S. Dabiri1, R. Raman2, J. Grooms3, D. Molina-Henry2
1. Sol Price Schaeffer Center & Alzheimer’s Therapeutic Research Institute, University of Southern California, USA; 2. Alzheimer’s Therapeutic Research Institute, University of Southern California, USA; 3. Department of Economics, Howard University, USA
Corresponding Author: Sanaz Dabiri, Sol Price Schaeffer Center & Alzheimer’s Therapeutic Research Institute, University of Southern California, USA sanazdab@usc.edu
J Prev Alz Dis 2024;6(11):1647-1672
Published online August 9, 2024, http://dx.doi.org/10.14283/jpad.2024.149
Abstract
BACKGROUND: Despite higher dementia prevalence in racial and ethnic minoritized communities, they are underrepresented in Alzheimer’s disease clinical trials. Community-based recruitment strategies are believed to yield positive outcomes in various fields, such as cancer and cardiovascular clinical trials, but their outcomes in Alzheimer’s disease and Related Dementias (AD/ADRD) require further study. In this systematic rapid review, we synthesized the available evidence on community-engaged recruitment strategies in enhancing participation in AD/ADRD clinical trials and observational study participation.
METHODS: We searched and identified studies describing a community-based recruitment approach for racial and ethnic minoritized communities across seven databases (Pubmed, OVID MEDLINE, Cochrane Central Register of Controlled Trials, CINAHL, PsychINFO, Web of Science, and EMBASE).
RESULTS: Out of 1915 screened studies, 49 met the inclusion criteria. Most studies employed multiple community-based recruitment approaches, including educational presentations, collaborations with community-based faith organizations, community advisory boards, and engagement with local clinics or health professionals. 52% of studies targeted more than one racial and ethnic minoritized population, primarily African Americans and then Hispanic/Latino. Gaps in knowledge about AD/ADRD, its increased risk among minoritized populations, distrust, and stigma were noted as barriers to research participation. Approximately 50% of the studies specified whether they evaluated their recruitment approaches, and in studies where approaches were evaluated, there was substantial heterogeneity in methods utilized.
CONCLUSION: The quality of available evidence on the use of community-based recruitment approaches to include racial and ethnic minoritized populations in AD/ADRD research, particularly in clinical trials, is limited. Systematic assessment of recruitment strategies is urgently needed to increase the evidence base around community-engaged recruitment approaches.
Key words: AD/ADRD clinical trial, recruitment, minoritized, disparities, community-based.
Introduction
In 2021, the US Department of Health and Human Services published a National Plan to treat Alzheimer’s disease and related dementias effectively (AD/ADRD) by 2025 (1). Despite a growing focus on AD/ADRD clinical trials, recruitment of participants continues to be a challenge. Older adults participating in clinical trials tend to be non-Hispanic White, have a higher socioeconomic status, are married, or have a partner (2). In this systematic rapid review, we focused on community-based AD/ADRD recruitment strategies to engage racial and ethnic minoritized communities. We followed the US Census official race categories: White, Black/African American, Asian American, American Indian/Alaska Native, Native Hawaiian/Pacific Islander. In terms of ethnicity, individuals with shared cultural ties to Latin America are often identified as Hispanic, Latino, Latina, or Latinx. For the purpose of this paper, we refer to this group of individuals as Hispanic/Latino.
The risk and prevalence of dementia are higher among racial and ethnic minoritized groups, including African American/Black and Hispanic/Latino (3-5). Nevertheless, a systematic review of randomized controlled trials (RCTs) reported that racial and ethnic minoritized communities remain underrepresented in AD/ADRD clinical trials (6). In a series of 6 AD/ADRD cooperative trials, only 5% Hispanic and 6% African American participants were enrolled (7). Similar findings have been observed in the AD/ADRD cohort or observational studies (8). Currently, Hispanic/Latino individuals account for 52% of the population growth in the US (9), and this population is expected to increase by almost double from 63.6 million to 111 million by 2060 (9, 10). Moreover, it is estimated that by 2044 half of the US population will identify as belonging to racial and ethnic groups other than non-Hispanic White (11). To ensure the generalizability of AD/ADRD research findings, there is a greater need for the participation of racial and ethnic minoritized communities in AD/ADRD clinical trials and observational studies.
Recruitment challenges have been well-documented and can fall under individual, social/environmental, or broader economic, institutional, and cultural barriers. Some of the reported challenges are the participants’ reluctance stemming from the risk-benefit analysis of concerns around the intervention (12), the presence of comorbid conditions (13), and restrictive study protocols defined by specific inclusion and exclusion criteria (14-16). Lack of awareness of available clinical trials, primary care physicians’ lack of resources to refer patients (17), and participants’ distrust (12, 18, 19) can contribute to apprehension around participation. Moreover, lengthy trial duration (13), transportation challenges (20), caregiver or study partner burden (21, 22), socioeconomic status, participants’ specific unmet cultural needs, and differences in perceived risk for AD/ADRD (19, 23) are additional recruitment challenges. The barriers to participation may also vary depending on the specific racial and ethnic minoritized groups. While the majority of individuals from minoritized communities believe medical research to be biased against their communities (24), the distrust may be more pronounced among African American participants because of the historical instances of unethical research and abuse of power. A systematic review identified the legacy of the Tuskegee Study, institutional racism and discrimination, concerns about the research process, and disregard for cultural norms among research teams as distinct barriers for African American individuals to enroll in any health-related research (25). On the other hand, Hispanic/Latino participants in a focus group identified that using low-literacy recruitment material, bi-lingual staff, and informing that immigration status would not impact the research participation among Hispanic/Latino adults (26). Asian American communities are diverse in history, culture, and language; however, they are often grouped together in research. Barriers to participation among Asian American adults may be more specific to their social context, such as the level of acculturation or family support (25). For individuals identifying as Pacific Islanders, barriers may be attributed to cultural insensitivity and concerns about data use (25). Therefore, differences in barriers, unique attributes, and lived experiences of the different populations should be considered when recruiting for AD/ADRD clinical trials and observational studies. For instance, in the case of African American research participants, increasing trust, diverse representation in research teams, and acknowledging past abuses have been strategies to facilitate recruitment (26).
Community outreach and the engagement of community partners and stakeholders may serve as facilitators for enhancing racial and ethnic minoritized participation in AD/ADRD clinical trials and non-intervention types of research (27). Community-based organizations may have better access to and build relationships with potential study participants. Such efforts have been successful in the context of cardiovascular disease prevention (28-30) and cancer clinical trials (28, 31, 32). For instance, in a quest to identify the most successful recruitment strategy to engage African American communities in clinical trials, Otado et al. reviewed all clinical trials at the Howard University Clinical Research Unit. The 50 reviewed studies involved cognitive aging, sickle cell disease, HIV, posttraumatic stress disorder, genetics, hypertension and diabetes, cancer, stress, substance use, and alcohol research. In this study, anecdotal reports from study coordinators suggested that community outreach yielded the highest recruitment outcome (28). Notably, community engagement exists on a spectrum, with different levels of community involvement, ranging from an outreach with minimal community involvement to a shared leadership that is based on a bi-directional relationship between researchers and community members (33). In response to the 2018 initiative by the National Institute on Aging urging additional research at developing and evaluating diversity among AD/ADRD research participants (34), multiple reviews have synthesized the current understanding of recruitment and retention strategies of racial and ethnic minoritized groups into all AD/ADRD research (35, 36). These reviews reported on all types of recruitment strategies in AD/ADRD research. Despite this breadth of information, there remains a knowledge gap in differentiating recruitment efforts between clinical trials and observational studies in the AD/ADRD field. While progress has been made in enrolling racial and ethnic minoritized individuals in AD/ADRD research, challenges persist in clinical trials where the aim is to evaluate a pharmacological agent. Given that AD/ADRD clinical trials face unique recruitment challenges, the recruitment strategies may contrast with those used for observational studies, which typically involve less invasive procedures and a lower burden on participants and their study partners. In this systematic rapid review, we narrowed our focus to the utilization of community-based recruitment methodologies to enhance the representation of racial and ethnic minoritized populations in AD/ADRD research. By doing so, we aimed to offer an updated understanding of the current state of the community-based recruitment approach within the recruitment science, shedding light on its effectiveness and challenges among AD/ADRD clinical trials compared to observational studies. Additionally, we examined the current evidence on participants’ attitudes toward AD/ADRD and persistent barriers to recruitment in AD/ADRD research despite using community-engaged recruitment strategies. Our systematic review sought to answer the following question: Are there discernible differences in the type of community-based recruitment strategies used for AD/ADRD clinical engagement (clinical trials and studies that assess interest in clinical trial participation) and observational engagement (non-interventional studies and studies gauging interest in non-interventional AD/ADRD research? We further delved into differences in strategies across racial and ethnic minoritized communities, identified knowledge gaps in the recruitment science of AD/ADRD in clinical trials and observational studies, and discussed potential approaches to fill these gaps.
Method
A rapid review is a knowledge synthesis approach that expedites the systematic review process by simplifying or omitting certain stages. This streamlined method allows for the timely and resource-efficient production of evidence synthesis. We conducted a rapid review of the literature using guidelines recommended by the Cochrane Handbook (37) over an eight-month period from 04/20/2023 to 12/12/2023. We registered the protocol of this rapid review in the International Prospective Register of Systematic Reviews (PROSPERO) database (ID: CRD42023427312) on 5/18/2023.
Study selection
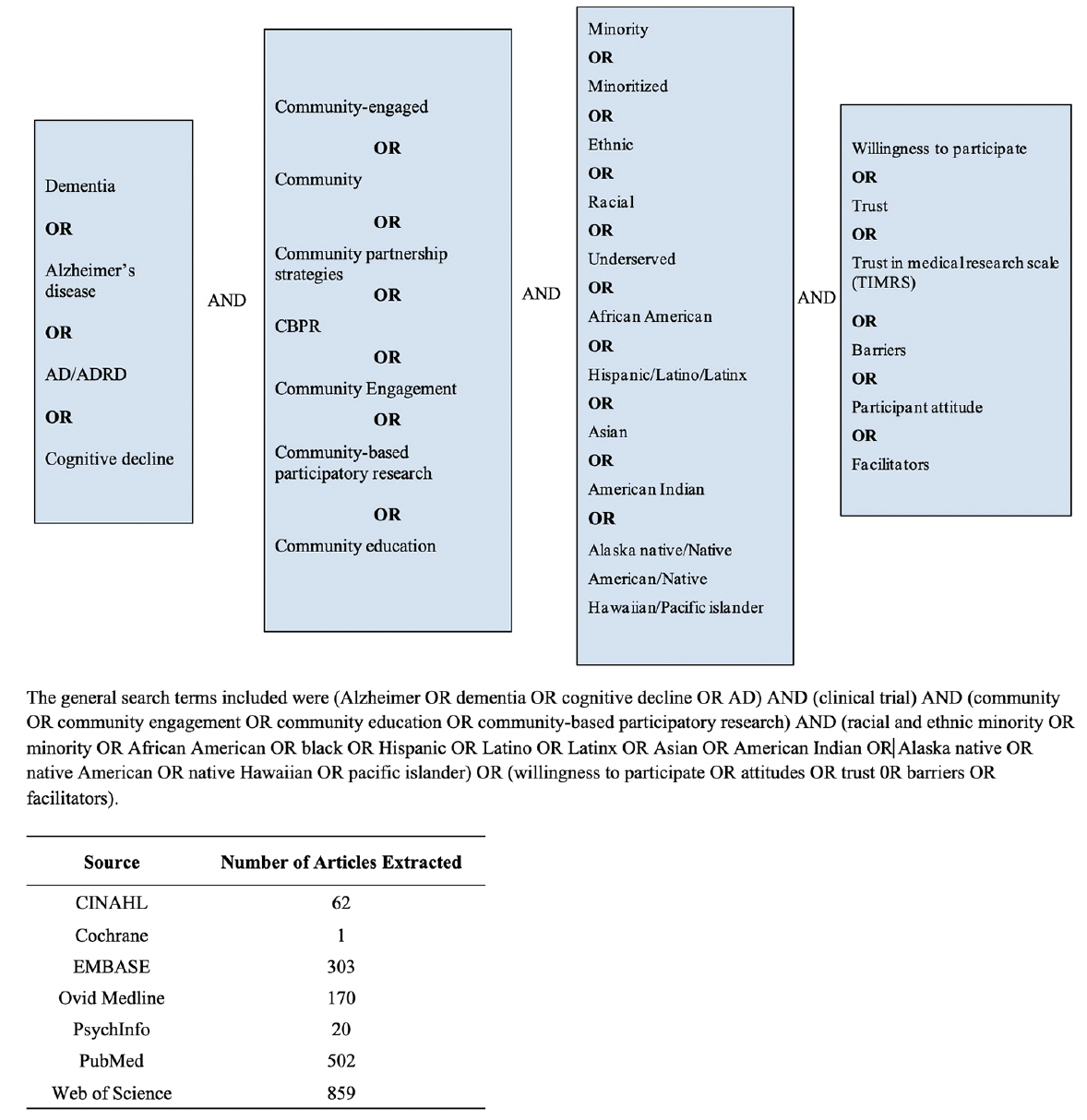
We searched across databases including: PubMed, OVID MEDLINE, Cochrane Central Register of Controlled Trials, CINAHL, PsychINFO, Web of Science, and EMBASE. We included any AD/ADRD-focused research published in English and a peer-reviewed journal that used at least one community-based recruitment approach to target participants from at least one racial and ethnic minoritized community (See Figure 1 for search terms and the number of articles extracted from each database). Community-based recruitment approaches were defined as any recruitment efforts that included community outreach that had a connection with community leaders, organizations, community church/faith-based groups, community health clinics, local businesses, community events, community senior centers, and community dementia services. Communities of focus were African American/Black, Hispanic/Latino, Asian/Asian American, American Indian or Alaska Natives, and Native Hawaiian or other Pacific Islander. Searches were not limited by time to ensure the capture of a more comprehensive understanding of community-based recruitment approaches and trace any emerging trends. Posters of conference abstracts were not included since the full description of the recruitment strategy would not be reported in the abstracts.
