S.A. Lott1, E. Streel2, S.L. Bachman3, K. Bode4, J. Dyer5, C. Fitzer-Attas6, J.C. Goldsack1, A. Hake7, A. Jannati8, R.S. Fuertes9, P. Fromy1
1. Digital Medicine Society (DiMe), Boston, MA, USA; 2. Altoida, Washington DC, USA; 3. VivoSense, Inc., Newport Coast, CA, USA; 4. Merck, Rahway, PA, USA; 5. Cumulus Neuroscience, Belfast, UK; 6. Cogniant Pte Ltd, Singapore and ClinMed LLC, Dayton, NJ, USA; 7. Eli Lilly and Company, Indianapolis, IN, USA; 8. Linus Health, Inc., Boston, MA, USA; Department of Neurology, Harvard Medical School, Boston, MA, USA; 9. Global Alzheimer’s Disease Office, Eisai, Spain
Corresponding Author: Sarah Averill Lott, Digital Medicine Society (DiMe), Boston, MA, USA, sarah.lott@dimesociety.org, 970-408-0780
J Prev Alz Dis 2024;5(11):1480-1489
Published online June 6, 2024, http://dx.doi.org/10.14283/jpad.2024.103
Abstract
Digital health technologies offer valuable advantages to dementia researchers and clinicians as screening tools, diagnostic aids, and monitoring instruments. To support the use and advancement of these resources, a comprehensive overview of the current technological landscape is essential. A multi-stakeholder working group, convened by the Digital Medicine Society (DiMe), conducted a landscape review to identify digital health technologies for Alzheimer’s disease and related dementia populations. We searched studies indexed in PubMed, Embase, and APA PsycInfo to identify manuscripts published between May 2003 to May 2023 reporting analytical validation, clinical validation, or usability/feasibility results for relevant digital health technologies. Additional technologies were identified through community outreach. We collated peer-reviewed manuscripts, poster presentations, or regulatory documents for 106 different technologies for Alzheimer’s disease and related dementia assessment covering diverse populations such as Lewy Body, vascular dementias, frontotemporal dementias, and all severities of Alzheimer’s disease. Wearable sensors represent 32% of included technologies, non-wearables 61%, and technologies with components of both account for the remaining 7%. Neurocognition is the most prevalent concept of interest, followed by physical activity and sleep. Clinical validation is reported in 69% of evidence, analytical validation in 34%, and usability/feasibility in 20% (not mutually exclusive). These findings provide clinicians and researchers a landscape overview describing the range of technologies for assessing Alzheimer’s disease and related dementias. A living library of technologies is presented for the clinical and research communities which will keep findings up-to-date as the field develops.
Key words: Digital health technologies, Alzheimer’s disease, assessment, digital medicine.
Abbreviations: AD: Alzheimer’s disease; ADRD: Alzheimer’s disease and related dementias; DATAcc: Digital Health Measurement Collaborative Community; DHT: Digital health technology.
Introduction
The uses for digital health technologies (DHTs) for clinical research, personalized medicine, and general wellness are well established and continue to expand (1, 2). In the field of Alzheimer’s disease and related dementias (ADRD), growth in the development and use of DHTs (3) is occurring alongside increased research and development of drugs for the prevention and treatment of Alzheimer’s disease (AD) and other dementia-inducing disorders including Lewy Body and frontotemporal dementias(4–6). The recent regulatory approval of some therapeutic agents targeting AD pathophysiology highlights the complexity of dementia management, as new treatment opportunities emerge alongside ongoing challenges in dementia research, clinical management, and holistic patient care (7). These challenges include identifying digital biomarkers that signal changes early in the disease course when intervention is more likely to provide a substantial impact; assessing clinical manifestations of dementia such as activities of daily living (ADLs), cognitive function, physical activity, and sleep; evaluating and demonstrating response to therapeutic interventions (8); and improving recruitment (including recruitment of diverse patient groups (9), retention, and assessment of participants in clinical trials). In addressing these challenges, clinicians and researchers will benefit from utilizing DHTs as fit for purpose tools that can play a strategic role in screening, monitoring, and longitudinal assessment in research, clinical, and real world settings.
Members of a global, multi-stakeholder working group convened by the Digital Medicine Society (DiMe) (10) met over several months to assess the characteristics of DHTs in ADRD populations, recognizing the immense opportunity for further research and clinical impact alongside the ever-increasing burden of Alzheimer’s disease and related dementias (11, 12). DiMe, a global non-profit dedicated to driving scientific progress and broad acceptance in digital medicine to redefine healthcare and improve lives, hosts the Digital Health Measurement Collaborative Community (DATAcc)(13), a U.S. Food and Drug Administration (FDA) Center for Diagnostic and Radiological Health (CDRH) collaborative community (14), and convenes a wide range of stakeholders and subject matter experts in pre-competitive forums to advance the field.
While others have examined the evidence for ADRD-relevant DHTs (15, 16), such efforts are often limited to a subset of available technologies or therapeutic domains, obscuring substantial portions of the DHT landscape and restricting opportunities for translation of the benefits of these tools across different dementias. To obtain a more comprehensive view, our ADRD working group aggregated multiple technologies across ADRD-therapeutic areas by surveying 20 years of peer-reviewed literature and then sourcing additional evidence from DHT users and developers. While we were primarily informed by the literature and had strict inclusion and exclusion criteria, our purpose in this exercise was to identify and collect digital health technologies in use for dementia measurement, not to interrogate the literature for quality or bias as one would expect in a traditional systematic literature review. Rather, our broad landscape assessment was literature-based and community supported, as the DiMe working group (comprised of ADRD experts from clinical, pharmaceutical, industry research and development, and patient advocacy domains; see acknowledgments for complete list of participating organizations) were given an opportunity to review the findings and identify additional technologies for consideration.
The results of this innovative effort are reported here, bringing an open science perspective and outlining the state of digital innovation in ADRD research. As a precompetitive group representing a range of interests, we do not promote any specific technology type, product, or developer in this analysis and therefore refrain from providing direct product examples in-text; a list of all sources that inform this overview is provided in the supplement so that interested readers may seek specific examples if desired. Here we provide a general overview advancing awareness of the range of technologies and their uses for dementia assessment.
Methods
Data Sources
The working group sought to identify DHTs with associated evidence of validity (17) and therefore started by turning to the peer-reviewed literature. In May 2023, we conducted a landscape review to identify DHTs being used for assessment in ADRD populations by the clinical and research communities. The search strings (see supplementary material) included terminology to identify DHTs, diagnostic and descriptive terms to focus on ADRD-relevant populations, and pertinent concepts of interest measured by a DHT including sleep health, memory, language, social function, oculomotor function, cognition, life space mobility, essential bodily functioning, and emotional or behavioral concerns. The selection of these concepts of interest was informed by literature focused on meaningful aspects of health(8, 18, 19) for ADRD stakeholders at large. We restricted search results to primary research studies with human participants published within the last 20 years. To avoid over-representation of any given technology brand, we intentionally did not include any name-brand affiliated keywords in the search terms, knowing this might mean relevant studies were omitted from the results if they included only brand identifiers for utilized technologies. The search string was performed within PubMed, APA PsycInfo, and Embase literature databases, and the results were then aggregated and deduplicated within reference management software Zotero (20).
