review. As (39) explained, a good analytic health economic evaluation method for answering the research question is a cost-effectiveness-analysis (CEA) or cost-utility-analysis (CUA). Thus, the CUA is a special form of CEA and uses quality-adjusted life-years (QALY) as comparator in contrast to the CEA that uses medical outcomes, both evaluation techniques are applicable for answering the research question. In general, the CEA uses information for health technologies and programs and applies economic principles for decision making, like allocation and cost-saving interventions. But CEA also shows cost-effectiveness in that sense that programs which are more effective than a comparator, could be also more costly. In this respect, the concept of willingness-to-pay (WTP) is applied. This means that a more costly but also more effective program might be seen as relatively cost-effective due to a given threshold (λ). International literature state that the λ from public financiers varies strongly, but at the mean 25,000 EUR per QALY is some proper threshold (47).
The basic principle of the Incremental Cost-Effectiveness-ratio (ICER) and the Incremental Net-Benefit (INB) are economic outcomes, that shared some common features, like a required pre-defined λ and the acceptance of quality-adjusted life years (QALYs). While ICER are more oriented towards a scientific decision-making, the INB is more oriented on practical issues:
(1) Incremental Cost-Effectiveness-ratio (ICER) = (incremental Cost)/(incremental Effectiveness)
A program could be strongly superior or cost-saving, when the effectiveness of prevention exceeds the effectiveness of non-intervention by a decrease of costs (south-east quadrant of the health economic evaluation plane (48)). But the program could be also superior or cost-effective when effectiveness of prevention exceeds the Standard of Care (SOC) – which could be also doing nothing – and has an ICER < λ (north-east quadrant). If the ICER > λ, economic evaluation describes the prevention program as inferior to the SOC, when the effectiveness of prevention is less than the effectiveness of SOC (by increased costs) or when the cost per gained QALY is higher than the accepted λ.
Another commonly used method from health economics is the Incremental Net-Benefit (INB), which shows if the program is cost-saving by increased outcomes (strong superior) or cost-effective (superior) under given λ. INB is calculated as followed:
(2) Incremental Net-Benefit (INB) = λ(incremental QALY) – (incremental Costs)
Here, it is clear that – under the condition of a given λ – a negative INB shows strong superiority/cost-saving of the program compared to the SOC.
Economic Secondary Outcomes
From an economic perspective, individuals that are seeking primary prevention want to maximize their health over their own utility and/or well-being over their lifetime (42). Hence, in prevention the estimated ICERs and input parameters are approximations from observations towards lifetime course. This implies that the economic evaluation of prevention programs must focus also strongly on coping with uncertainty and therefore sensitivity-analysis became quite important (39, 49).
Results
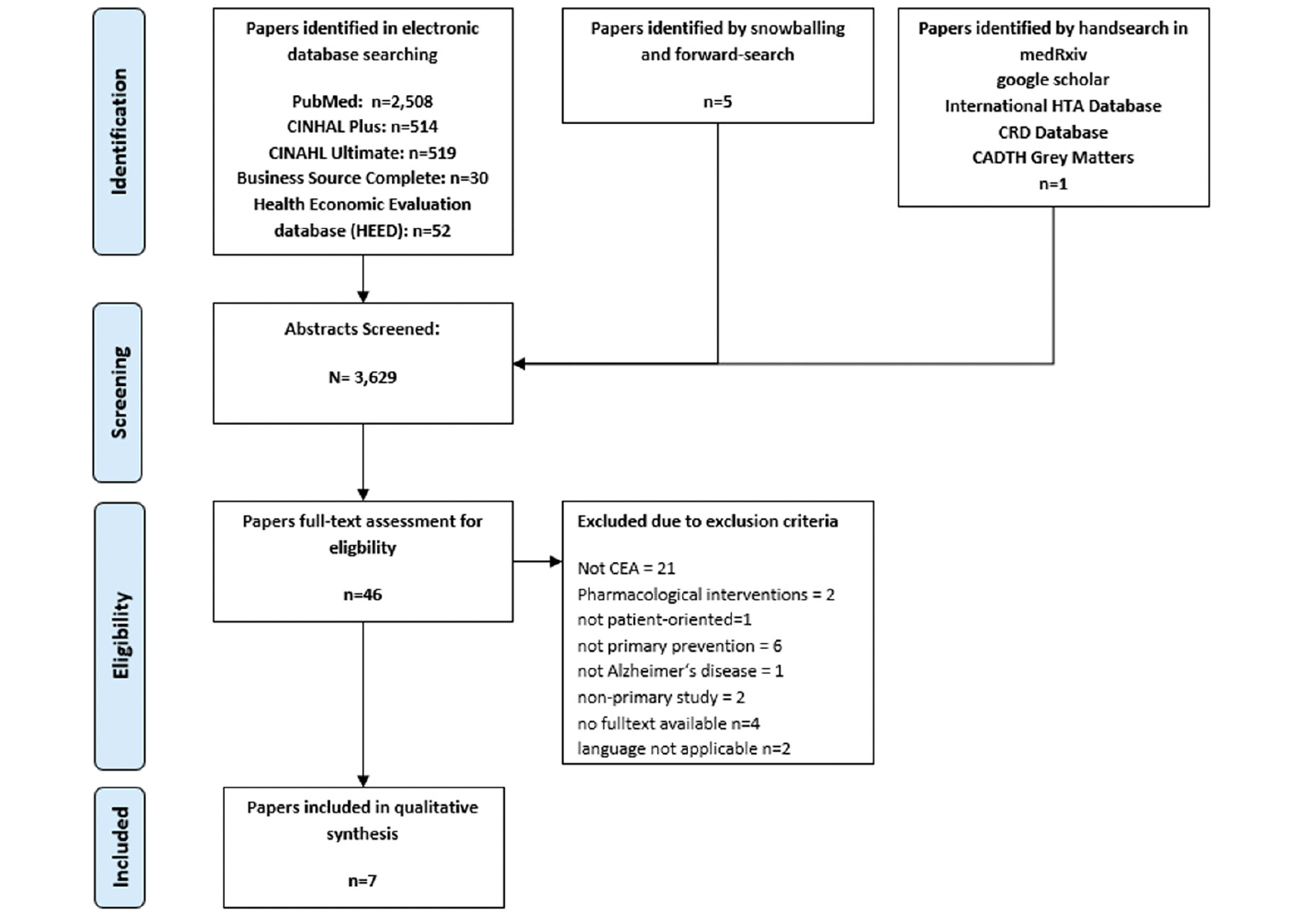
As the Flow-Chart (figure 1) shows, the literature search yielded 3,629 publications. After screening the abstracts 46 papers met inclusion criteria for full-text screening. After removing 39 due to the missing the scope of this review seven studies were included for the qualitative synthesis of this review (40, 41, 50–54). In searching for grey literature one study protocol was identified, which could not include into the review, because the cost-effectiveness analysis was not undertaken yet (55). The descriptives of the included studies are presented in table 1.