We included two types of published articles: 1. Studies that reported community-based recruitment efforts to increase racial and ethnic minoritized communities in AD/ADRD clinical trials or observational studies. 2. Recruitment studies that used community-based recruitment efforts to explore racial and ethnic minoritized communities’ attitudes and perceptions about AD/ADRD participation in clinical trials or observational studies.
A clinical trial, as defined by the NIH (38), is a research study where one or more human subjects are assigned to one or more interventions to assess the effects on health-related outcomes. In our review, we focused on community-based recruitment approaches in AD/ADRD research, including studies that assessed barriers and facilitators of clinical trial participation among racial and ethnic minoritized communities. We categorized both recruitment studies for AD/ADRD clinical trial engagement and AD/ADRD clinical trial studies together as Clinical Engagement. Observational studies, on the other hand, are those in which the investigator records observations and analyzes data without assigning participants to a specific intervention. These studies may focus on observing risk factors, natural history, or variations in disease progression and treatment without implementing an intervention (38). We grouped both observational studies and those gauging interest in non-interventional research participation together as Observational Engagement.
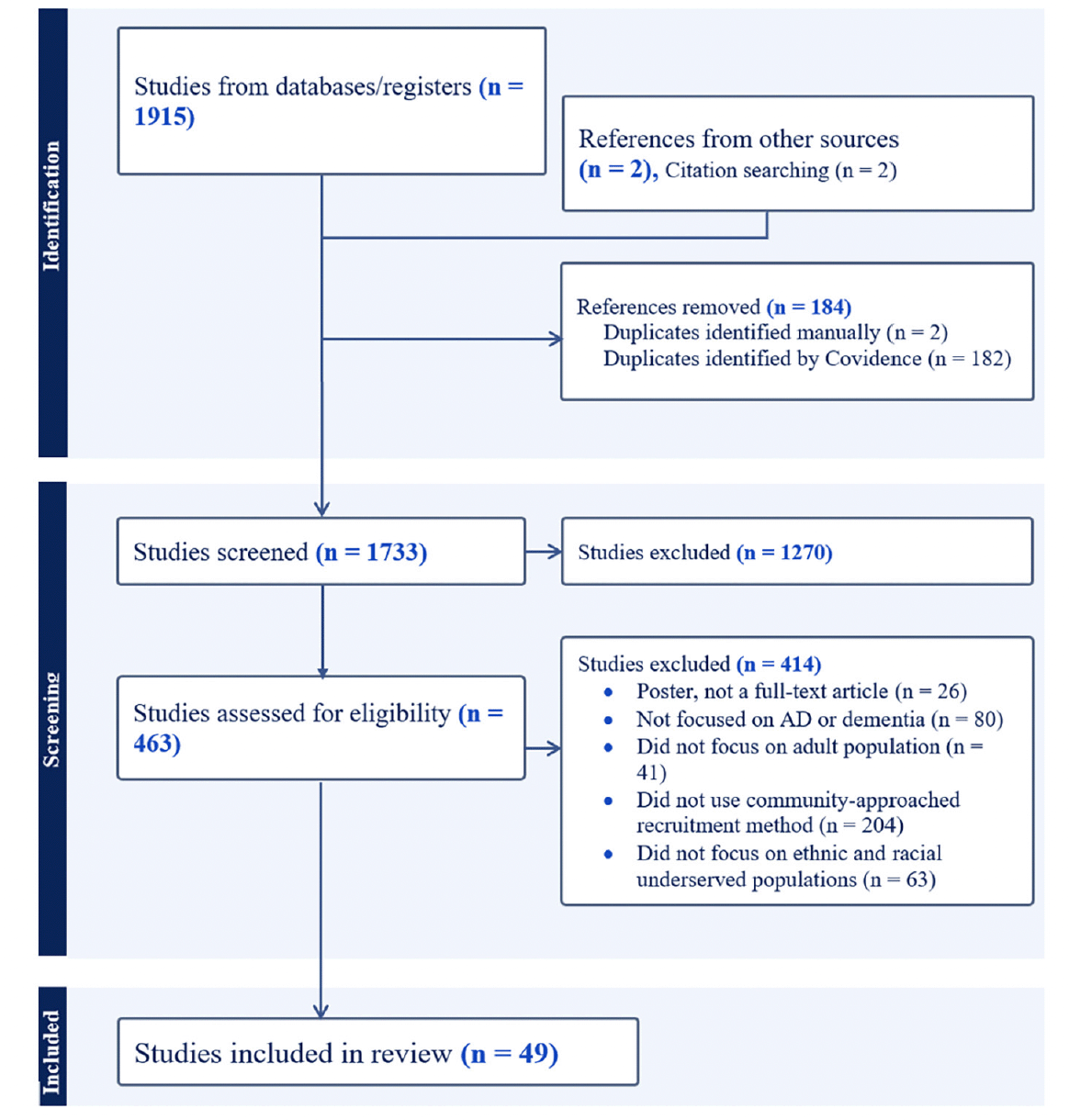
References were imported to EndNote, and initial duplicates were removed. Electronic results were imported and screened using Covidence Systematic Review Software (2019). Duplicates were identified and excluded once the references were imported on Covidence. The consort diagram in Figure 2 details the screening and selection process of included and excluded studies. Studies were included if they described a recruitment approach that involved the community, and the focus of recruitment was on increasing participation of community members from different ethnic and racial groups. Furthermore, studies that included measures of knowledge of AD/ADRD, trust in the medical and research community, beliefs about AD/ADRD research, and willingness to participate were captured to summarize available evidence within the selected studies on participants’ attitudes and perceptions toward AD/ADRD clinical trials and observational studies.
Studies were excluded if their target population did not specifically use a community-approached recruitment method to recruit racial and ethnic minoritized communities and if the study was not AD/ADRD related
Data extraction
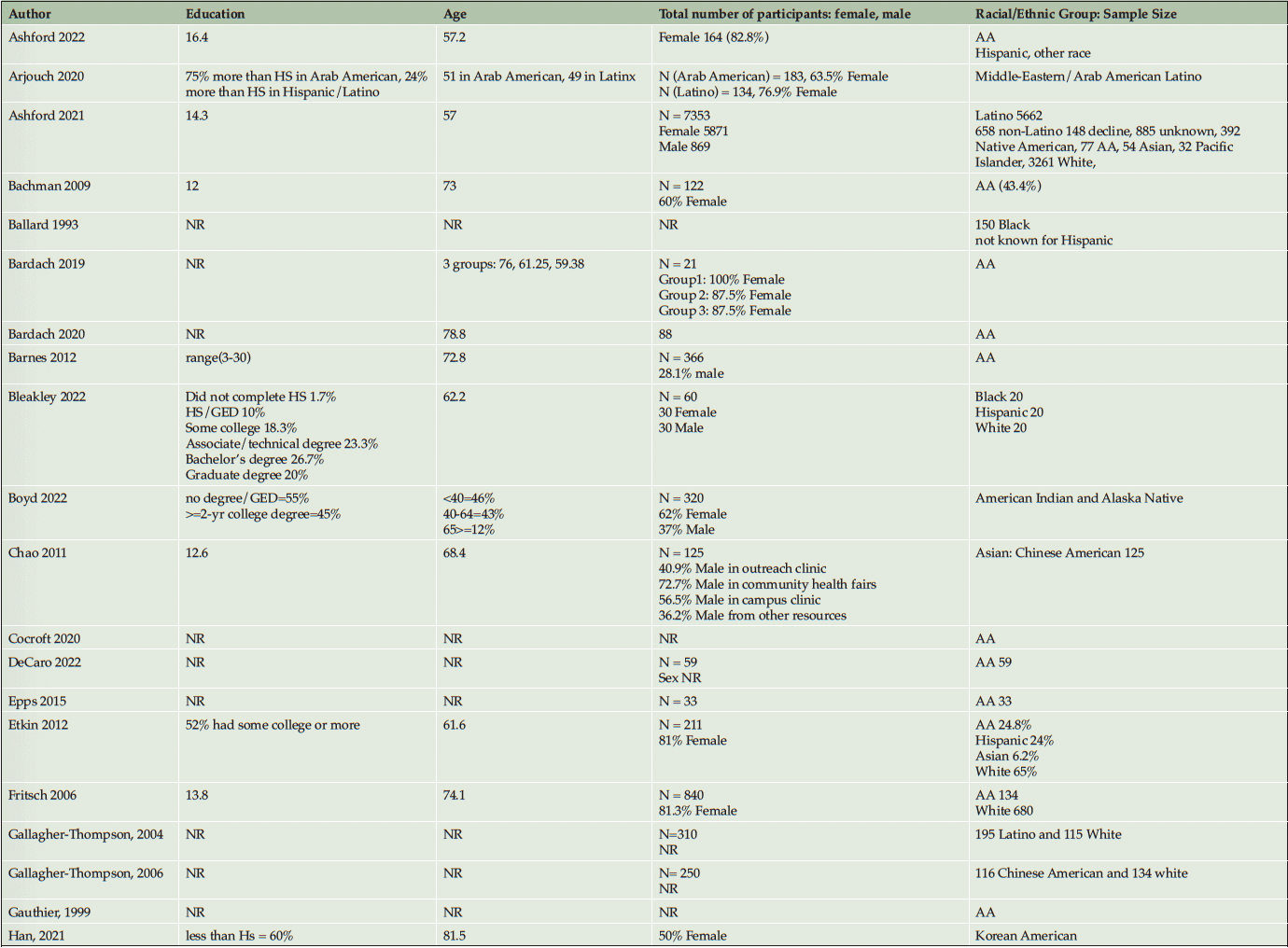
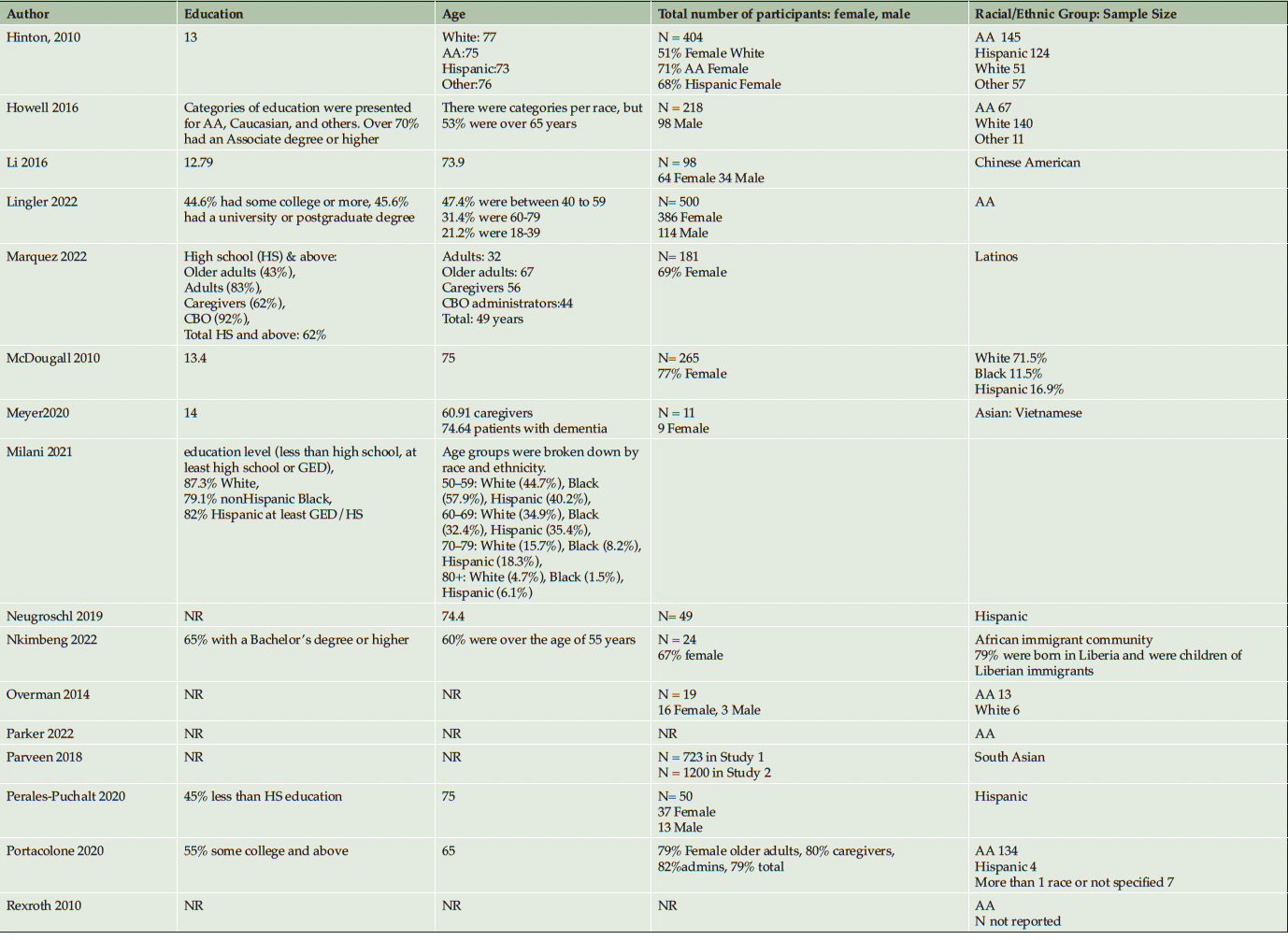