Literature Review Screening
Identified articles were screened by two reviewers (SAL, PF), with a random sample of 5% co-reviewed to ensure harmony on inclusion/exclusion standards. Articles were included or excluded based on the criteria outlined in Table 1.
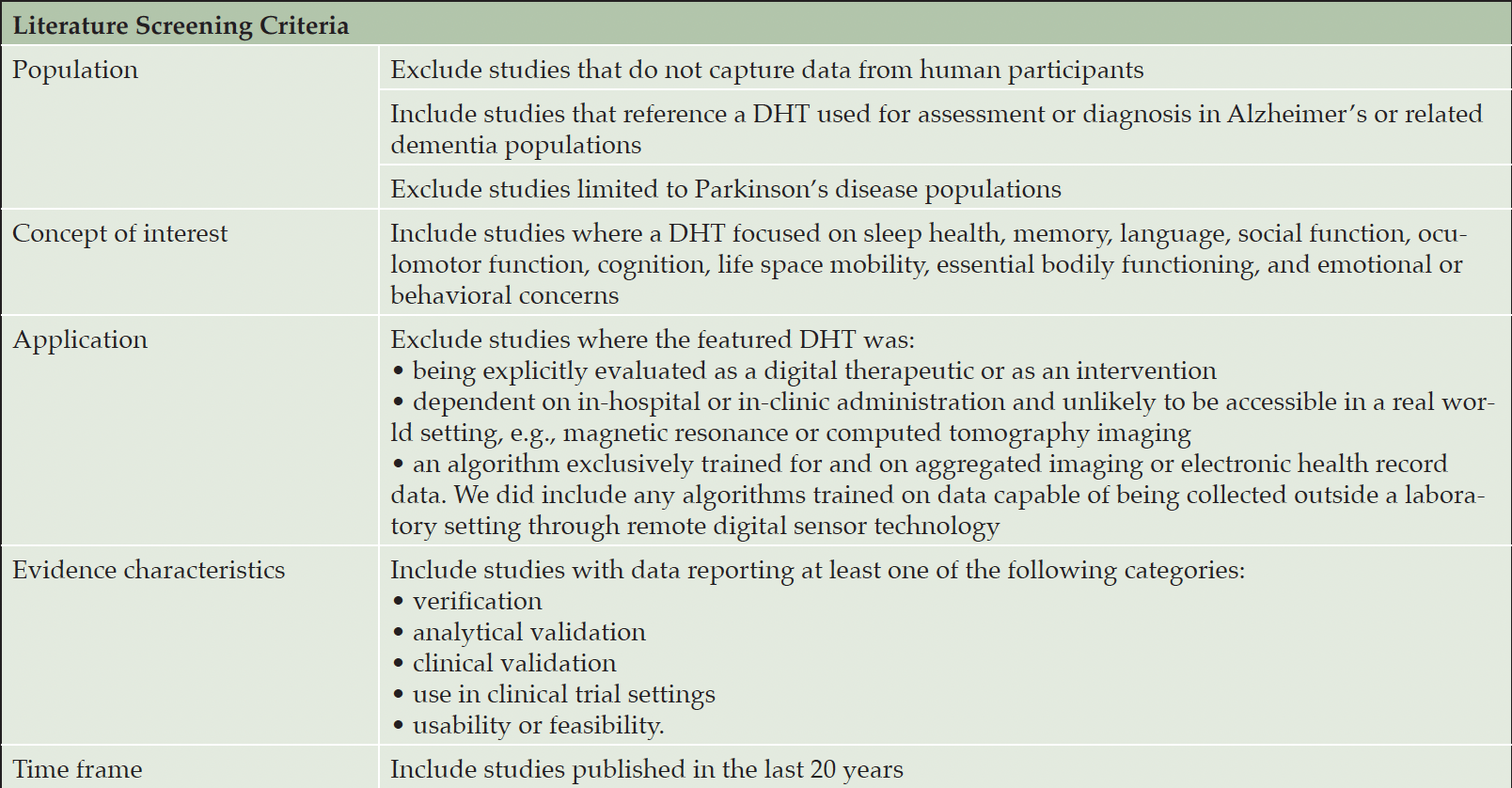
Table 1. Inclusion/exclusion criteria applied to papers identified in literature search or returned by survey
Extraction
From each qualifying manuscript, we extracted the name and manufacturer of the technology being utilized; the technology type, form factor, and wear location; the reported therapeutic category and population descriptors as described verbatim by study authors; and the general health concepts and outcomes under assessment. Categories used elsewhere in assessing and categorizing DHTs informed our classification labels (21, 22) for technology types and health outcomes. Technology types were sorted into ambient (standalone, not worn on the body), wearable (worn on the body for any period of time), implantable (surgically placed), or ingestible (swallowed); while form factor descriptions were as provided by study authors in the text. Broad health outcome categories were sorted into activities of daily living, mental health, neurocognitive, neurological or sensory, physical activity, and sleep. More specific health outcome category labels were derived from study descriptors and are also reported. Lastly, we labeled evidence according to the type of data it reported: verification, analytical validation, clinical validation, and usability or feasibility (17). When extracting these evidence types and other characteristics such as diagnostic classifications, study populations descriptors, or outcome measures, we adhered to author/publication verbiage as closely as possible with the goal of collating rather than interpreting labels or assessing individual pieces of evidence as to their rigor or performance.
Community Sourcing
ADRD subject matter experts in DHT development, clinical trials, and clinical care from the DiMe ADRD project team (10) reviewed and added to the list of technologies created during extraction, to identify additional technologies not captured in our literature search. Developers of identified DHTs were contacted by email (when contact information was accessible) and provided a survey via a Qualtrics (23) link where they could return peer reviewed articles, conference posters or presentations, regulatory documents, datasets, open-source algorithms, or other supporting materials for their technologies in line with the V3 framework (17). If a developer responded and provided any evidence, that data was assessed against the same inclusion and exclusion standards for the literature presented in Table 1 and included in our results if eligible. Finally, the survey link and a description of the library were shared on LinkedIn during World Alzheimer’s Month in an effort to solicit evidence for ADRD DHTs from the broader scientific and digital health communities (September, 2023).
Results
The combined literature search resulted in an initial dataset of 1,743 manuscripts; after screening we identified 153 eligible articles related to 117 unique DHTs. We reviewed and omitted 13 technologies that were clearly obsolete and discontinued, bringing the results from the literature to 102 DHTs. Project partners reviewed the complete list and made us aware of an additional 57 technologies, resulting in a potential total of 159 unique DHTs for ADRD assessment. Surveys were disseminated via email to technology developers of the additional technologies to request evidence so their technologies could be considered for inclusion; as of this writing, 42 survey submissions featuring 17 technologies were returned. Survey responses included peer-reviewed publications, conference poster and oral presentation materials, and documentation of regulatory approvals. After assessing all returned materials for eligibility as outlined in Table 1, our total results included 172 pieces of evidence representing 106 unique DHTs. A list of the results of eligible manuscripts/evidence and the respective technologies therein is available in the supplementary material.