Study designs of included studies
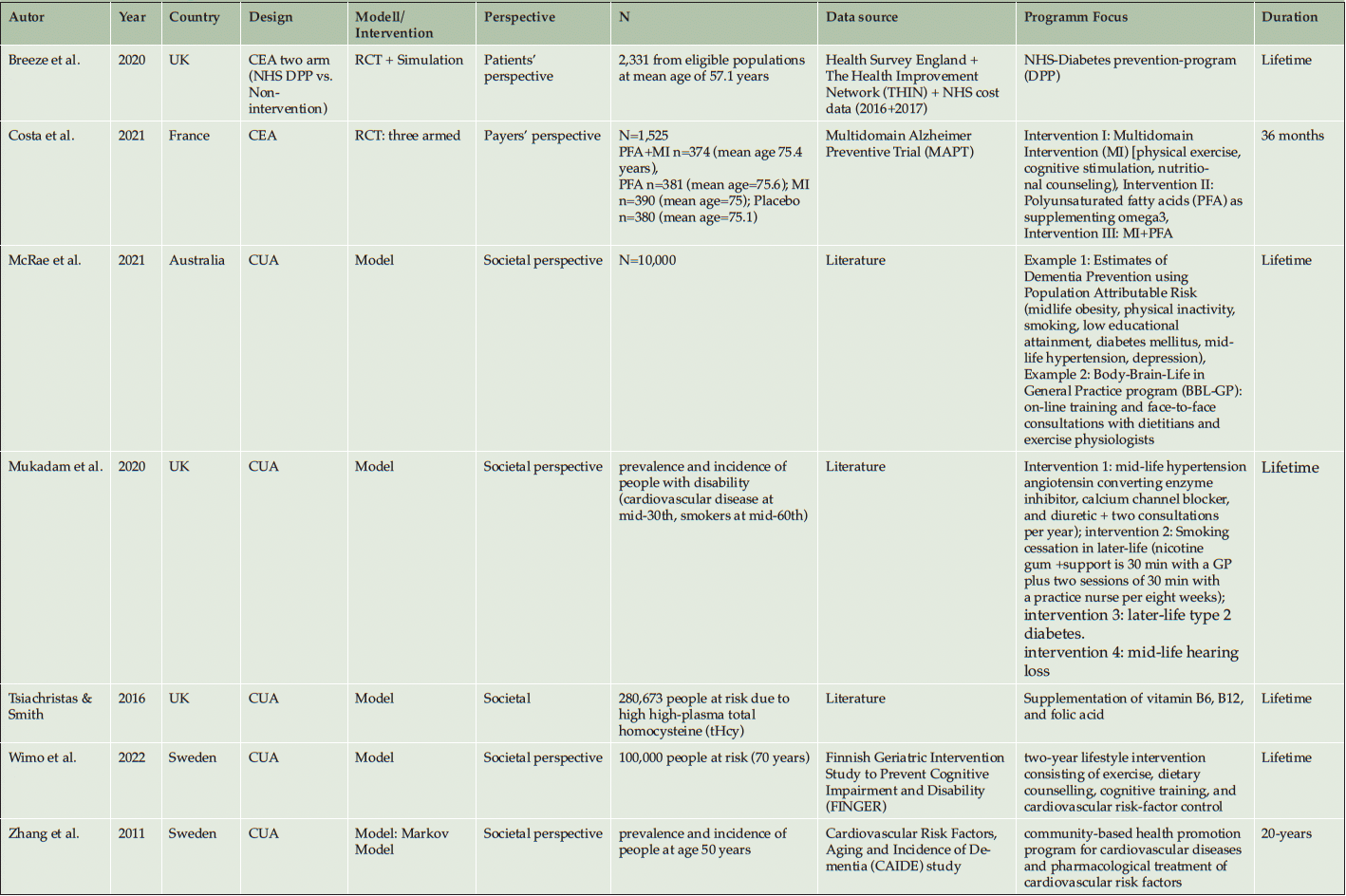
From the seven included studies, three studies were conducted in the United Kingdom (50, 53, 54), two in Sweden (40, 41), one in France (51), and one in Australia (52). Six studies used economic model simulation approaches (40, 41, 51–54). Two used information alongside a randomized controlled trial (RCT) (50, 51), while five studies inform their CEA with literature and secondary data from register and/or public health authorities (40, 41 ,52–54). The duration of the Randomized Controlled Trial was 36 months (51) inclusive of the follow-up. The second RCT study used model-based simulations to calculate the CUA for a lifetime (50). Nearly all model simulation uses the lifetime perspective (40, 50, 52–54), Zhang et al. (2011) simulated the CUA up to 20 years.
Sample and Population at risk
In the Literature, different methodologies to define ‘People at risk’ are applied. Firstly, there are differences in the Age definition, because nearly all studies include people at age around 60 or older, only the cardiovascular interventions in Mukadam et al. (2020) focused on people at the age of 35, and Zhang et al. (2011) focused on people above 50 years of age. Secondly, some studies defining the target population via a validated metric to define them reliable, but the definitions vary for Population Attributable Risks (PAR) or people at risk. Breeze et al. (2020), Wimo et al. (2022) and Zhang et al. (2011) had a clear inclusion of the population via the ‘Cardiovascular Risk factors, Aging and Dementia’ (CAIDE) Risk. Costa et al. (2021) included all persons with at least one contact with a dementia service center and a Mini-Mental-Score-Examination over 24. Breeze et al. (2020) defines risk factors within a population of diabetes patients from the NHS-database. McRae et al. (2020) use an approach of people at risk, defined via a set of known risk factors of dementia, e.g. midlife obesity, physical inactivity, smoking, low educational attainment, diabetes mellitus, midlife hypertension, and depression. Also, Mukadam et al. (2020) defines people at risk with other risk factors (body-mass index [BMI] ≥25 kg/m²; diabetes; hypertension; cardiovascular diseases, including stroke and heart diseases).