A single-reviewer data extraction approach was adopted in light of the limited time and resources available for this review. This decision aimed to warrant the timely completion of the review while maintaining a focus on the essential aspects of the evidence synthesis. To ensure the reliability and accuracy of the review, quality control measures were implemented, including the use of standardized data extraction and quality assessment tools, and periodic consultation with a secondary reviewer for critical stages. We adopted the data extraction item checklist in the Cochrane Handbook (37) for this review. Specifically, we extracted information regarding participants’ age, education, targeted minoritized population, sample size, study design, specific community-based recruitment approach, measures of participants’ perceptions and attitudes toward AD/ADRD research, inclusion/exclusion criteria, study aims, outcome variables, geographical location of recruitment, and key findings.
Data quality assessment
Data quality was assessed using the Quality Assessment Tool for quantitative studies (39), which categorizes the assessment as weak, moderate, and strong based on eight criteria, including selection bias, study design, confounder, blinding, data collection methods, participant attrition, intervention integrity, and analysis. Joanna Briggs Institute Checklist for Qualitative Research (40) was used for qualitative and descriptive studies. Each item on the checklist helps the reviewer evaluate the extent to which the study meets established qualitative research standards, contributing to an overall assessment of the study’s reliability and validity. During the full-text screening, the quality of each article was assessed through all eight domains of the Quality Assessment Tool or the ten items of the Joanna Briggs Institute Checklist to check whether the items on the Qualitative Assessment Tool or Joanna Briggs Institute checklist were addressed in the articles.
Results
The primary outcome of this review was to synthesize available evidence on differences in community-based recruitment strategies for AD/ADRD clinical trials versus observational studies. The secondary outcome was synthesizing available evidence on these strategies’ differences across racial and minoritized communities. Additionally, we explored participants’ attitudes and perceptions toward AD/ADRD research.
Our database searches yielded 1915 studies, and two additional studies were identified by cross-referencing. After removing duplicates, 1270 studies were excluded during title and abstract screening. A further 414 studies were excluded during full-text screening because they were either posters, lacked community-based recruitment methods, or were not focused on the AD/ADRD research. Additionally, articles lacking adult participants and articles that did not have an emphasis on recruiting racial and ethnic minoritized communities were also excluded (see Figure 2). We identified 49 studies that focused strictly on community-engaged recruitment strategies, emphasizing recruiting at least one racial and ethnic minoritized group (see Figure 2). Out of 49 studies, 11 focused on clinical trial recruitment or assessed willingness to participate in clinical trials (clinical engagement), and the rest of the articles were AD/ADRD observational studies (observational engagement).