Populations Represented
Our results captured a wide variety of ADRD population descriptors as reported by study authors or DHT developers (Table 2). The classifications most represented include Mild Cognitive Impairment (MCI) in 39% of evidence records and 49% of technologies, followed by the general classification of Alzheimer’s disease indicated in 31% of evidence records and 40% of technologies.
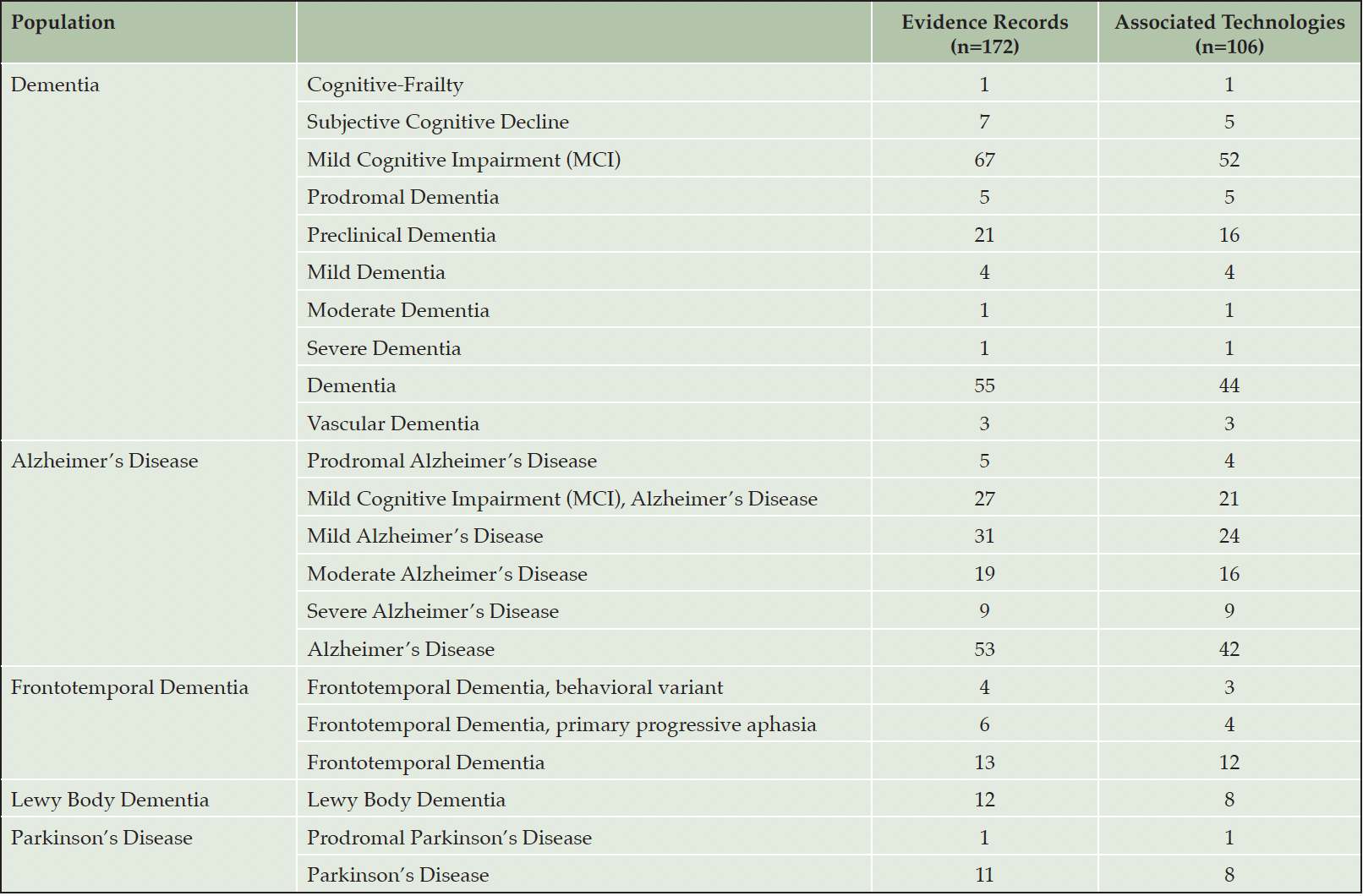
Table 2. Frequencies of population areas of focus, as reported in manuscripts. Many pieces of evidence reference multiple diagnostic classifications
Technology Types, Form Factors, and Health Concepts
Ambient (or non-wearable sensor technologies) are the most prevalent technology type, representing 61% of included DHTs and 68% of the evidence (Table 3). This category includes environmental or stand alone sensors as well as software and application-based technologies which are not tied to a given device. 83% of ambient technologies fall into the software and application category. Wearable sensor devices represent 32% of DHTs and 26% of evidence. Seven percent of technologies incorporate both wearable and ambient technologies. The most common form factors are software and applications designed for a tablet or smartphone (42% DHTs; 45% of evidence) and sensors worn on the body but not as a smartwatch (e.g. attached via a strap or brace) (19% DHTs; 16% of evidence). Cameras and contactless sensors are also strongly represented in the ambient category (collectively 13% DHTs; 14% evidence), while smartwatches represent a smaller portion of the results (8% DHTs; 5% evidence). Most studies feature commercially available DHTs (89% of evidence) though we included experimental/noncommercial DHTs if relevant to our population and concepts of interest (11% evidence). Our results also returned examples of “smart home” applications with a combination of different technology types designed to passively monitor life space mobility such as cameras, movement detecting radar, or pressure mats.