Type of interventions
Two studies applied a diabetes specific prevention program and looked at the effects of these programs on the reduced risk for dementia (50, 53). Two further studies investigate the reduction of cardiovascular risk factors and how these programs could reduce the risk of developing dementia (40, 41). All included studies, irrespective of their specific focus, use behavioral or food supplement prevention programs as their intervention focus (40, 41, 50–54).
Costing methodology
Costing methodology is focused on direct cost four studies using unit costs (40, 41, 51). Four studies used average cost from the programs (50, 52–54). One focused on the patient’s perspective (50), another on the payer’s perspective (51), and five focused on the societal perspective (40, 41, 52–54).
Outcome measures of the included trials
Six studies used the generic ‘European Quality of Life Index in five-dimensions’ (EQ-5D) from the EuroQol-Group for calculating QALYs (40, 41, 50, 52–54). Costa et al. (2021) used a dementia-specific index that combines four cognitive tests (51). These tests were (1) free and total recall of the Free and Cued Selective Reminding Test, (2) Minim-Mental-Score-Examination (MMSE) orientation items, (3) the Digit Symbol Substitution Test score from the Revised Wechsler Adult Intelligence Scale, and (4) the Category Naming Test. The outcomes were measured via their number of standard deviations above or below the mean (z-score). They defined a decrease of a z-score of 0.3 as clinically relevant.
Narrative synthesis of findings
Intervention Costs
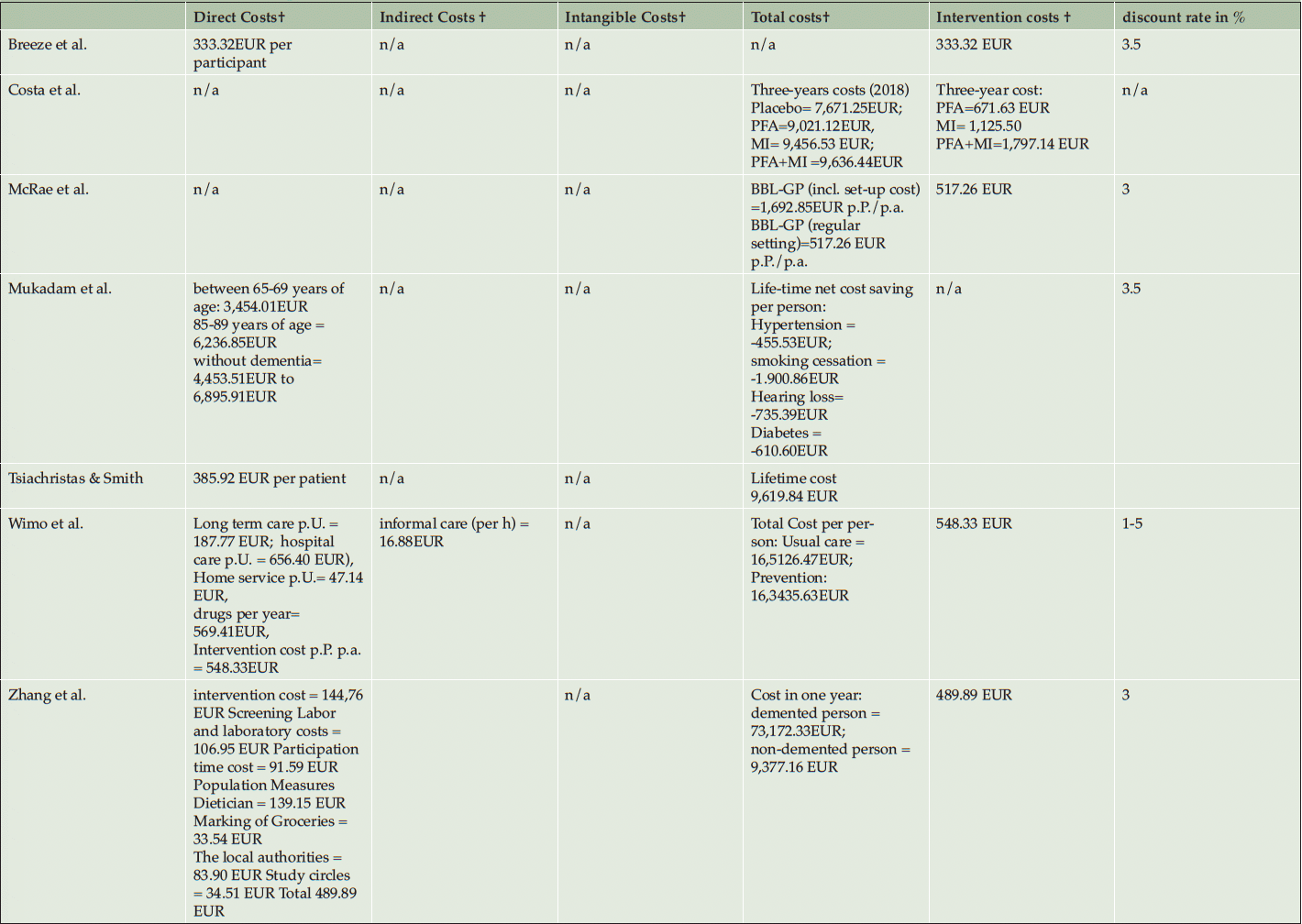
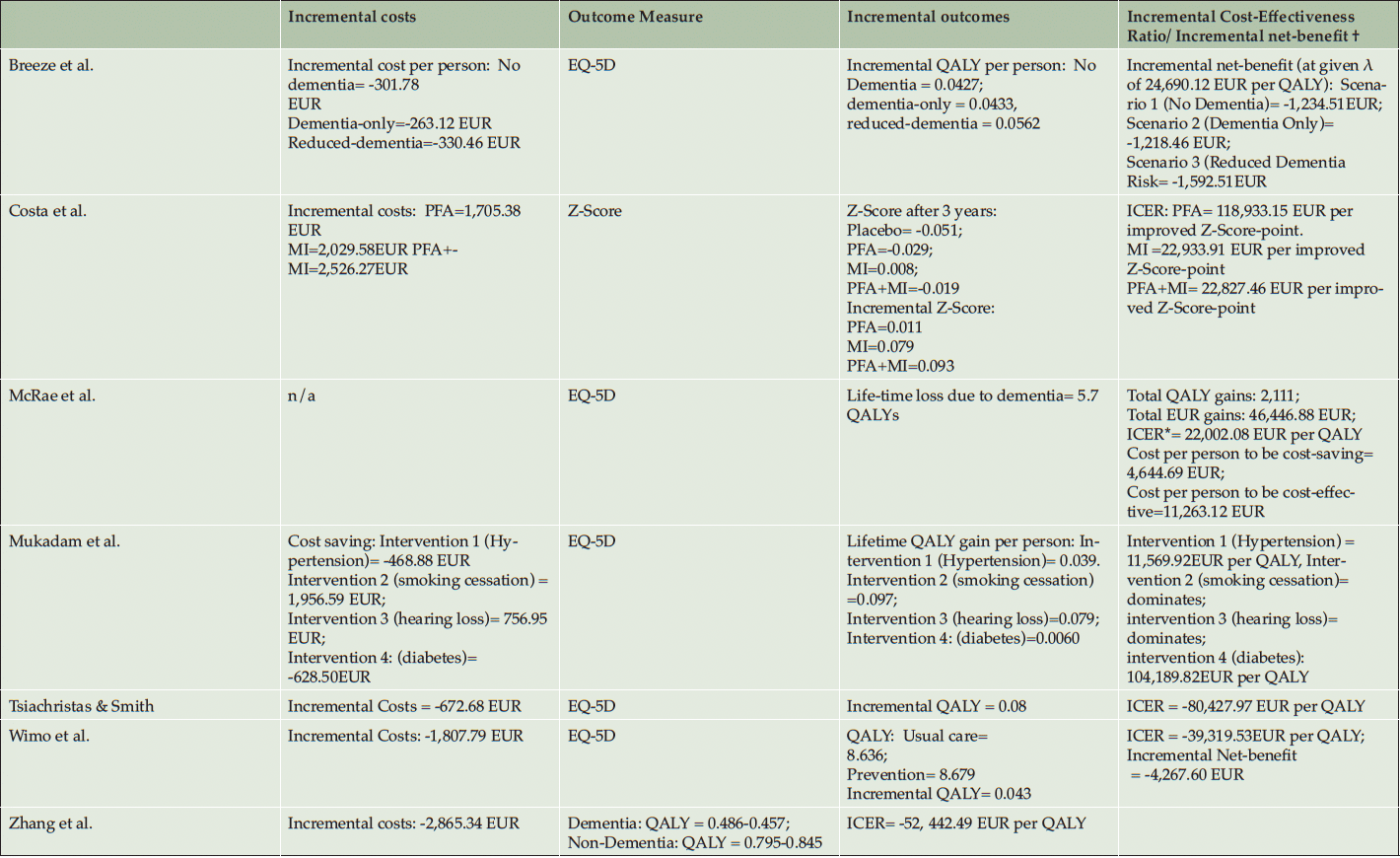
Table 3 shows the costing and the variation of direct, indirect, intangible, and total costs of the prevention interventions. Reporting of costs for the intervention are varying depending on the intensity of the interventions but the average intervention cost was 472,20 EUR per Person (SD=74.06 EUR) ranging from 333.32 EUR to 548.33 EUR (40, 41, 50, 52). The intervention cost of Costa et al. (2021) and Kato et al. (2022) were reported as total cost over the intervention period and could therefore not be included in the calculation of the average intervention costs. Mukadam et al. did not report intervention costs and compared instead the health expenditures for people with and without dementia (53).
*self-calculated ; † PPP to 2022 in EUR
Incremental QALYs and QALYs/gained
The QALYs gained per person due to prevention are 0.08 (SD=0.08) at mean, whereof Zhang et al. (2011) reports an incremental QALY of 0.388. Most studies report incremental QALY between 0.04 and 0.06, whereof Zhang et al. (2011) reports a significant outlier. Hence, the number of people at risk is quite high in the older age population, the total economic and societal impact of this QALY gain will increase in aging societies due to the aggregated QALYs/gained of avoided cases in increasing numbers of dementia (40, 56). Outcomes are found in table 4.