Characteristics of included studies
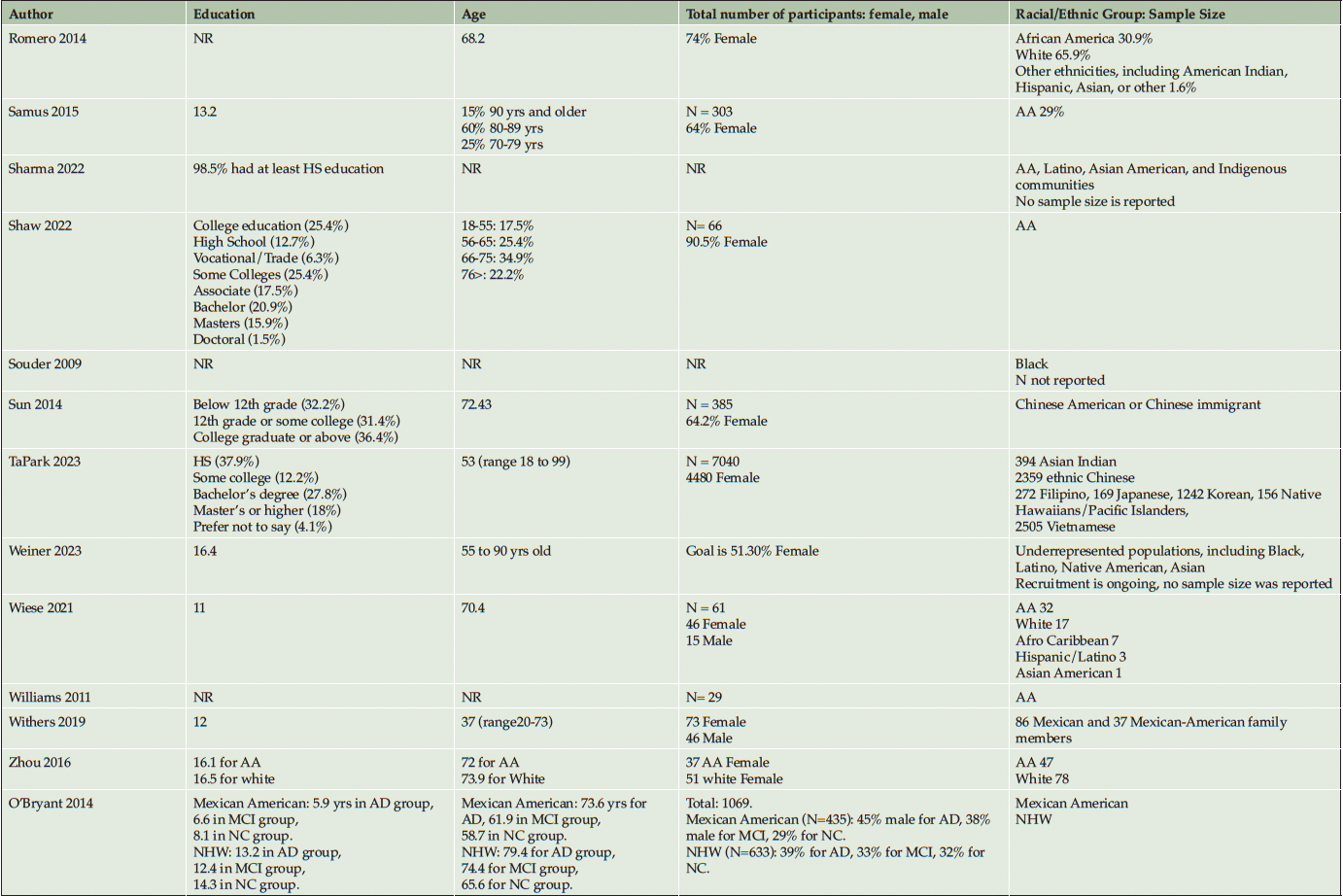
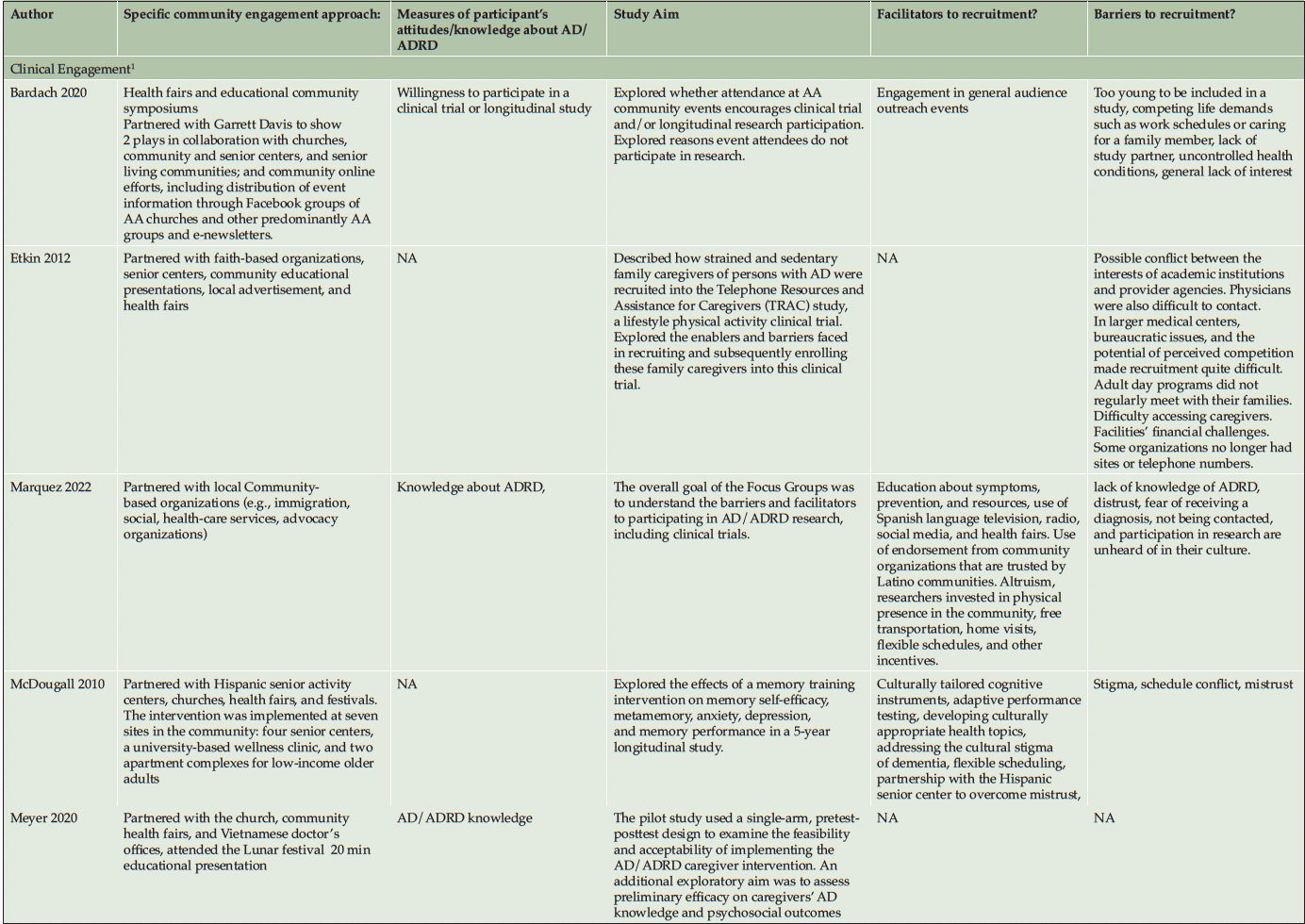
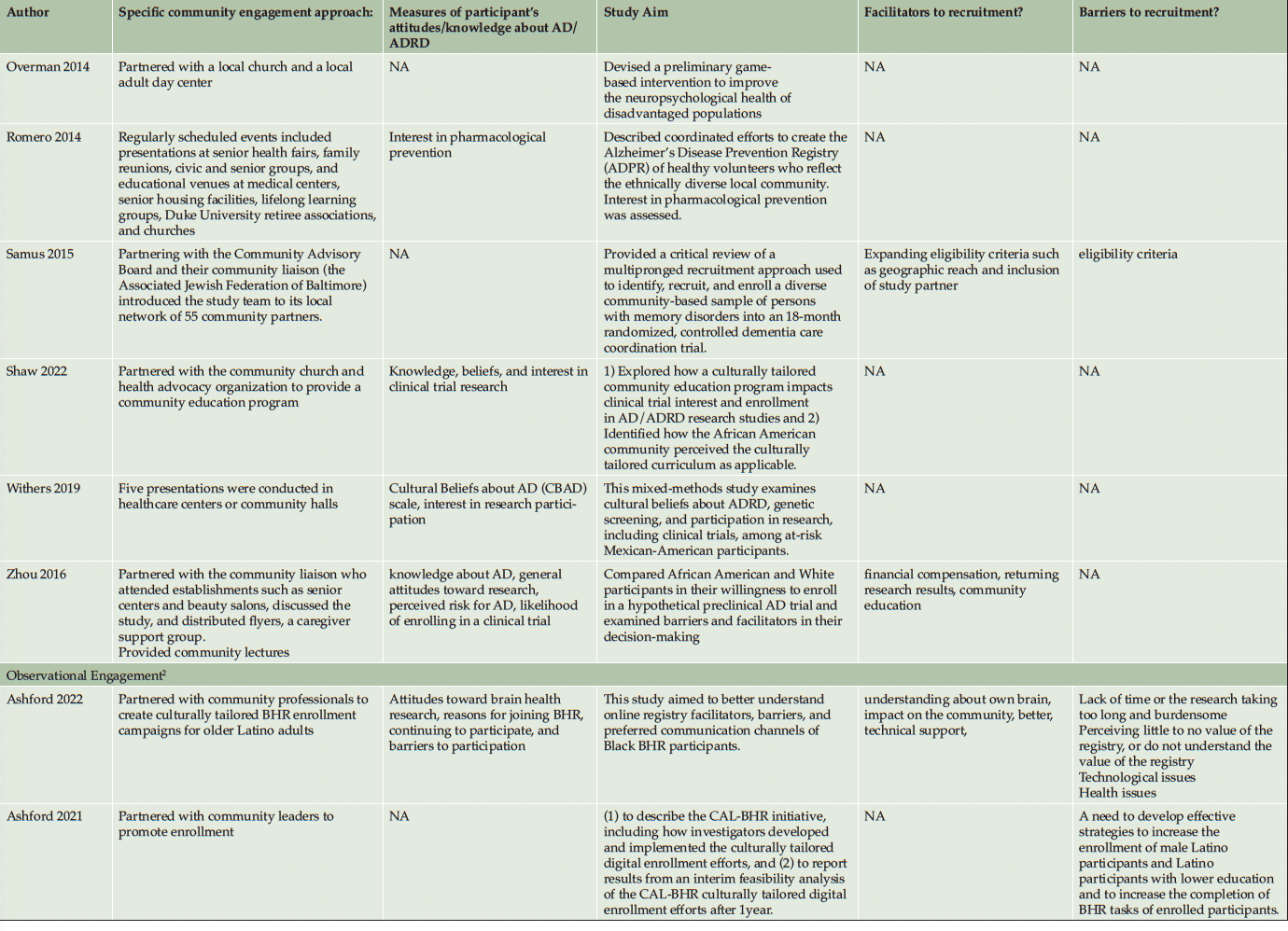
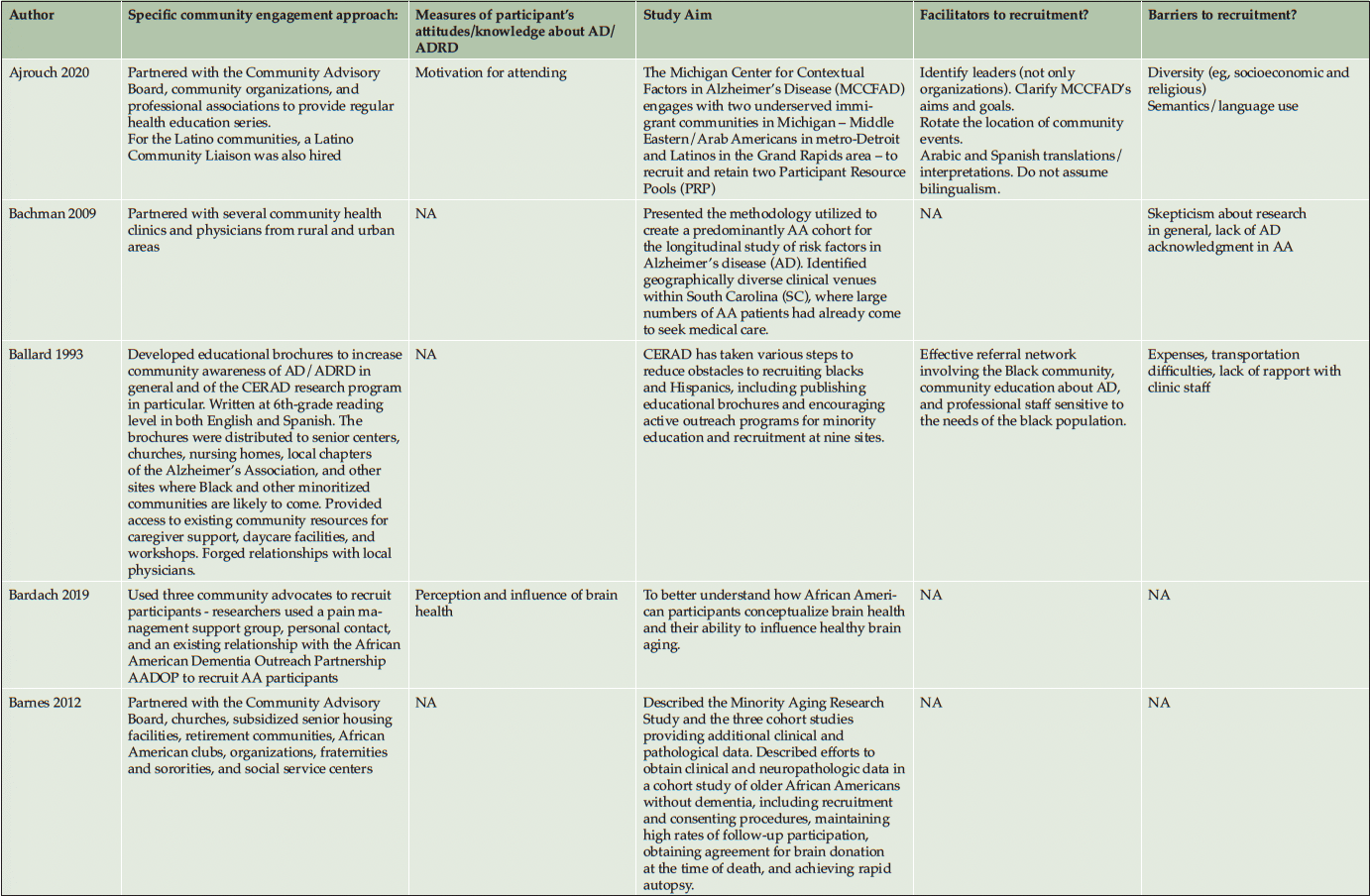
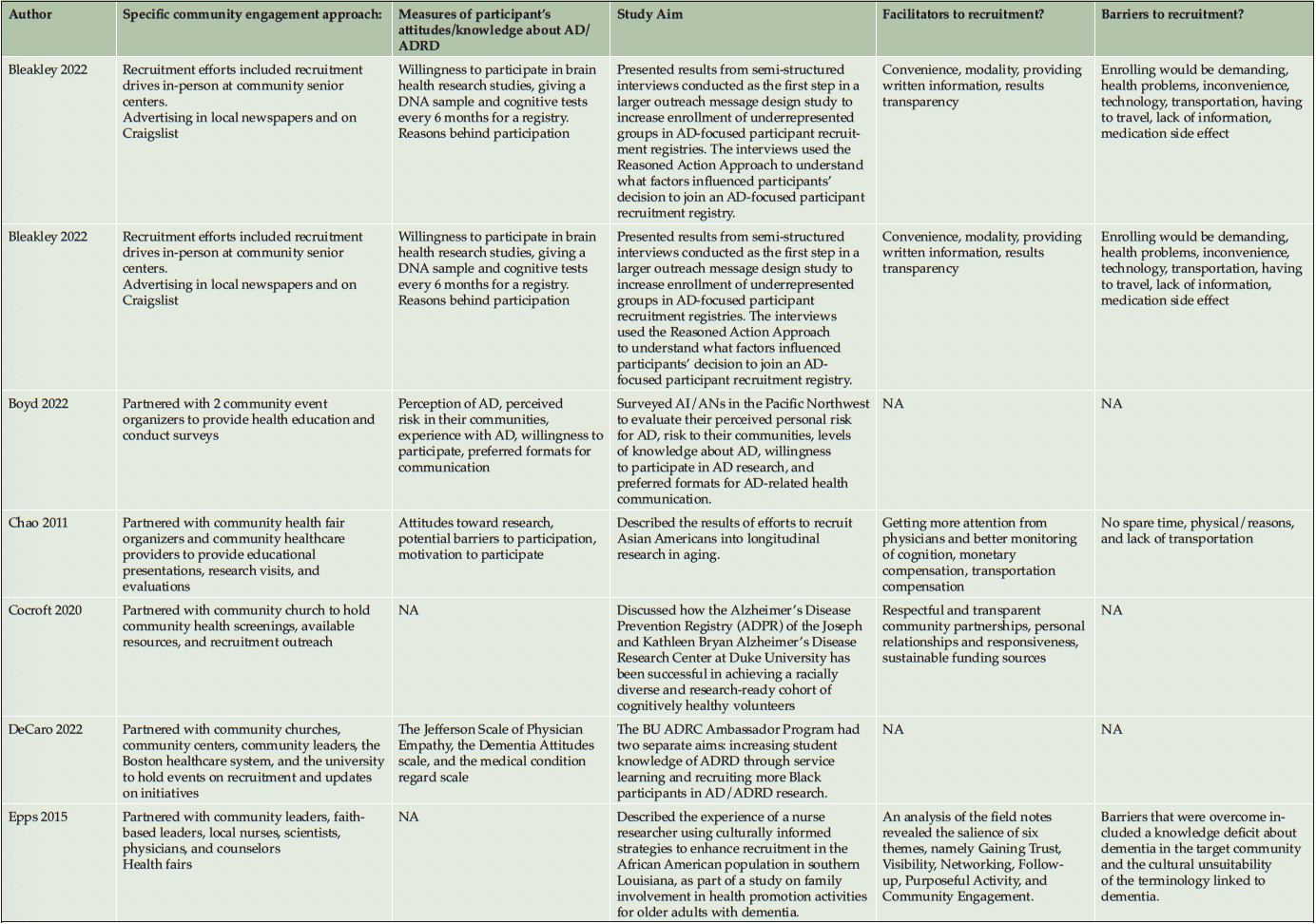
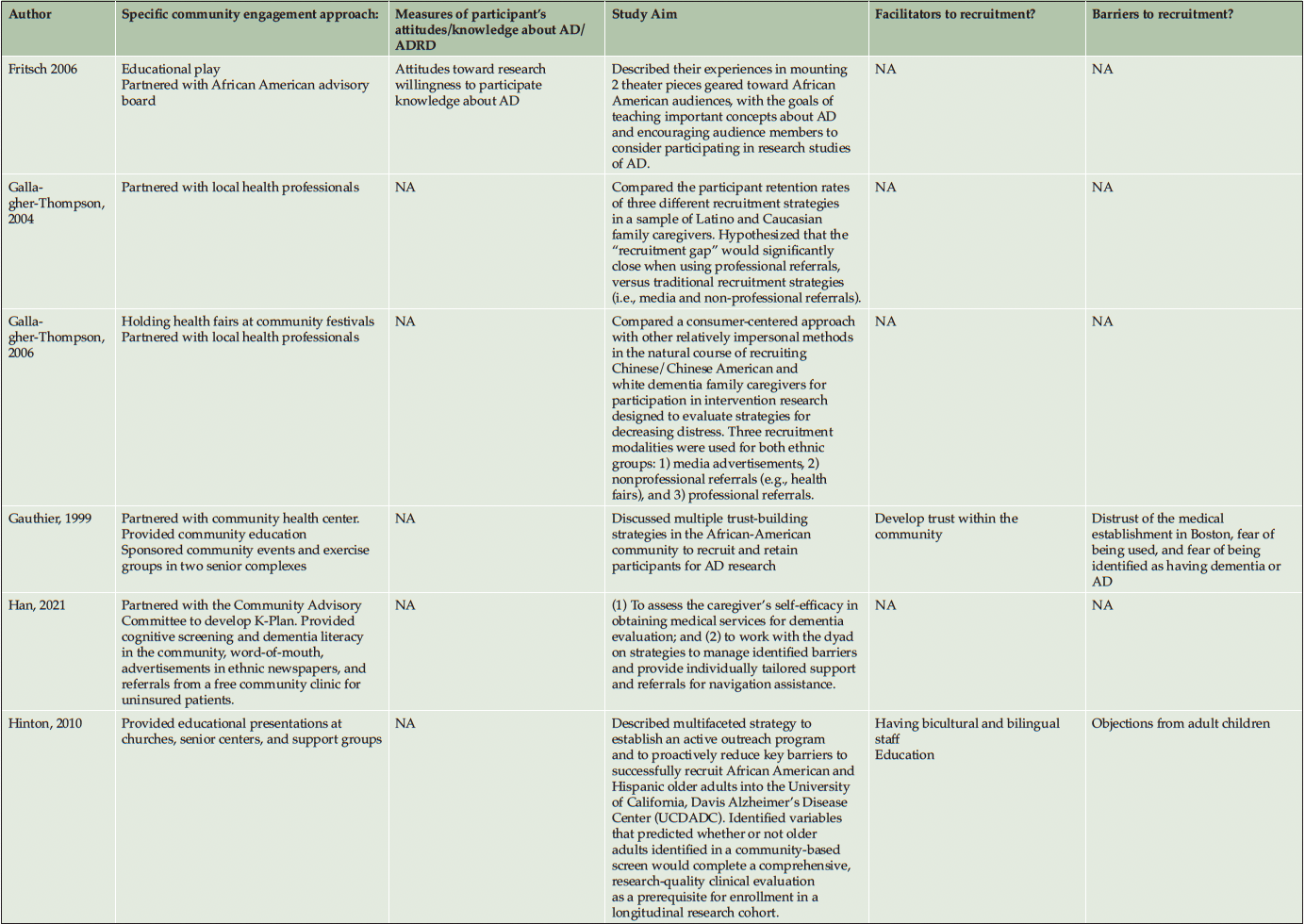
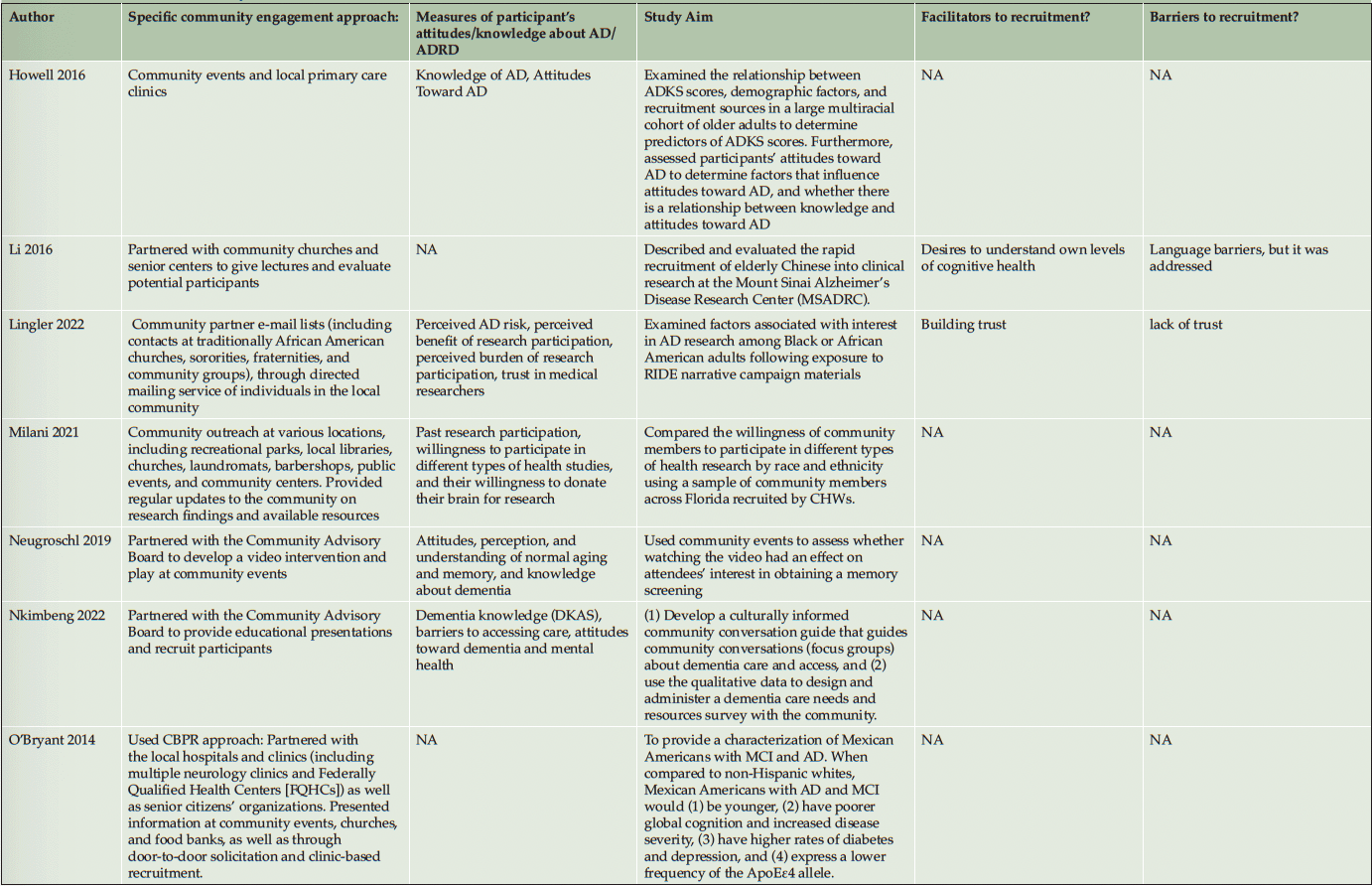
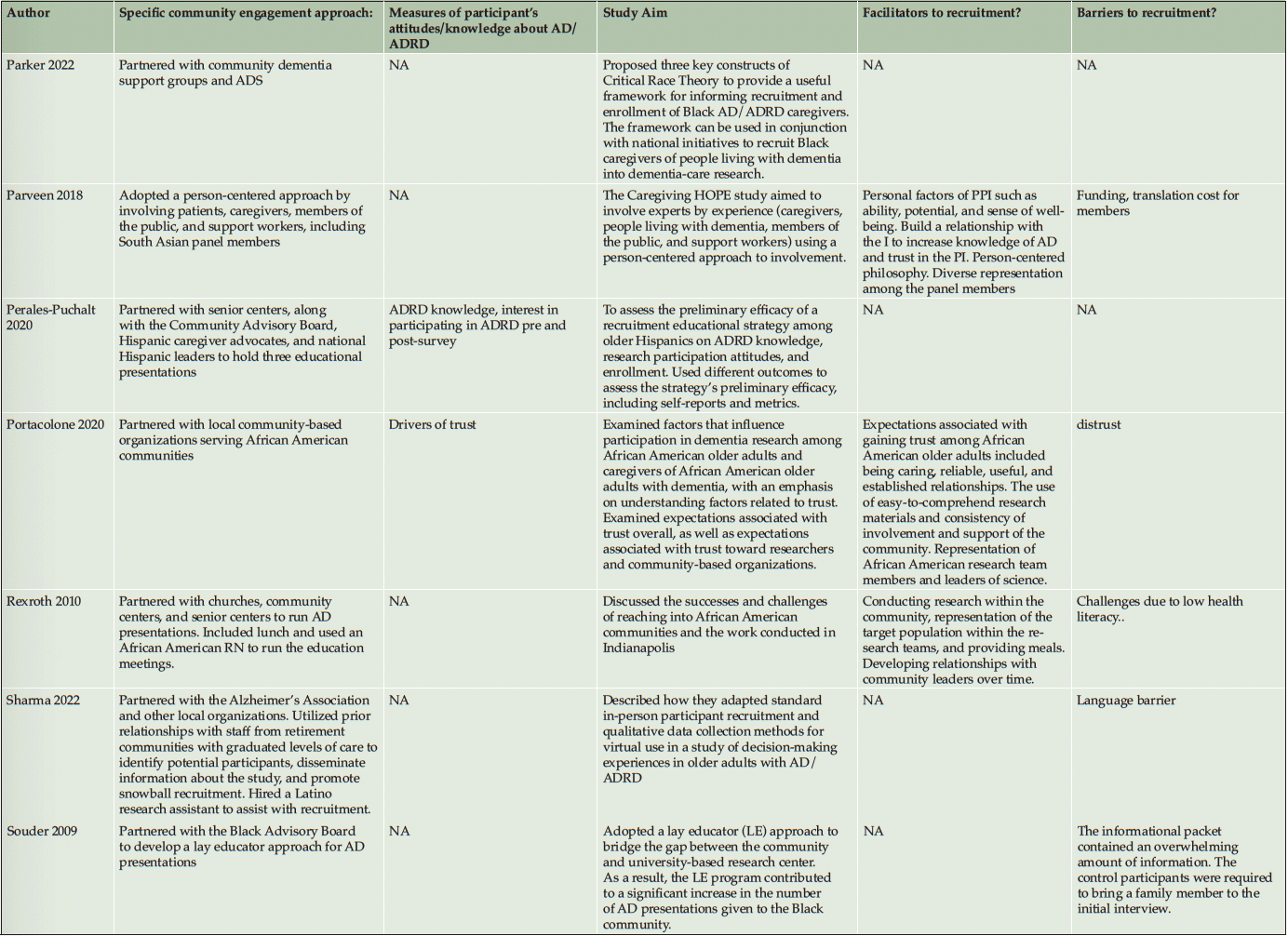
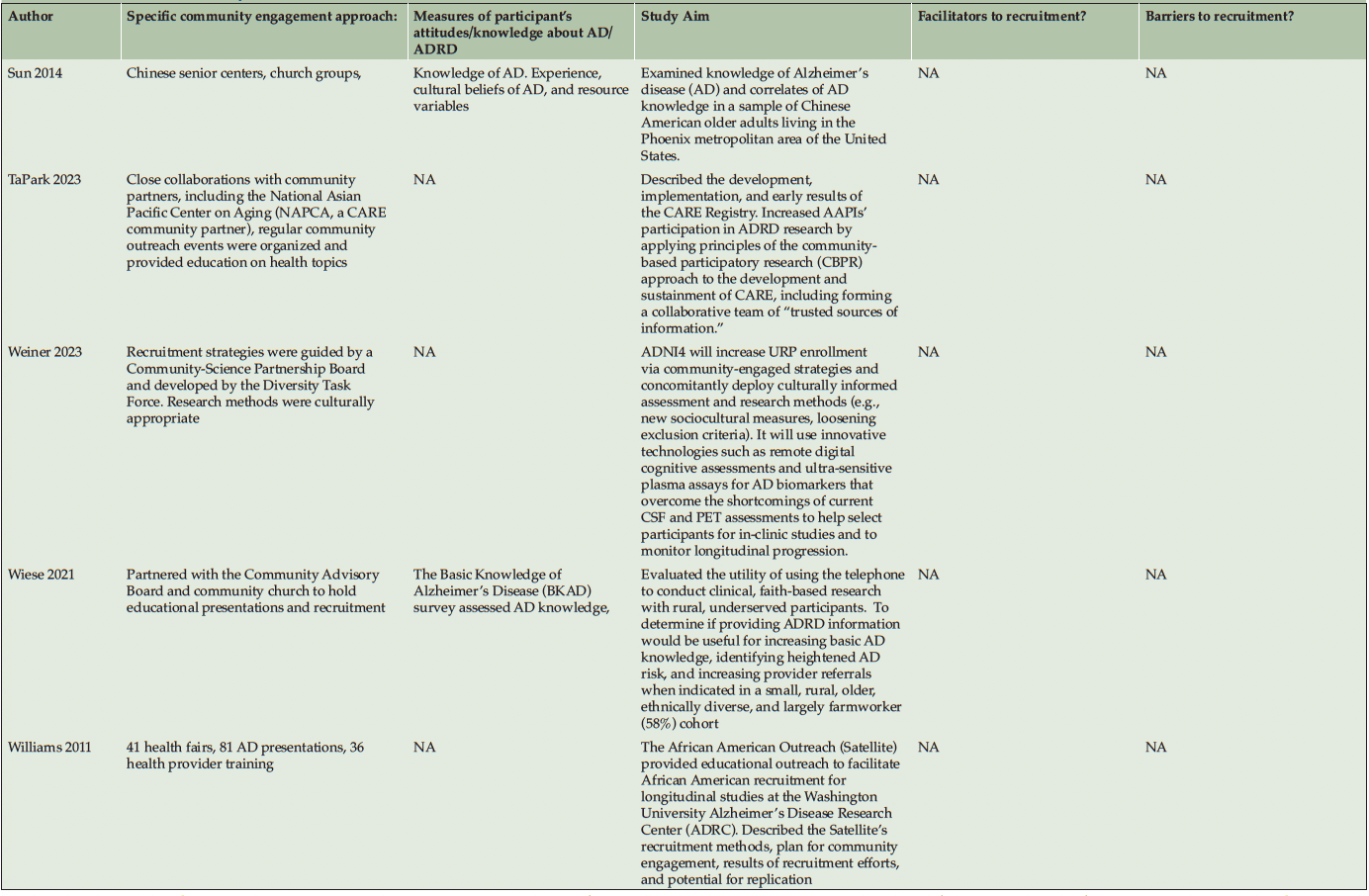
Ten studies (20%) utilized a qualitative approach (5, 41-49) without numeric outcomes, employing methods such as in-depth interviews, case studies, or written reports. Thirty studies (61%) used a quantitative approach (14, 50-78) with at least one numeric outcome. Five articles (10%) were descriptive studies, such as research protocols (79-83). There were four (8%) mixed-method studies (84-87). Of the 49 studies, 24 (49%) collected participants’ attitudes and beliefs about AD/ADRD and research participation. Thirty-nine (80%) studies used more than one recruitment approach. For instance, 21 studies (43%) participated in community events collaborating with community faith-based groups or local health professionals. Ten (20%) studies formed partnerships with a Community Advisory Board and another community organization, such as a church or a local health clinic, to create educational programs at churches, senior centers, and community events. Twenty-four studies (49%) reported a measure of participants’ attitudes and knowledge about AD/ADRD research. Fourteen studies (29%) identified barriers or facilitators to recruiting racial and ethnic minoritized populations, delineated by study participants (5 studies, 36%), community partners/liaisons (3 studies, 6%), or the investigators (6 studies, 12%). The characteristics of these studies are included in the following section and summarized in Table 1 and Table 2.
• African American = AA, Non-Hispanic White = NHW
1. Clinical trials or studies that assessed participants’ attitudes or perceptions regarding AD/ADRD clinical trial participation; 2. Non-Clinical Trials/Observational Studies/Descriptive Studies or studies that assessed participants’ attitudes or perceptions regarding AD/ADRD research participation
Data synthesis
Geographical Location
The locations of the studies varied. Eleven studies (22%) did not disclose the location of recruitment and enrollment of sample populations. We found only one study in the UK (46) that focused on racial and ethnic minoritized communities outside of the US. The distribution varied widely, with a large portion of the studies (30%) conducted in California. Some places were in major urban centers such as New York City, Los Angeles, and Chicago), while others were in a mix of urban and rural areas (Kentucky, central Texas). Only six studies (12%) described the location of their recruitment efforts as rural areas, with 80% reporting the