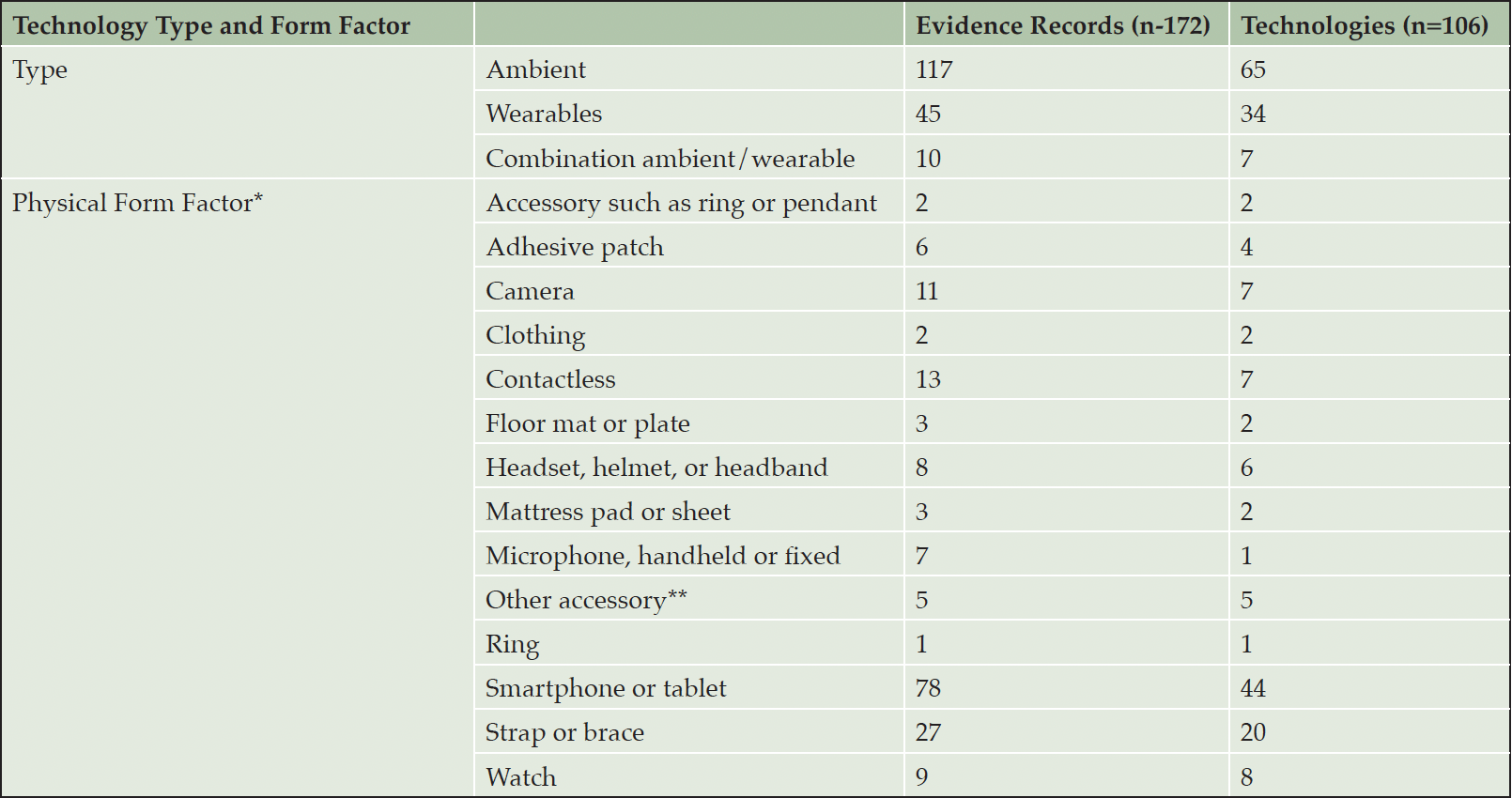
Table 3. Prevalent technology types and form factors. Ambient includes environmental and software or application-based technologies
* not mutually exclusive **digitized pen, wearable pendant
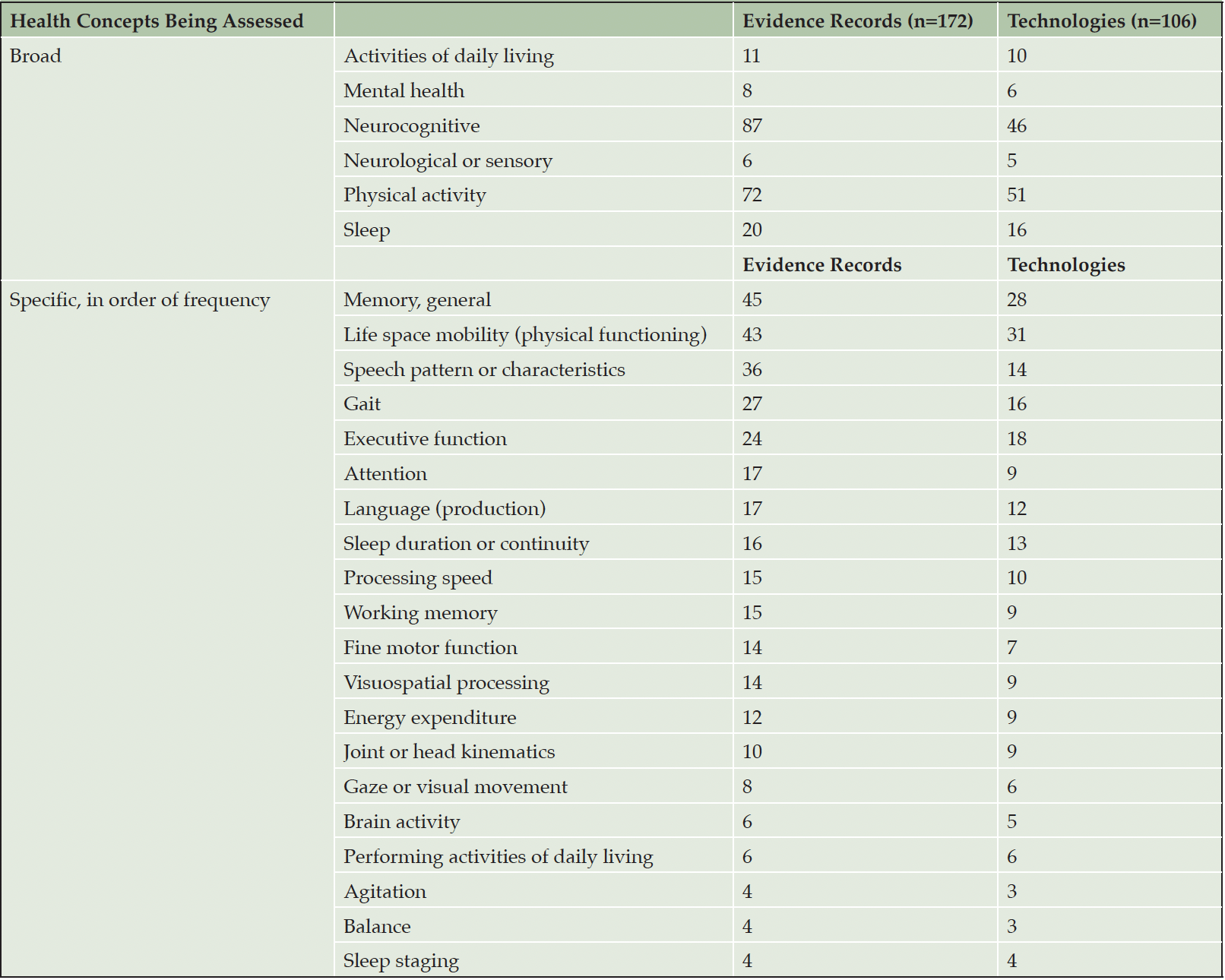
DHTs are classified into the health concepts of interest they assess, including but not limited to activities of daily living; mental health, neurocognitive function, neurological or sensory function, physical activity, and sleep (Table 4). The most frequent concept of interest is neurocognitive function with 43% of technologies and 51% of the evidence, but physical activity represents a larger portion of DHTs (48%) and is addressed in 42% of evidence. Measured outcomes as reported in included studies were analyzed to categorize the evidence into more specific health concepts. Memory is the concept of interest addressed most often as a primary focus of 26% of DHTs and 26% of evidence, but life space mobility, speech patterns and characteristics, gait and mobility, sleep, and several subdomains of memory and cognitive function are also well represented (Table 4).