Incremental Cost-Effectiveness Ratios and Incremental Net-Benefit
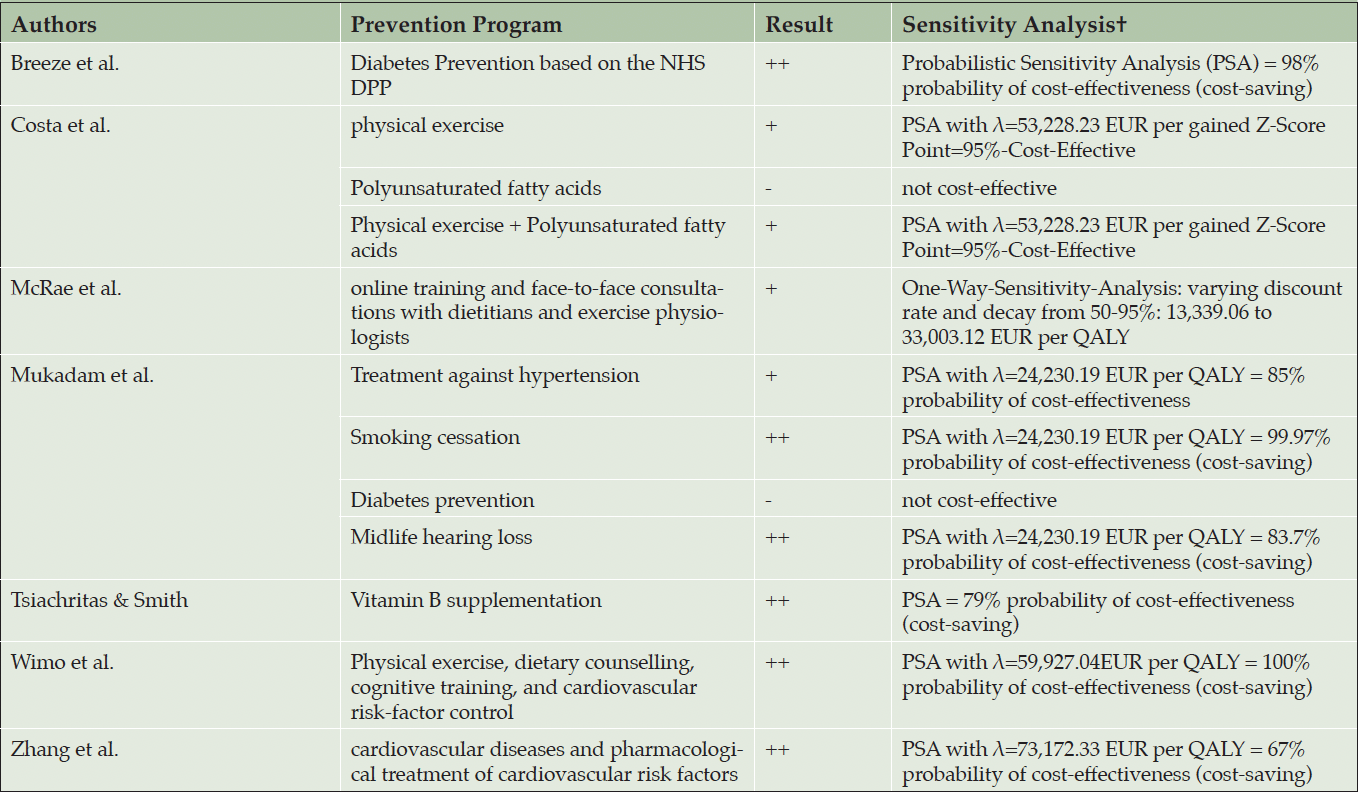
The ICERs/INB are shown in table 3. The reported ICERs vary between -80,427.97 EUR per QALY (54) and 104,189.82 EUR per QALY (53). This means that per QALY gained the intervention saves 80,427.97 EUR in the best-case scenario or cost 104,189.82 EUR in the worst-case scenario. Costa et al. calculated positive ICERs for physical exercise, polyunsaturated fatty acids, and for a combination of both interventions. Costa et al. (2021) use z-scores as effectiveness-measure, ICERs vary between 22,827.46 EUR per gained z-score-point for the combination preventions program and 118,833.15 EUR per gained z-score-point for the polyunsaturated fatty acids supplement strategy. Breeze et al. (2020) calculated INB based on a given λ of 24,690.12 EUR per QALY and showed cost-effectiveness in all dementia prevention strategies from -1,218.46 EUR to -1,592.51 EUR. Thereof, six out of twelve ICERs/INB showed cost-saving potentials of prevention programs. Most cost-saving potentials showed the multidomain interventions (40,41) and the supplementation vitamin B (54).
Sensitivity analyses
All included studies applied a sensitivity analysis, whereof eleven of twelve CEAs/CUAs use a probabilistic sensitivity analysis (PSA). Hence, the λ is varying in the assumption of the north-east quadrant and we want to give some insights towards the uncertainty of the studies. While the studies which identified ICERs at the cost-saving quadrant, nearly all ICER were plotted in the south-west quadrant (40, 41, 50, 53, 54). This implies that the estimations are quite robust. The estimated probabilities from the studies with an ICER in the north-east quadrant show different results. Diabetes intervention (53) and the polyunsaturated fatty acids (51) show robust non-cost-effective results, Kato et al. showed that based on a λ of 42,757.26 EUR per QALY the physical exercises may be cost-effective as well. Also, all multidomain interventions showed robust cost-effective results with an ICER under λ of 25,000 EUR per QALY (40, 41, 50–53).
† PPP to 2022 in EUR; ++ strong superior; + superior at WTP of 25,000 EUR per QALY; – inferior at WTP of 25,000 per QALY; – – strong inferior
Discussion