community church as their leading community partner (43, 73, 76, 78) to engage rural communities. In addition to this partnership, studies in rural areas used educational programs (73, 76, 86, 78) as community outreach, forged more relationships with local health professionals (78, 43) for referrals, and participated in health fairs (78,4 3). Among studies that described their recruitment location as urban (32 studies, 65%), 55% participated in health fairs and other community events, 45% used education as an outreach strategy, 33% partnered with local health professionals and local health clinics, and 30% formed relationships with community churches, and 24% developed their community-based recruitment strategies in partnership with their Community Advisory Boards. Locations in the Intermountain West and part of the Midwest of the USA were not represented among the studies reviewed. The approximate area of recruitment efforts is available in the supplemental file, indicating where studies reported their geographic locations.
Characteristics of studies target populations
Of 49 studies, 23 (47%) focused on more than one racial and ethnic minoritized population, with 13 studies (23%) also recruiting non-minoritized individuals (Non-Hispanic White adults). Most studies reported on recruiting African American adults in both clinical (8 studies, 73%) and observational engagement (20 studies, 53%) categories. Three studies (27%) reported on Hispanic/Latino adults in clinical engagement, and 12 studies (32%) in observational engagement. Two studies (18%) reported on Asian participants in clinical engagement, and nine studies (24%) in observational engagement. Five studies (13%) reported on Native American/Alaskan Native or Pacific Islander populations among observational engagement.
Thirty studies (61%) reported that most participants identified as female. Eight studies (16%) did not report the sex of the participants. Overall, we observed that aside from race and ethnicity, other attributes of the target populations, such as education, age, sex, occupation, and income, were not consistently described. Only 31 studies (63%) reported educational attainment and 34 studies (69%) reported their participants’ age/age group. Table 1 summarizes the characteristics of the sample populations.
Community-based recruitment strategies
Recruitment strategies were variable, but 21 studies (43%) used community outreach in the form of educational presentations to raise community awareness and recruit participants. Twenty-one studies (43%) used different types of community events, including health fairs (3, 41, 83, 43, 50, 49, 51, 56, 58, 60, 62, 71, 74), cultural events (55, 63, 50, 66), and community fairs (47, 48, 81, 71), to raise awareness and enroll participants. Thirteen studies (27%) reported partnering with the local clinic, such as free community clinics (61), neurology clinics and Federally Qualified Health Centers (78), local wellness clinics (50), neighborhood health