V3, other evidence standards in action
The V3 framework (17) codifies best practices for evaluating verification, analytical validation, and clinical validation to determine whether DHTs are fit for purpose. Briefly, these processes evaluate whether sensor-based technologies and their algorithm(s) measure and interpret what they intend to measure, and provide metrics that are clinically or functionally meaningful in the stated context of use (17). To reflect these standards, we categorized evidence to indicate when it reports verification, analytical validation, clinical validation, and/or usability. Most of the current evidence reports validation: 34% of the evidence reports analytical validation while a majority (69%) reports evidence for clinical validation within ADRD populations. 19% percent of the evidence reports demonstrated usability or feasibility within ADRD populations. Just three manuscripts reported verification. These categories are not mutually exclusive; a single manuscript might report on multiple aspects.
Discussion
To the best of our knowledge, this review is the first to broadly document the growing landscape of DHTs available for assessment within the ADRD space. DHTs focused on cognition and memory are unsurprisingly prevalent in our results. However, we identified several other concepts of interest in the literature, underscoring the versatility DHTs offer for holistic assessment of ADRD populations. When examining technology types, mobile or software applications are prevalent and their broad scope is notable. We find these technologies are being used by researchers and clinicians to assess multiple aspects of neurocognition (including several types of memory, attention, executive functioning, and processing speed); speech production and language characteristics; fine motor functioning; oculomotor behavior; sleep; gait, mobility, or balance; life space mobility dynamics; and emotional or other behavioral patterns. Given the ubiquity of smartphones and web-based options for assessment, these findings indicate a large capacity for sensitive and ongoing patient assessment or monitoring in clinical and real world settings, including the critical opportunity to reach patients not previously accessible to research or therapeutic settings. A majority (89%) of our results feature commercially available DHTs, emphasizing that a range of technological solutions are available and in use by clinicians and researchers even as additional development and innovation continues.
Biomarkers, early detection opportunities, and digital phenotyping
Our results indicate researchers are leveraging DHTs to identify digital biomarkers and behavioral changes that can provide early indicators of functional and cognitive changes in both clinical and remote settings. Examples include but are not limited to wearable headbands for in-home EEG assessment to monitor sleep, sophisticated analysis of speech patterns and vocal dynamics as captured by smartphone speakers, cameras tracking patterns in narrow applications (gaze/eye movement) as well as broad (behavioral) patterns in the home; and inertial sensors worn on the lower back, chest, arm, or leg to monitor mobility patterns. These technologies detect subtle functional and behavioral differences that can differentiate patients from healthy controls and discriminate between disease stages. When tablet or smartphone apps, wearables, and other DHTs are used in aggregate, the results can be quite illustrative, providing a “digital phenotype” (24) that captures a comprehensive in-situ portrait of someone’s daily functional and cognitive status, providing a better window into a patient’s emotional state, social patterns, and physical experiences to generate research insights and guide clinical care in ways that are meaningful to patients (25). “Smart home” applications show promise in this regard as well, with personalized and multimodal monitoring that might include cameras that track life space mobility paired with bed pressure mats to detect sleep patterns, restlessness, or agitation. When these technologies detect signals such as behavioral and activity changes, a digital phenotype can be established. Such setups are currently utilized in a variety of ways including discriminating between disease states (26), detecting agitation or apathy (27), unobtrusively monitoring response to therapeutics in care settings (28), and improving the selection of candidates for clinical research purposes (29).
Clinical research
The importance for understanding the landscape of ADRD-based DHTs is apparent when considering clinical research. Broadly, DHTs can improve trials by (a) reducing within-participant measurement noise (30), ultimately leading to improved statistical power, smaller trials (29), and faster paths to regulatory judgment of new therapies and (b) decentralizing assessments, enabling the participation of individuals located in more diverse geographical areas and therefore acquiring a more representative and diverse sample of the population under study (9). (For explication of this approach, see Sliwinski, 2008) (31). Although there is a recent example elsewhere in clinical research using a DHT as a primary clinical endpoint (32), this is not current practice in the ADRD space. However, the growing acceptance of DHTs as secondary endpoints in ADRD research (33, 34) is slowly moving the field towards this possibility (35, 36). Studies employ DHTs for monitoring response to treatment in a variety of ways, such as using actigraphy to discern impacts of Mevidalen on activity and sleep within Lewy Body dementia cohorts (37, 38) or using smartwatches to detect suvorexant-induced changes in sleeping patterns for persons with Alzheimer’s experiencing insomnia (39).
Digital neuropsychology
The dominance of technologies assessing memory (short term, long term, working), executive function, attention, processing speed, verbal fluency, and visuospatial processing document an increasingly digital shift within neuropsychological assessment. Clinicians see value in the opportunity to assess functional status in real world settings (40) even as they grapple with concerns around ecological validity (25, 41) due to variations in technology, computer literacy, environmental factors, and the presence or lack of supervision by a trained observer (41, 42). While further research is needed to assuage these concerns, data continues to emerge illustrating that innovative digital assessment modalities can successfully discriminate between healthy controls and individuals in early stages of Alzheimer’s disease (43). Such DHTs provide clear advantages with regard to scalability and increasing access to patients for whom an in-person assessment may not be feasible (41), as well as the potential for real world and longitudinal evidence gathering (44) in populations where early detection is key. Many of the ambient technologies captured in this analysis were smartphone or tablet applications designed to analyze and interpret cognition or fine motor function to detect cognitive decline or discriminate between dementia states. Some examples include digital tasks testing aspects of cognition such as working memory, processing speed, or episodic memory (45); executive function (46); reaction time (47); speech or text patterns (48, 49); or pressure of a digital pen(50) on a device.
Wearables versus ambient sensors
Wearable sensors generally (32%), and smartwatches in particular (8%) were less represented in our landscape review than ambient technologies. Given their abundance and relative accessibility, wearable sensors can play an important role in the sensitive assessment of many ADRD-relevant measures, including mobility (51) (including but not limited to gait, life space pattern changes, and fall detection) and other biomarkers such as late-onset essential tremor (52) or heart rate variability (3). However, the lower proportion of wearables to ambient technologies may be a reflection of the versatility and advantages of the latter for dementia populations. For example, ambient sensors may be more appropriate for populations where memory or awareness concerns might make adherence with wearables a challenge. The aforementioned smart home possibilities with cameras, pressure sensors, and/or smart speakers/smartphones that detect changes in behavioral patterns or vocal biomarkers offer a powerful modality for real world continuous data collection while posing minimal burden on patients or caregivers.