Complementary to Walsh et al. (2022) and the Cochrane Reviews (11, 57) we found that the (cost)effectiveness for physical interventions is given for people at risk but has inconclusive evidence for people with (pre)symptomatic dementia. Also, our results are in line with the broader study of Walsh et al. (2022) which found a cost-saving tendency by reducing the risk factors in a healthy population. Coming closer to prodromal or clinical relevant states of dementia, the interventions of reducing risk factors and behavioral lifestyle change became less (cost)effective (23, 58). While one paper of the review reveals that the more the risk comes to a prodromal state, the more cost-effective prevention strategies are, we cannot confirm this result with evidence from our systematic review (40, 58, 59). Compared to CEAs in interventions at prodromal state, the intervention for People at risk is clearly cost-effective, whereof studies at population with already mild cognitive impairment did not reach cost-effectiveness even at higher WTP-thresholds (23, 51–53).
Beside this, the program focus is also crucial. We could verify the assumptions of Rosenberg et al. (2020) that multimodal interventions are likely to be cost-saving, where single interventions tend to be cost-effective (60). Our studies showed that, the effectiveness of a prevention program drives the ICER more than the cost side. Here, our results are verifying Walsh et al. (2022), which also conclude a tendency of cost-effectiveness for physical exercise interventions but is also in favor for multidomain interventions. An explanation may be that targeting more than one risk factor may have synergizing effects (11). Likewise, Breeze et al. (2020) and Lin et al. (2014) this review showed that dementia prevention is cost-saving when the population is clearly defined as ‘people at risk’ with the aim to prevent from the prodromal state. Nonetheless, the definition of People at risk must be clearly specified in current economic studies.
This insight may be more relevant under the conditions of prevention at the prodromal state (screening), than under the risk-factor approach (prevention) (61). Here, this review is in line with the guideline of the National Institute for Health and Care Excellence (35) and the WHO position paper for brain health (10). This implies a core message for further economic evaluation of prevention of dementia: Focusing on primary prevention, the focus should be on ‘people at risk for developing dementia’ by using valid instruments like the CAIDE risk score.
From an economic perspective we also want to stress that the Willingness-to-pay threshold must be clarified as well, hence, we found that the assumptions of λ are also varying on a broad extend in different countries and may be higher for aged cohorts and higher severity of the disease (62). This makes the interpretation of ICERs in the north-east quadrant complicated, also because the stated λ for the US seems to be double from European conditions (47, 63).