centers (81, 79, 63), community health professionals (49, 72, 3, 43, 79, 56), local chapter of Alzheimer’s Association, Alzheimer’s Disease Research Center (ADRC; 83), and other local healthcare agencies (60) as another commonly reported recruitment approach. Ten studies (20%) partnered with a local Community Advisory Board to develop recruitment material or to disseminate information. Among partner organizations, study investigators engaged with local businesses, community care programs, community liaisons, African American clubs, community support groups, senior centers, local media, and libraries. A large proportion of the studies (16 studies, 33%) described partnering with community faith-based organizations to disseminate study materials, hold educational presentations or identify potential participants.
Among our 11 reviewed articles in the clinical engagement category, five implemented a nonpharmaceutical intervention (e.g., lifestyle physical activity (58), cognitive training (68, 50)) that also included caregivers as their target populations. For instance, McDougall (50) partnered with Hispanic senior activity centers, churches, health fairs, and festivals to increase African American and Hispanic participation. At seven community sites, they randomly assigned participants to either a self-efficacy memory intervention or a comparison group with structured lectures on health improvements. In another study, Overman et al. (68) collaborated with a local church and adult center to focus on African American recruitment. They tested a game-based intervention to improve the neuropsychological health of older adults. Etkin et al. (58) implemented community participatory research and partnered with a faith-based and other senior service organization to increase the number of African American, Hispanic/Latino, and Asian American participants in their caregiver lifestyle physical activity clinical trial. Meyer et al. (66) collaborated with a local church and Vietnamese physicians, actively engaging in health fairs and Lunar festivals to host educational presentations aimed at recruiting Vietnamese American participants. Their objective was to implement a single-arm pretest-posttest design to test a psychosocial intervention for Vietnamese American caregivers of individuals with AD/ADRD. Samus et al. (72) analyzed a multifaceted community-based recruitment approach involving gatekeepers and community outreach to enroll racial and ethnic minoritized populations into an 18-month randomized controlled dementia care coordination trial. The rest of the clinical engagement studies assessed attitudes and willingness toward clinical trial participation. For instance, Romero et al. (71) held community events for educational presentations at health fairs, family reunions, civic groups, medical centers, and senior housing facilities to increase African American participants in the ADPR registry and assessed participants’ interest in a pharmacological prevention trial.