Diagnostic classifications
Our results encompass a wide spectrum of dementia diagnoses and severity classifications. However, we note the prevalence of Alzheimer’s disease in our results compared to Frontotemporal dementias and Lewy Body dementia, as well as the prevalence of diagnoses like mild cognitive Impairment or general dementia as compared to results that specifically examined populations with moderate or advanced diagnoses. This suggests an opportunity for DHT developers and researchers to study additional uses and clinical validity within populations that are more narrowly defined within disease severity class, or in dementias other than Alzheimer’s disease.
Digital Measurement Products Library: A living resource
The digital health technology landscape evolves rapidly. Technologies from this review with peer-reviewed evidence published 2020-present are collated into the Library of Digital Measurement Products, an open access resource hosted by DiMe (53) for the clinical, research, and developer communities. While our landscape analysis included evidence published between 2003-2023 featuring DHTs that are both commercial and experimental, the library is a living resource intended to assist researchers and clinicians who may be considering a current and specific use case. Thus, it describes DHTs that are commercially available on the market at the time of entry into the database, and is restricted to peer-reviewed evidence published from 2020-present. Evidence is tagged according to the study type by which it is reported in the published literature as verification, analytical validation, clinical validation, or usability (17). Categorical fields are filterable so the end user can sort and view evidence according to a specific technology or technology type, therapeutic area or concept of interest, or by reported evidence type. This library aligns with the mission of the Digital Health Measurement Collaborative Community (13, 14) to share knowledge and resources to advance digital medicine and ultimately improve patient lives; we encourage the wider ADRD community to join our collective efforts to ensure diverse evidence-supported DHTs are represented in this important resource. A link to submit evidence for potential inclusion into the library is available to the public on DiMe’s Library of Digital Measures Products web page (53); beginning mid-2024, the library will be updated quarterly via review of community submissions, revisiting of the published literature, and removal of outdated evidence.
Limitations
While our results provide a broad overview of DHTs for ADRD assessment across 20 years of literature, we intentionally excluded brand-name identifiers from our search strings to avoid bias by disproportionately representing some market technologies over others. Additionally, we omitted search terms specific to Parkinson’s disease dementia because it has differentiating features from other dementias, and therefore will be the focus of a similar exercise in future research. Due to this intentional limiting of some search terms, we do not claim the search results to be exhaustive. As we conducted this exercise to aggregate DHTs and their uses within ADRD, with a focus on extracting technologies rather than conducting a formal appraisal of the literature, we report these findings as a technology landscape analysis rather than a traditional systematic review.
Lastly, members of the DiMe working group made the authorship team aware of additional technologies which were not identified/discovered via the literature search. While this carries a risk of bias (primarily that some additional technologies were presumably overlooked due to being unknown to project partners), we considered that the benefits of greater inclusion outweighed this potential drawback. We reached out to solicit supporting evidence for these, but received evidence back for a small portion. Consequently, many of the technologies identified during partner review are not included in this analysis, as we lacked the associated evidence necessary to report on them.
Conclusion
DHTs show great promise for ADRD populations by enabling objective, continuous, and repeatable measurements of functioning and symptoms in a range of settings. Ambient, application-based, and wearable technologies offer vast real world and longitudinal evidence generation possibilities, as well as the opportunity to take traditional neuropsychological and neurocognitive assessment out of the clinic or laboratory and into homes and care settings. They offer additional information to facilitate clinical decision making, and provide opportunities for clinical research to identify earlier signals of disease onset, monitor disease trajectory, or detect response to therapeutic treatment at a time when demand is increasing for precision medicine that better identifies and targets appropriate candidates for care and intervention, particularly in the prodromal or earliest stages (54, 55). These technologies continue to elucidate insights about domains such as cognitive function, sleep, mobility, language changes, and social and behavioral trends which will prove invaluable alongside traditional dementia research and management. This review confirms the multifaceted uses for and significant potential of DHTs as clinicians and researchers seek clarity and insights beyond the reach of traditional assessment modalities.
Acknowledgments: We appreciate the support of our collaborating partners and experts who comprise the DATAcc by DiMe ADRD Project working group and supported this manuscript: Abbvie; Altoida, Alzheimer’s Drug Discovery Foundation, Aural Analytics, Biogen, BioSensics, Boston University, Cambridge Cognition, Cogniant Pte Ltd, Cognito Therapeutics, Cogstate, Cumulus Neuroscience, Eisai, Eli Lilly & Company, Gates Ventures, ki:elements, Koneksa, Linus Health, Luca Health, Medidata, Merck, Northwestern University, Oregon Health & Science University, Roche, Sage Bionetworks, VivoSense, Winterlight Labs.
Author Contributions: All authors contributed to the conceptual development of this manuscript. All authors have approved the final submitted version and agree to be accountable for all aspects of the work presented here.