Unfortunately, the studies address mostly aging populations and include people above 60 years of age. But we also stress that cost-effectiveness of interventions is related to the age of the target population (18). Nearly all included studies focus on people above 60 years of age. Specific towards younger cohorts Mukadam et al. (2020) and Breeze et al. (2020) showed that prevention at middle-age may be also have some high cost-saving potential. These results are in line with the econometric analysis of Baal et al. (2016) and the Cochrane Review (11) on the effectiveness of multidomain interventions. This may be crucial to focus either on the people at risk in aged populations or focus on the general reduction of risk factors in a healthy population at middle-age (11, 21, 57). A general prevention strategy over the entire life course seem not appropriate and a well-timed intervention or reduction of risk factors may be indicated for effective prevention programs (11). But some evidence is that for both populations, the cost-saving potential is at the primary prevention of dementia and not on mitigation of a prodromal state (64). Defining the target group with the best-evidence guidelines, e.g., NICE (2015), LANCET-Commission on Dementia prevention (12), or the WHO guideline (8), would help to increase the validity of the economic evidence.
Limitations
This review showed indeed some heterogeneity in focus of the intervention, which limits the possibility of reporting results in more detail. Hence, results of all programs showed a tendency for multimodal prevention programs. Based on the critical appraisal by Hafdi, et al. (2021) nearly all included studies have some performance bias due to missing blinding. Other insights for further health economic research for good CEA may be that the people at risk should be defined consistent via a standardized risk assessment (for example CAIDE). Some other limitation of the included studies is a strong overlapping in authors of the included studies. Even if this overlapping may consider a bias, the methodological differences, manly based of different data sources weaken the issue of reporting bias. Nonetheless, further systematic reviews should take this issue into account. Language restrictions in the screening process may also constraining the results, but from the current state of the art literature and using different databases, this seems to be some minor limitation of this review.
Conclusion
This review resulted in three main findings: (i) Prevention programs for preventing people at risk from developing dementia can be cost-effective and cost-saving, (ii) multimodal prevention interventions could be cost saving when the risk factors are defined alongside the evidence from WHO and LANCET, and (iii) there are some indications that life-style prevention in middle-age may be a good strategy for cost-effective prevention strategies. Therefore, further CEA specialized on prevention in middle-aged cohorts may be indicated. Like the Cochrane Review by Hafdi et al. (2021) conclude the right time for intervention seems to be essential for effectiveness of prevention and this was also found for cost-effectiveness in this review. Concluding cost-effectiveness and even cost-saving potential due to multidomain interventions for prevention of dementia must be supported by further research and high-quality economic evaluations alongside good designed multicenter RCTs. The results of this scoping review highlight the cost-saving potential of preventive dementia measures if they are targeting people at risk but not people who are already showing mild symptoms and if the target population is clearly defined and addressed. Consequently, preventive dementia measures seem to be a feasible way to reduce the burden of predicted increasing costs of dementia.
Ethical Approval: Not applicable.
Competing interests: The authors declare that they have no competing interests.
Authors’ contributions: AB, SA and MH developed the search strategy and the study design. AB was responsible for the health economic frame, SA, and MH for the dementia specific questions. Search process and inclusion were done equally by all three. Data extraction and calculations were done by AB and were cross checked by SA and MH. Writing the manuscript was undertaken by all three. All three authors were contributing substantially towards the interpretation of results. We want to thank Anne Busch and Siegfried Eisenberg for their friendly review of this manuscript.
Availability of data and materials: Data are available from the corresponding author on requested.
Funding: This work was supported by the research grants from the Lower Austrian government in form of a research-professorship. Open access funding provided by IMC University of Applied Sciences Krems
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Jönsson L. The personal economic burden of dementia in Europe. The Lancet Regional Health – Europe. 2022;20:100472. doi:10.1016/j.lanepe.2022.100472
2. Jönsson L, Tate A, Frisell O, Wimo A. The Costs of Dementia in Europe: An Updated Review and Meta-analysis. PharmacoEconomics. 2023;41(1):59-75. doi:10.1007/s40273-022-01212-z
3. Pedroza P, Miller-Petrie MK, Chen C, et al. Global and regional spending on dementia care from 2000–2019 and expected future health spending scenarios from 2020–2050: An economic modelling exercise. eClinicalMedicine. 2022;45:101337. doi:10.1016/j.eclinm.2022.101337
4. Wimo A, Seeher K, Cataldi R, et al. The worldwide costs of dementia in 2019. Alzheimer’s & Dementia. 2023;19(7):2865-2873. doi:10.1002/alz.12901
5. Liss JL, Seleri Assunção S, Cummings J, et al. Practical recommendations for timely, accurate diagnosis of symptomatic Alzheimer’s disease (MCI and dementia) in primary care: a review and synthesis. J Intern Med. 2021;290(2):310-334. doi:10.1111/joim.13244
6. Andrieu S, Coley N, Lovestone S, Aisen PS, Vellas B. Prevention of sporadic Alzheimer’s disease: lessons learned from clinical trials and future directions. The Lancet Neurology. 2015;14(9):926-944. doi:10.1016/S1474-4422(15)00153-2
7. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. The Lancet. 2015;385(9984):2255-2263. doi:10.1016/S0140-6736(15)60461-5
8. WHO. WHO Guidelines: Risk Reduction of Cognitive Decline and Dementia. WHO Press; 2019.
9. WHO. Global Status Report on the Public Response to Dementia. WHO Press; 2021.
10. WHO. Optimizing Brain Health across the Life Course: WHO Position Paper. WHO Press; 2022.
11. Hafdi M, Hoevenaar-Blom MP, Richard E. Multi-domain interventions for the prevention of dementia and cognitive decline. Cochrane Dementia and Cognitive Improvement Group, ed. Cochrane Database of Systematic Reviews. 2021;2021(11). doi:10.1002/14651858.CD013572.pub2
12. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396(10248):413-446. doi:10.1016/S0140-6736(20)30367-6
13. Sabbagh MN, Perez A, Holland TM, et al. Primary prevention recommendations to reduce the risk of cognitive decline. Alzheimer’s & Dementia. 2022;18(8):1569-1579. doi:10.1002/alz.12535
14. Auer S, Gamsjäger M, Donabauer Y, Span E. Stage specific retrogenetic training for persons with dementia: Importance of psychological features in the different disease stages. In: Schloffer H, Prang E, Frick A, eds. Memory Training: Theoretical and Practical Basics [Gedächtnistraining: Theoretische Und Praktische Grundlagen]. Springer; 2015:181-187.