As described in the previously mentioned articles, the majority of clinical engagement studies (10 studies, 90%) employed multiple community-based recruitment strategies. Participating in community events emerged as the most frequently used approach (7 studies, 64%), followed by community church partnership (5 studies, 45%), educational presentations (5 studies, 45%), collaboration with local health professionals and health clinics (3 studies, 27%) and engagement with Community Advisory Boards (1 study, 9%). In contrast, studies in the observational engagement category prioritized educational presentations (15 studies, 39%) and participating in community events (15 studies, 39%), followed by collaboration with community churches (11 studies, 29%), health professionals or community health clinics (11 studies, 29%), and community advisory boards (9 studies, 24%).
Table 2 lists specific community-engaged recruitment strategies for each study. Recruitment strategies across racial and ethnic groups exhibited a high degree of consistency, indicating minimal variation among the groups. However, among the reviewed observational studies, recruitment materials were developed in different languages, such as Chinese (56, 85, 60, 64), Spanish (14, 51, 76, 80, 62, 70), and Arabic (51), to recruit Asian American, Hispanic/Latino, and Middle-Eastern participants. Including bilingual research team members (45, 64, 85) was another approach specific to engaging Hispanic/Latino and Asian American participants.
Recruitment approach evaluation
Twenty-five studies (51%) reported the impact of their recruitment approaches. However, the evaluation method or the specification of a “successful’’ recruitment approach was not consistent across the clinical or observational engagement studies. Overall, only six studies (12%) compared different recruitment methods to report which approach yielded the highest number of enrollments. Compared to traditional recruitment strategies such as media and non-professional referrals, forming collaborative relationships with community healthcare agencies showed an increase in the recruitment of Hispanic/Latino (14) and Chinese American (60) individuals. Meyer et al. (66) compared different outreach efforts, such as community partner agencies, community festivals, community presentations, and other types of effort, such as word-of-mouth or referrals. Their findings showed community partner agencies produced the majority of study referrals (66). Zhou et al. (77) compared different recruitment outcomes from community talk, ADRC Registry, community liaison, community referrals, Banner Alzheimer’s Prevention Initiative Registry, caregiver support program, and clinical referrals, and found that through community liaison, they were able to recruit more African American participants. Samus et al. (72) compared five recruitment strategies: community liaison, community organizations that either sent letters about the study or distributed study materials, Johns Hopkins dementia research registries, and general community outreach. Their findings showed that the majority of African-American participants were referred by their community liaison. Thirteen studies (26%) monitored how many racial and ethnic minoritized participants they recruited and contributed to the success of the recruitment strategy. Finally, six studies (12%) reported that they either met their recruitment target or surpassed their goal.
AD/ADRD clinical trial recruitment has rigorous inclusion criteria compared to AD/ADRD observational research. Therefore, recruitment approaches that are successful in engaging racial and ethnic minoritized communities may not necessarily work in AD/ADRD clinical trials. However, the limited evidence that we reviewed demonstrated that through multiple community engagements with community liaisons such as community churches and community health clinics, and attending community events such as health fairs, researchers met/surpassed their target racial and ethnic recruitment goal (58, 72) or they evaluated community-engaged recruitment method by comparing their outreach with other types of recruitment approaches (53, 66, 71).
Most studies did not describe using theory to guide their recruitment strategies. Eight studies (16%) reported using frameworks with observable patterns, such as adopting recruitment approaches that are culturally sensitive, engaging the community, and having tailored messaging. Among clinical engagement studies, Samus et al. (72) crafted a recruitment methodology anchored in community-based participatory research (88) and gatekeeper outreach models (89), which cultivated active participation and trust within local communities. Etkin et al. (58) combined social marketing principles with community-based participatory research (88), utilizing precise messaging and community engagement to improve recruitment efforts. Shaw et al. (73) devised a culturally-tuned educational program through the Cultural Accommodation Model, recognizing elements such as religion, spirituality, and diet as key to involving African American communities.
Among observational engagement studies, Bleakley et al. (5) adopted the Reasoned Action Approach ((RAA (90)) to identify cognitive and motivational determinants that affect individuals’ decisions to participate in AD/ADRD studies. Wiese et al. (76) used the faith-based participatory model (FMM) to involve religious leaders in shaping programs to fit the congregation’s needs and values. They also trained community members to serve as faith-based health educators and utilized local pictures, resources, and language to ensure the material was culturally appropriate. Parker et al. (69) implemented Critical Race Theory in devising recruitment methods for African American/Black caregivers of individuals with AD/ADRD, aiming to counteract systemic racial inequities in healthcare by using culturally appropriate and customizable recruitment materials to reach and recruit racial and ethnic minoritized communities. Sun (85) applied the Explanatory Model (91) and the Common-Sense Model (92) to gain insights into how individuals perceive disease, blending lay health education and cultural considerations into recruitment practices. Lastly, Parveen et al. (46) based their recruitment strategy on the concept of patient and public involvement, proposed by Arnstein (93) and Morrow et al. (94), ensuring that collaboration with caregivers, those with dementia, and community experts was central.
Moreover, only a few clinical (44, 58, 66, 71, 72, 77) and observational studies (14, 39, 45, 55, 56, 59, 66, 78, 80, 96, 97) reported whether the relationships researchers formed with the community were new or existing prior to their research projects. However, the number of articles with existing community partnerships (11 studies, 22%) exceeded the number of newly formed relationships (7 studies, 14%).
Participants’ attitudes and perceptions
Out of 24 studies (49%) that measured participants’ attitudes toward dementia and AD/ADRD research, five (20%) were clinical engagement studies (44, 53, 66, 71, 73). The specific assessments were the basic knowledge of AD (BKAD) (96), 30-item Alzheimer’s disease knowledge scale (ADKS) (97), 13-item epidemiology/etiology disease scale (EDS) (98), dementia knowledge (DKAS)(99), barriers to accessing care, drivers of trust, trust in medical researchers, perceived risk for AD/ADRD, perceived benefits from participation, cultural beliefs about AD (CBAD)(86), willingness to participate, willingness to donate brain for research, motivation to participate, and preferred formats for communication. Many of the elicited attitudes toward dementia in all racial and ethnic minoritized groups suggested that knowledge of AD was lacking (43, 44, 55, 63, 82, 84-86, 70, 77). In contrast to non-Hispanic White individuals, racial and ethnic minoritized communities exhibited a lower perceived risk of AD/ADRD. For example, only half of AI/AN participants thought AD/ADRD was a major health problem among AI/ANs, while 77% of the participants viewed AD/ADRD as a major health problem for the general public (55). In older Chinese American adults, being male and having lower education levels were related to less AD/ADRD knowledge (85). Similarly, African American participants had a lower AD/ADRD knowledge compared to White participants, and AD knowledge was also associated with education and attitudes toward AD/ADRD prevention and treatment (63). Hispanic/Latino participants also reported a lack of knowledge, fear of receiving a diagnosis, and cultural stigma regarding research participation as their barriers to joining either an observational (52, 62) or clinical trial study (44).
As expected, distrust of the research community (47, 65, 79, 81, 95), knowledge deficits (5, 43, 79, 82, 52, 44), and logistical issues such as transportation, schedule conflict, or lack of time were among the highest reported barriers in the studies (5, 41, 56, 80, 46, 87, 95). Other widely cited barriers were stigma (86, 95), health challenges (5, 41, 56, 52), and language barriers (48, 51, 64). Barriers specific to reviewed clinical trial studies from the research teams’ perspectives included difficulties in engaging physicians, bureaucratic hurdles in larger medical centers, reliance on adult day program staff for family contact, working with distant caregivers, and financial constraints hindering agency support (58). Among observational studies, researchers reported objections from adult children and their decision to veto parents’ enrollment in the study (62).
Among the observational and clinical trial articles, some barriers were addressed by attempts to overcome the broader institutional and cultural issues. Engaging in strategies to improve trust appeared to be the most important facilitator, especially for African American participants. In a qualitative study (47), African American participants suggested researchers and academic institutions invest in the health and well-being of African American communities, involve African American researchers in research teams, and enhance information sharing between research institutions and African American communities to gain their trust. Community education and enhancing AD/ADRD knowledge by increasing diverse representation in the research teams, addressing community needs, and using culturally appropriate and easy-to-comprehend recruitment materials also seemed to facilitate the recruitment approach. Ballard et al. (1993) (80) reported that involving the African American community, community education about AD/ADRD, and research teams being sensitive to the needs of the African American community were the key facilitators of increasing enrollment over a two-year period of active recruitment. Similarly, McDougall et al. (2010) (95) used culturally tailored cognitive instruments, culturally appropriate health topics, reducing the cultural stigma of dementia, adaptive performance testing, and scheduling as essential facilitating strategies to overcome mistrust and increase Hispanic/Latino and African American participation. Table 2 lists all barriers and facilitators that were reported in the studies.
Data Quality
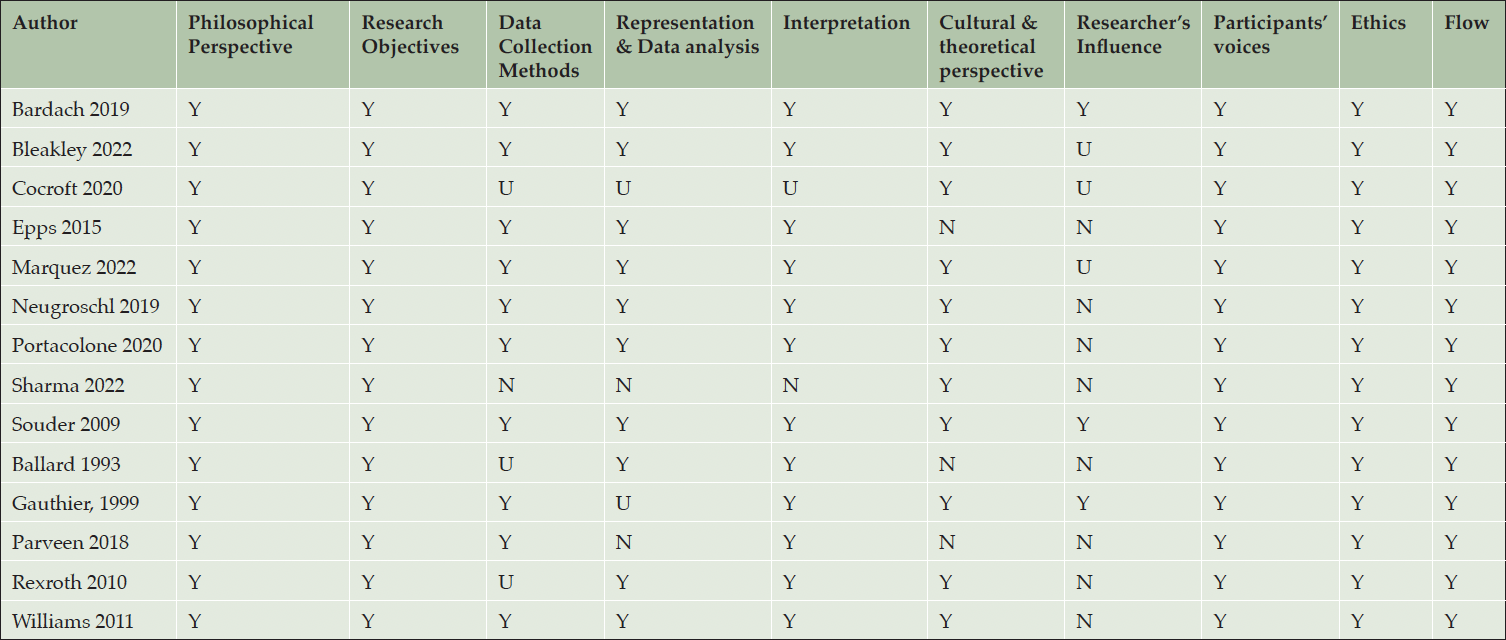
The quality of studies varied. We used the Joanna Briggs Institute checklist to appraise the methodological quality of qualitative or descriptive studies. All studies stated the research methodology and objectives, represented participants’ voices, had evidence of ethical approval, and conclusions appeared to be based on analysis or interpretation of the data. Out of 14 studies (29%), two met all ten quality criteria. Six studies (12%) met nine criteria, two (4%) met eight criteria, and the remaining four (8%) met at least four criteria. The majority of articles lacked clarity regarding the researchers’ cultural or theoretical orientations and did not acknowledge the potential bidirectional influence between the researchers and their studies. Additionally, there was a discrepancy between the selected research methodologies and the representation of the analyses (see Table 3).