Conflict of interest: SAL is an employee of the Digital Medicine Society; ES is an employee of Altoida and holds company stock; SLB is an employee of VivoSense, Inc; KB is an employee of Merck & Co and Co-Chair of Mobile in Clinical Trials Conference; JD is an employee of Cumulus Neuroscience, holds company stock, & is coauthor on a pending patent with other Cumulus Neuroscience employees; CFA receives consulting income from Cogniant Pte Ltd.; JCG is an employee of the Digital Medicine Society; AH is an employee of Eli Lilly & Co. and holds company stock; AJ is an employee of Linus Health, Inc. and is listed as an inventor on pending patents on digital biomarkers of cognition and digital assessments for early diagnosis of dementia; RSF is an employee of Eisai Farmaceutica SA; PF is an employee of the Digital Medicine Society, reports consulting income from Sarepta, IQVIA, Clarivate, Open Health, has a leadership or fiduciary role with International Society of Quality of Life, and is president of consulting company SeeingTheta.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Perry B, Herrington W, Goldsack JC, et al. Use of Mobile Devices to Measure Outcomes in Clinical Research, 2010-2016: A Systematic Literature Review. Digit Biomark 2018;2:11–30; https://doi.org/10.1159/000486347
2. Abernethy A, Adams L, Barrett M, et al, 2022.The Promise of Digital Health: Then, Now, and the Future. NAM Perspect. https://doi.org/10.31478/202206e
3. Kourtis LC, Regele OB, Wright JM, Jones GB, 2019. Digital biomarkers for Alzheimer’s disease: the mobile/ wearable devices opportunity. NPJ Digit Med. https://doi.org/10.1038/s41746-019-0084-2
4. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med 2023;388:9–21; https://doi.org/10.1056/NEJMoa2212948
5. Watts KE, Storr NJ, Barr PG, Rajkumar AP. Systematic review of pharmacological interventions for people with Lewy body dementia. Aging Ment Health 2023;27:203–216; https://doi.org/10.1080/13607863.2022.2032601
6. Trieu C, Gossink F, Stek ML, Scheltens P, Pijnenburg YAL, Dols A. Effectiveness of Pharmacological Interventions for Symptoms of Behavioral Variant Frontotemporal Dementia: A Systematic Review. Cogn Behav Neurol 2020;33:1–15; https://doi.org/10.1097/WNN.0000000000000217
7. Vaz M, Silva V, Monteiro C, Silvestre S. Role of Aducanumab in the Treatment of Alzheimer’s Disease: Challenges and Opportunities. Clin Interv Aging 2022;17:797–810; https://doi.org/10.2147/CIA.S325026
8. Manta C, Patrick-Lake B, Goldsack JC. Digital Measures That Matter to Patients: A Framework to Guide the Selection and Development of Digital Measures of Health. Digit Biomark 2020;4:69–77; https://doi.org/10.1159/000509725
9. Improving and Increasing Diversity, Equity, and Inclusion in Digitized Clinical Trial Design and Implementation, 2023. In: Digital Medicine Socie-ty. https:// dimesociety.org/access-resources/diversity-equity-inclusion/. Accessed 1 Oct 2023
10. Digital Measures: Alzheimer’s Disease and Related Dementias, 2023. In: Digital Medicine Society: DATAcc by DiMe. https://datacc.dimesociety.org/ digital-measures-adrd/. Accessed 15 Feb 2024
11. Li X, Feng X, Sun X, Hou N, Han F, Liu Y. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2019. Front Aging Neurosci 2022;14:937486; https://doi.org/10.3389/fnagi.2022.937486
12. Tahami Monfared AA, Byrnes MJ, White LA, Zhang Q. The Humanistic and Economic Burden of Alzheimer’s Disease. Neurol Ther 2022;11:525–551; https://doi.org/10.1007/s40120-022-00335-x
13. Digital Medicine Society DATAcc by the Digital Medicine Society (DiMe). https://datacc.dimesociety.org/. Accessed 31 Jan 2024
14. Center for Devices, Radiological Health (2024) Collaborative Communities: Addressing Health Care Challenges Together. In: U.S. Food and Drug Ad-ministration. https://www.fda.gov/about-fda/cdrh-strategic-priorities- and-updates/collaborative-communities-addressing-health-care-challenges together. Accessed 15 Feb 2024
15. Curcic J, Vallejo V, Sorinas J, et al. Description of the Method for Evaluating Digital Endpoints in Alzheimer Disease Study: Protocol for an Exploratory, Cross-sectional Study. JMIR Res Protoc 2022;11:e35442; https://doi.org/10.2196/35442
16. Muurling M, Hinds C, Wu Y, et al, 2022. User experience and compliance rates of remote monitoring technologies in Alzheimer’s disease: Preliminary results of the RADAR-AD study. Alzheimers Dement. https://doi.org/10.1002/ alz.064507
17. Goldsack JC, Coravos A, Bakker JP, et al. Verification, analytical validation, and clinical validation (V3): the foundation of determining fit-for-purpose for Biometric Monitoring Technologies (BioMeTs). NPJ Digit Med 2020;3:55; https://doi.org/10.1038/s41746-020-0260-4
18. DiBenedetti DB, Slota C, Wronski SL, et al. Assessing what matters most to patients with or at risk for Alzheimer’s and care partners: a qualita-tive study evaluating symptoms, impacts, and outcomes. Alzheimers Res Ther 2020;12:90; https://doi.org/10.1186/s13195-020-00659-6
19. Hauber B, Paulsen R, Krasa HB, Vradenburg G, Comer M, Callahan LF, Winfield J, Potashman M, Hartry A, Lee D, Wilson H. Assessing what matters to people affected by Alzheimer’s disease: a quantitative analysis. Neurology and Therapy. 2023 Apr;12(2):505-27; https://doi.org/10.1007/s40120-023-00445-0
20. Corporation for Digital Scholarship Zotero.
21. Olaye IM, Belovsky MP, Bataille L, et al. Recommendations for Defining and Reporting Adherence Measured by Biometric Monitoring Technol-ogies: Systematic Review. J Med Internet Res 2022;24:e33537; https://doi.org/10.2196/33537
22. Sharma Y, Djambazova KV, Marquez CD, Lyden K, Goldsack JC, Bakker JP, 2023. A systematic review assessing the state of analytical valida-tion for connected, mobile, sensor-based digital health technologies. bioRxiv. https:// doi.org/10.1101/2023.05.22.23290371
23. Qualtrics, 2015. Qualtrics XM – Experience Management Software.
24. Torous J, Kiang MV, Lorme J, Onnela J-P. New Tools for New Research in Psychiatry: A Scalable and Customizable Platform to Empower Data Driven Smartphone Research. JMIR Ment Health 2016;3:e16; https://doi.org/10.2196/mental.5165
25. Hackett K, Giovannetti T. Capturing Cognitive Aging in Vivo: Application of a Neuropsychological Framework for Emerging Digital Tools. JMIR Aging 2022;5:e38130; https://doi.org/10.2196/38130
26. Akl A, Taati B, Mihailidis A. Autonomous unobtrusive detection of mild cognitive impairment in older adults. IEEE Trans Biomed Eng 2015;62:1383– 1394; https://doi.org/10.1109/TBME.2015.2389149
27. Au-Yeung W-TM, Miller L, Beattie Z, May R, Cray HV, Kabelac Z, Katabi D, Kaye J, Vahia IV. Monitoring Behaviors of Patients With Late-Stage Dementia Using Passive Environmental Sensing Approaches: A Case Series. Am J Geriatr Psychiatry 2022;30:1–11; https://doi.org/10.1016/j.jagp.2021.04.008