15. Boada M, Rodrigo A, Jessen F, et al. Complementary pre-screening strategies to uncover hidden prodromal and mild Alzheimer’s disease: Results from the MOPEAD project. Alzheimer’s & Dementia. 2022;18(6):1119-1127. doi:10.1002/alz.12441
16. El-Hayek YH, Wiley RE, Khoury CP, et al. Tip of the Iceberg: Assessing the Global Socioeconomic Costs of Alzheimer’s Disease and Related Dementias and Strategic Implications for Stakeholders. J Alzheimers Dis. 2019;70(2):323-341. doi:10.3233/JAD-190426
17. Hampel H, Vergallo A, Iwatsubo T, et al. Evaluation of major national dementia policies and health-care system preparedness for early medical action and implementation. Alzheimer’s & Dementia. 2022;18(10):1993-2002. doi:10.1002/alz.12655
18. Lin PJ, Yang Z, Fillit HM, Cohen JT, Neumann PJ. Unintended Benefits: The Potential Economic Impact Of Addressing Risk Factors To Prevent Alzheimer’s Disease. Health Affairs. 2014;33(4):547-554. doi:10.1377/hlthaff.2013.1276
19. Nickel F, Barth J, Kolominsky-Rabas PL. Health economic evaluations of non-pharmacological interventions for persons with dementia and their informal caregivers: a systematic review. BMC Geriatr. 2018;18(1):69. doi:10.1186/s12877-018-0751-1
20. Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer’s disease: a new lexicon. The Lancet Neurology. 2010;9(11):1118-1127. doi:10.1016/S1474-4422(10)70223-4
21. Walsh S, Brain J, Mukadam N, et al. A systematic review of the cost-effectiveness of community and population interventions to reduce the modifiable risk factors for dementia. Maturitas. 2022;166:104-116. doi:10.1016/j.maturitas.2022.09.002
22. Braun A, Kurzmann P, Höfler M, Haber G, Auer S. Cost of care for persons with dementia: using a discrete-time Markov chain approach with administrative and clinical data from the dementia service Centres in Austria. Health Econ Rev. 2020;10(1):29. doi:10.1186/s13561-020-00285-w
23. Green C, Handels R, Gustavsson A, et al. Assessing cost-effectiveness of early intervention in Alzheimer’s disease: An open-source modeling framework. Alzheimer’s & Dementia. 2019;15(10):1309-1321. doi:10.1016/j.jalz.2019.05.004
24. Nguyen KH, Comans TA, Green C. Where are we at with model-based economic evaluations of interventions for dementia? a systematic review and quality assessment. Int Psychogeriatr. 2018;30(11):1593-1605. doi:10.1017/S1041610218001291
25. Solomon A, Mangialasche F, Richard E, et al. Advances in the prevention of Alzheimer’s disease and dementia. J Intern Med. 2014;275(3):229-250. doi:10.1111/joim.12178
26. Handels R, Wimo A. Health-economics of dementia prevention using modelling. In: Irving K, Hogervorst E, Oliveira D, Kivipelto M, eds. New Developments in Dementia Prevention Research. State of the Art and Future Possibilities. Routledge; 2020:170-180.
27. Sopina E, Sørensen J. Decision modelling of non-pharmacological interventions for individuals with dementia: a systematic review of methodologies. Health Econ Rev. 2018;8(1):8. doi:10.1186/s13561-018-0192-8
28. Leicht H, Heinrich S, Heider D, et al. Net costs of dementia by disease stage: Net costs of dementia by disease stage. Acta Psychiatrica Scandinavica. 2011;124(5):384-395. doi:10.1111/j.1600-0447.2011.01741.x
29. Spackman E, Kadiyala S, J. Neumann P, L. Veenstra D, D. Sullivan S. Measuring Alzheimer Disease Progression with Transition Probabilities: Estimates from NACC-UDS. CAR. 2012;9(9):1050-1058. doi:10.2174/156720512803569046
30. Deb A, Thornton JD, Sambamoorthi U, Innes K. Direct and indirect cost of managing alzheimer’s disease and related dementias in the United States. Expert Review of Pharmacoeconomics & Outcomes Research. 2017;17(2):189-202. doi:10.1080/14737167.2017.1313118
31. Knapp M, Iemmi V, Romeo R. Dementia care costs and outcomes: a systematic review: Costs and outcomes-review. Int J Geriatr Psychiatry. 2013;28(6):551-561. doi:10.1002/gps.3864
32. Duan Y, Lu L, Chen J, et al. Psychosocial interventions for Alzheimer’s disease cognitive symptoms: a Bayesian network meta-analysis. BMC Geriatr. 2018;18(1):175. doi:10.1186/s12877-018-0864-6
33. Peña-Longobardo LM, Rodríguez-Sánchez B, Oliva-Moreno J, Aranda-Reneo I, López-Bastida J. How relevant are social costs in economic evaluations? The case of Alzheimer’s disease. Eur J Health Econ. 2019;20(8):1207-1236. doi:10.1007/s10198-019-01087-6
34. Schneider U, Kleindienst J. Monetising the provision of informal long-term care by elderly people: estimates for European out-of-home caregivers based on the well-being valuation method. Health Soc Care Community. 2016;24(5):e81-e91. doi:10.1111/hsc.12250
35. NICE. Dementia, disability and frailty in later life – mid-life approaches to delay or prevent onset. Published online 2015.
36. Mangialasche F, Kivipelto M, Solomon A, Fratiglioni L. Dementia prevention: current epidemiological evidence and future perspective. Alzheimers Res Ther. 2012;4(1):6. doi:10.1186/alzrt104
37. Kivipelto M, Solomon A, Ahtiluoto S, et al. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): Study design and progress. Alzheimer’s & Dementia. 2013;9(6):657-665. doi:10.1016/j.jalz.2012.09.012
38. Baal PHM van, Hoogendoorn M, Fischer A. Preventing dementia by promoting physical activity and the long-term impact on health and social care expenditures. Preventive Medicine. 2016;85:78-83. doi:10.1016/j.ypmed.2016.01.013
39. Handels R, Wimo A. Challenges and recommendations for the health-economic evaluation of primary prevention programmes for dementia. Aging & Mental Health. 2019;23(1):53-59. doi:10.1080/13607863.2017.1390730
40. Wimo A, Handels R, Antikainen R, et al. Dementia prevention: The potential long-term cost-effectiveness of the FINGER prevention program. Alzheimer’s & Dementia. Published online July 16, 2022:alz.12698. doi:10.1002/alz.12698
41. Zhang Y, Kivipelto M, Solomon A, Wimo A. Cost-Effectiveness of a Health Intervention Program with Risk Reductions for Getting Demented: Results of a Markov Model in a Swedish/Finnish Setting. JAD. 2011;26(4):735-744. doi:10.3233/JAD-2011-110065
42. Hall J. Disease Prevention, Health Care, and Economics. In: Glied S, Smith PC, eds. The Oxford Handbook of Health Economics. Oxford University Press; 2011:555-577.
43. Munn Z, Pollock D, Khalil H, et al. What are scoping reviews? Providing a formal definition of scoping reviews as a type of evidence synthesis. JBI Evidence Synthesis. 2022;20(4):950-952. doi:10.11124/JBIES-21-00483
44. Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. International Journal of Evidence-Based Healthcare. 2015;13(3):141-146. doi:10.1097/XEB.0000000000000050
45. Tricco AC, Lillie E, Zarin W, et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol. 2016;16(1):15. doi:10.1186/s12874-016-0116-4
46. Wallace BC, Small K, Brodley CE, Lau J, Trikalinos TA. Deploying an interactive machine learning system in an evidence-based practice center: abstrackr. In: Proceedings of the 2nd ACM SIGHIT Symposium on International Health Informatics – IHI ’12. ACM Press; 2012:819. doi:10.1145/2110363.2110464
47. McDougall JA, Furnback WE, Wang BCM, Mahlich J. Understanding the global measurement of willingness to pay in health. Journal of Market Access & Health Policy. 2020;8(1):1717030. doi:10.1080/20016689.2020.1717030
48. Black WC. The CE Plane: A Graphic Representation of Cost-Effectiveness. Med Decis Making. 1990;10(3):212-214. doi:10.1177/0272989X9001000308
49. Briggs AH, Claxton K, Sculpher MJ. Decision Modelling for Health Economic Evaluation. Oxford University Press; 2006.
50. Breeze P, Thomas C, Thokala P, Lafortune L, Brayne C, Brennan A. The Impact of Including Costs and Outcomes of Dementia in a Health Economic Model to Evaluate Lifestyle Interventions to Prevent Diabetes and Cardiovascular Disease. Med Decis Making. 2020;40(7):912-923. doi:10.1177/0272989X20946758
51. Costa N, Mounié M, Pagès A, et al. The Cost-Effectiveness of Three Prevention Strategies in Alzheimer’s Disease: Results from the Multidomain Alzheimer Preventive Trial (MAPT). J Prev Alz Dis. Published online 2021:1-11. doi:10.14283/jpad.2021.47
52. McRae I, Zheng L, Bourke S, Cherbuin N, Anstey KJ. Cost-Effectiveness of Dementia Prevention Interventions. J Prev Alz Dis. Published online 2020:1-8. doi:10.14283/jpad.2020.71
53. Mukadam N, Anderson R, Knapp M, et al. Effective interventions for potentially modifiable risk factors for late-onset dementia: a costs and cost-effectiveness modelling study. The Lancet Healthy Longevity. 2020;1(1):e13-e20. doi:10.1016/S2666-7568(20)30004-0
54. Tsiachristas A, Smith AD. B-vitamins are potentially a cost-effective population health strategy to tackle dementia: Too good to be true? Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2016;2(3):156-161. doi:10.1016/j.trci.2016.07.002
55. Eggink E, Hafdi M, Hoevenaar-Blom MP, et al. Prevention of dementia using mobile phone applications (PRODEMOS): protocol for an international randomised controlled trial. BMJ Open. 2021;11(6):e049762. doi:10.1136/bmjopen-2021-049762
56. Zarate JM. Identifying people at risk for Alzheimer’s disease. Nat Neurosci. 2021;24(11):1503-1503. doi:10.1038/s41593-021-00953-y
57. Forbes D, Forbes SC, Blake CM, Thiessen EJ, Forbes S. Exercise programs for people with dementia. Cochrane Dementia and Cognitive Improvement Group, ed. Cochrane Database of Systematic Reviews. 2015;2015(4). doi:10.1002/14651858.CD006489.pub4
58. Kato G, Doi T, Arai H, Shimada H. Cost-effectiveness Analysis of Combined Physical and Cognitive Exercises Programs Designed for Preventing Dementia among Community-dwelling Healthy Young-old Adults. Phys Ther Res. 2022;25(2):56-67. doi:10.1298/ptr.E10153
59. Davis JC, Hsiung GYR, Liu-Ambrose T. Challenges moving forward with economic evaluations of exercise intervention strategies aimed at combating cognitive impairment and dementia. Br J Sports Med. 2011;45(6):470-472. doi:10.1136/bjsm.2010.077990
60. Rosenberg A, Mangialasche F, Ngandu T, Solomon A, Kivipelto M. Multidomain Interventions to prevent cognitive impairment, Alzheimer’s disease: From Finger to WORLD-WIDE Fingers. J Prev Alz Dis. Published online 2020:1-8. doi:10.14283/jpad.2019.41
61. Saito M, Shimazaki Y, Nonoyama T, Ohsugi K. Association Between Oral Health and the Medical Costs of Dementia: A Longitudinal Study of Older Japanese. Am J Alzheimers Dis Other Demen. 2021;36:1533317521996142. doi:10.1177/1533317521996142
62. Phelps CE, Lakdawalla DN. Methods to Adjust Willingness-to-Pay Measures for Severity of Illness. Value in Health. 2023;26(7):1003-1010. doi:10.1016/j.jval.2023.02.001
63. Neumann PJ, Cohen JT, Weinstein MC. Updating Cost-Effectiveness — The Curious Resilience of the $50,000-per-QALY Threshold. N Engl J Med. 2014;371(9):796-797. doi:10.1056/NEJMp1405158
64. Solomon A, Ngandu T, Kivipelto M. From prediction to dementia prevention. In: Irving K, Hogervorst E, Oliveira D, Kivipelto M, eds. New Developments in Dementia Prevention Research. State of the Art and Future Possibilities. Routledge; 2019:13-26.
© The Authors 2024