28. Thomas NWD, Beattie Z, Marcoe J, Wright K, Sharma N, Mattek N, Dodge H, Wild K, Kaye J. An Ecologically Valid, Longitudinal, and Unbi-ased Assessment of Treatment Efficacy in Alzheimer Disease (the EVALUATE-AD Trial): Proof of-Concept Study. JMIR Res Protoc 2020;9:e17603; https://doi.org/10.2196%2F17603
29. Wu C-Y, Beattie Z, Mattek N, Sharma N, Kaye J, Dodge HH. Reproducibility and replicability of high-frequency, in-home digital biomarkers in reducing sample sizes for clinical trials. Alzheimers Dement 2021; 7:e12220 https://doi.org/10.1002/trc2.12220
30. Öhman F, Hassenstab J, Berron D, Schöll M, Papp KV. Current advances in digital cognitive assessment for preclinical Alzheimer’s disease. Alzheimers Dement 2021;13:e12217; https://doi.org/10.1002/dad2.12217
31. Sliwinski MJ. Measurement-burst designs for social health research. Soc Personal Psychol Compass 2008;2:245–261; https://doi.org/10.1111/j.1751-9004.2007.00043.x
32. Bellerophon Announces Top-Line Data from Phase 3 REBUILD Clinical Trial of INOpulse® for Treatment of Fibrotic Interstitial Lung Disease, 2023. In: Bellerophon Therapeutics, Inc. https://investors.bellerophon.com/news releases/news-release-details/bellerophon-announces-top-line-data-phase-3- rebuild-clinical. Accessed 5 Jun 2023
33. O’Hanlon CE., Farmer CM., Ryan J., Ernecoff N., 2023. Clinical Outcome Assessments and Digital Health Technologies Supporting Clinical Trial Endpoints in Early Parkinson’s Disease: Roundtable Proceedings and Roadmap for Research. RAND https://doi.org/10.7249/CFA2550-1
34. Perry B, Kehoe L, Swezey T, Le Masne Q, Goldhahn J, Staley A, Corneli A (2023) How Much Evidence Is Enough? Research Sponsor Expe-riences Seeking Regulatory Acceptance of Digital Health Technology-Derived Endpoints. Digit Biomark 2023;7:45–53; https://doi.org/10.1159/000529878
35. Masanneck L, Gieseler P, Gordon WJ, Meuth SG, Stern AD. Evidence from ClinicalTrials.gov on the growth of Digital Health Technologies in neurology trials. NPJ Digit Med 2023;6:23; https://doi.org/10.1038%2Fs41746-023-00767-1
36. Brem A-K, Kuruppu S, de Boer C, et al. Digital endpoints in clinical trials of Alzheimer’s disease and other neurodegenerative diseases: chal-lenges and opportunities. Front Neurol 2023;14:1210974; https://doi.org/10.3389/fneur.2023.1210974
37. Wang J, Battioui C, McCarthy A, et al. Evaluating the Use of Digital Biomarkers to Test Treatment Effects on Cognition and Movement in Patients with Lewy Body Dementia. J Parkinsons Dis 2022;12:1991–2004; https://doi.org/10.3233/jpd-213126
38. Chen C, Kowahl NR, Rainaldi E, Burq M, Munsie LM, Battioui C, Wang J, Biglan K, Marks WJ Jr, Kapur R. Wrist-worn sensor-based mea-surements for drug effect detection with small samples in people with Lewy Body Dementia. Parkinsonism Relat Disord 2023;109:105355; https://doi.org/10.1016/j.parkreldis.2023.105355
39. Svetnik V, Wang T-C, Ceesay P, et al. Pilot evaluation of a consumer wearable device to assess sleep in a clinical polysomnography trial of suvorexant for treating insomnia in patients with Alzheimer’s disease. J Sleep Res 2021;30:e13328; https://doi.org/10.1111/jsr.13328
40. Parsons T, Duffield T. Paradigm Shift Toward Digital Neuropsychology and High-Dimensional Neuropsychological Assessments: Review. J Med Internet Res 2020;22:e23777; https://doi.org/10.2196/23777
41. Van Patten R. Introduction to the Special Issue – Neuropsychology from a distance: Psychometric properties and clinical utility of remote neuro-cognitive tests. J Clin Exp Neuropsychol 2021;43:767–773; https://doi.org/10.1080/13803395.2021.2021645
42. Lunardini F, Luperto M, Romeo M, et al. Supervised Digital Neuropsychological Tests for Cognitive Decline in Older Adults: Usability and Clinical Validity Study. JMIR Mhealth Uhealth 2020;8:e17963 https://doi.org/10.2196/17963
43. Muurling M, de Boer C, Vairavan S, et al. Augmented reality versus standard tests to assess cognition and function in early Alzheimer’s disease. NPJ Digit Med 2023;6:234 https://doi.org/10.1038/s41746-023-00978-6
44. Hampshire A, Ballard C, Williams G, 2020. Computerized neuropsychological tests undertaken on digital platforms are cost effective, achieve high engagement, distinguish and are highly sensitive to longitudinal change: Data from the PROTECT and GBIT studies. Alzheimers Dement. https://doi. org/10.1002/alz.041122
45. Thompson LI, Harrington KD, Roque N, Strenger J, Correia S, Jones RN, Salloway S, Sliwinski MJ. A highly feasible, reliable, and fully remote protocol for mobile app-based cognitive assessment in cognitively healthy older adults. Alzheimers Dement 2022;14:e12283; https://doi.org/10.1002/dad2.12283
46. Tarnanas I, Tsolaki A, Wiederhold M, Wiederhold B, Tsolaki M. Five year biomarker progression variability for Alzheimer’s disease dementia prediction: Can a complex instrumental activities of daily living marker fill in the gaps? Alzheimers Dement 2015;1:521–532; https://doi.org/10.1016%2Fj.dadm.2015.10.005
47. Seixas AA, Rajabli F, Pericak-Vance MA, Jean-Louis G, Harms RL, Tarnanas I. Associations of digital neuro-signatures with molecular and neuroimaging measures of brain resilience: The altoida large cohort study. Front Psychiatry 2022;13:899080; https://doi.org/10.3389/fpsyt.2022.899080
48. Yeung A, Iaboni A, Rochon E, et al. Correlating natural language processing and automated speech analysis with clinician assessment to quan-tify speech language changes in mild cognitive impairment and Alzheimer’s dementia. Alzheimers Res Ther 2021;13:109; https://doi.org/10.1186%2Fs13195-021-00848-x
49. Fraser KC, Rudzicz F, Rochon E. Using text and acoustic features to diagnose progressive aphasia and its subtypes. Interspeech 2013. https://doi. org/10.21437/interspeech.2013-514
50. Kim KW, Lee SY, Choi J, Chin J, Lee BH, Na DL, Choi JH. A Comprehensive Evaluation of the Process of Copying a Complex Figure in Early- and Late Onset Alzheimer Disease: A Quantitative Analysis of Digital Pen Data. J Med Internet Res 2020;22:e18136; https://doi.org/10.2196%2F18136
51. Cullen A, Mazhar MKA, Smith MD, Lithander FE, Ó Breasail M, Henderson EJ, 2022. Wearable and Portable GPS Solutions for Monitoring Mobility in Dementia: A Systematic Review. Sensors . https://doi.org/10.3390/s22093336
52. Wang X, St George RJ, Bai Q, Tran S, Alty J, 2022. New horizons in late-onset essential tremor: a pre-cognitive biomarker of dementia? Age Ageing. https:// doi.org/10.1093/ageing/afac135
53. Library of Digital Measurement Products, 2024. In: DATAcc by DiMe. https:// datacc.dimesociety.org/digital-measurement-library/. Accessed 27 Feb 2024
54. Forloni G. Alzheimer’s disease: from basic science to precision medicine approach. BMJ Neurol Open 2020;2:e000079; https://doi.org/10.1136%2Fbmjno-2020-000079
55. Lukiw WJ, Vergallo A, Lista S, Hampel H, Zhao Y, 2020. Biomarkers for Alzheimer’s Disease (AD) and the Application of Precision Medicine. J Pers Med. https://doi.org/10.3390/jpm10030138
© The Authors 2024