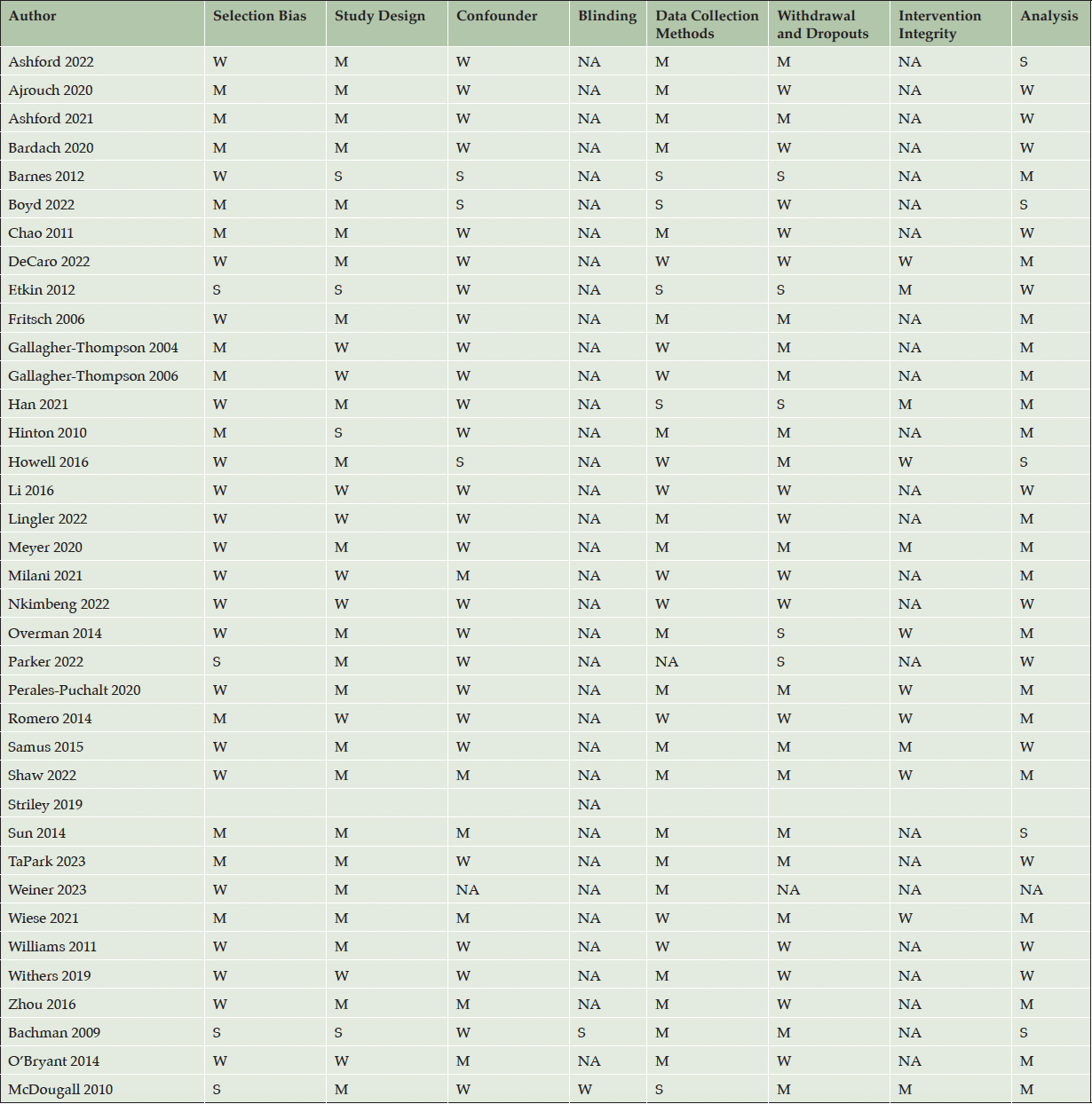
We utilized the Quality Assessment Tool for quantitative studies. The majority of 36 quantitative studies received a «weak» rating due to high risk of bias, including selection bias (n = 20, 56%), study design (n = 9, 25%), confounders (n = 26, 72%), data collection method (n = 10, 28%), withdrawal and dropouts (n = 14, 39%), intervention integrity (n = 7, 19%), and analysis (n= 12, 33%). Tables 3 and 4 demonstrate the results of quality assessments.
Y= Yes, N= No, U= Unclear; Philosophical Perspective: Is there congruity between the stated philosophical perspective and the research methodology? Research Objectives: Is there congruity between the research methodology and the research question or objectives?; Data Collection Methods: Is there congruity between the research methodology and the methods used to collect data?; Representation & Data analysis: Is there congruity between the research methodology and the representation and analysis of data?; Interpretation: Is there congruity between the research methodology and the interpretation of results?; Cultural & theoretical perspective: Is there a statement locating the researcher culturally or theoretically?; Researcher’s Influence: Is the influence of the researcher on the research, and vice-versa, addressed?; Participants’ voices: Are participants, and their voices, adequately represented?; Ethics: Is the research ethical according to current criteria or, for recent studies, and is there evidence of ethical approval by an appropriate body?; Flow: Do the conclusions drawn in the research report flow from the analysis, or interpretation, of the data?
Strong = S, Moderate = M, Weak = W
Discussion
Our rapid review suggested that strategies were variable among clinical trials and observational studies that utilized community-based recruitment approaches. Efforts to increase AD/ADRD awareness and recruitment outcomes by participating in community events, such as health fairs, were predominantly used in clinical trials. Whereas, efforts aimed at enhancing AD/ADRD knowledge through providing community educational presentations and participating in community events were equally employed by observational studies. Most of the included studies formed alliances with community faith-based organizations and some with community health professionals to recruit racial and ethnic minoritized populations. However, the limited evidence that we reviewed suggested that clinical trials did not report establishing relationships with local health professionals/health clinics as frequently as they did with faith-based organizations. Indeed, difficuly in engaging with community physicians was one of the noted barriers in our reviewed studies. Collaboration with local healthcare providers can enhance clinical trial awareness and may also reduce the distrust in pharmacological interventions if the referrals come from local physicians with existing relationships with the community.
Evidence of recruitment success can be challenging when using a community-based approach, as most studies can conduct multiple concurrent recruitment strategies. Therefore, teasing which strategy yielded the best recruitment outcome may be difficult. The absence of a “best” single strategy suggests that a multifaceted approach to recruitment, incorporating various strategies, may be more appropriate. Approximately half of the reviewed studies evaluated their recruitment strategies, with varying evaluation methods reported for “successful” community-based approaches. Comparison of recruitment methods for enrollment rates was limited. Monitoring numbers of recruitment of racial and ethnic minoritized participants was more common as a metric of success, and several studies reported they met or exceeded their recruitment targets. A commonality observed among these studies was the utilization of multiple recruitment strategies. Rigorous randomized community-based recruitment strategies should compare and identify the most successful approach to recruiting racial and ethnic minoritized communities in AD/ADRD clinical trials. Community-based approaches require building relationships between the research team and community members, which requires time and effort. However, studies are mandated by sponsors to submit summary results within a certain time period. Given the fact that community-based recruitment outcomes may not immediately occur, this becomes specifically challenging for clinical trial studies as they have enrollment timelines, and thus, evaluating what efforts encouraged participation in a rigorous way becomes additionally burdensome.
Multiple studies reported utilizing existing or new relationships within the community they were attempting to recruit. However, most studies did not explicitly describe whether their relationship with their focus community organizations or gatekeepers was new or whether they had an existing rapport. Understanding the nature of the relationship with the community would provide insights into the optimal community-based recruitment approach conducive to fostering favorable recruitment outcomes. Based on our findings, quantitative studies had a substantial risk of selection bias, study design, lack of consideration of confounders, and insufficient description of attrition. Furthermore, we observed that the majority of reviewed AD/ADRD observational and clinical trial studies provided a brief description of the recruitment strategies. As researchers strive for greater inclusivity in recruiting older adults, it’s important for the guidance around recruitment reports and metrics to reflect the needs of diverse communities and strive for equitable representation in clinical research. Furthermore, frameworks and theory-based models can help guide researchers and readers to better understand the recruitment methodology and advance community-based recruitment science.
Although the majority of reviewed clinical trials and observational studies reported the approximate geographical areas of recruitment efforts, 22% of the studies did not report this information. Geographical locations, characteristics of the research area, and whether it is a rural or urban area can provide additional insights about socio-ecological factors influencing participants’ willingness to join AD/ADRD research. Our reviewed studies suggested that there may be a lack of geographical diversity, which can also limit the generalizability of recruitment outcomes. To facilitate increasing representation of racial and ethnic minoritized community members in AD/ADRD clinical trials, we need additional evidence of protocols and community-based recruitment strategies to expound on the success of community-based recruitment methodology. Implementing pragmatic clinical trial designs that are embedded in healthcare systems (100) could facilitate increased access to racial and ethnic minoritized communities that are hardly reached by researchers but exist in the healthcare systems. Overall, evidence-based and culturally sensitive recruitment approaches could benefit the external validity of studies.
Limitations
The current rapid review should be interpreted in the context of several limitations. Using one reviewer to ensure data extraction and quality assessment in a short turnaround time would have introduced biases. Such a design can increase susceptibility to selection bias, potential overlook of relevant studies, and incomplete evidence synthesis. The absence of quality evaluation by another reviewer is indeed a drawback. The rapid nature of the current review may have compromised rigor, potentially resulting in a trade-off between efficiency and comprehensiveness. Despite this limitation, this rapid review adhered to the systematic review protocol and followed the steps from study identification across several databases to quality assessment, all accomplished within a short timeframe.
Conclusion
This review identified a number of recruitment strategies that utilized community-based approaches to increase the participation of minoritized communities. We found that using a multiprong recruitment approach that addresses barriers such as building trust by forming coalitions and partnerships with community-based organizations, using culturally sensitive strategies, and being receptive to the community’s needs may yield more fruitful recruitment outcomes. Such recruitment efforts can simultaneously address multiple levels of individual and institutional barriers. It is imperative that AD/ADRD clinical investigators continue their efforts to increase recruitment and engagement of racial and ethnic diversity in AD/ADRD clinical trials, as including such minoritized populations would yield more information on prospective variation in intervention outcomes. Our study reviewed AD/ADRD research studies that utilized community-based recruitment strategies to increase the representation of racial and ethnic minoritized populations with limited evidence of community engagement in recruitment in AD/ADRD clinical trials. Publishing clinical trial protocols and feasibility studies can increase evidence of community-based recruitment science. In addition, incorporating recruitment evaluation into AD/ADRD clinical trial protocols would enhance consistency in recruitment methodologies and facilitate systematic data collection on best practices.
With more evidence of community engagement as a viable recruitment strategy to increase racial and ethnic representation in AD/ADRD clinical trials, we can advance recruitment science by establishing a standardized community-based recruitment methodology.
Acknowledgements: This work was supported by an award from the American Heart Association Grant #946223/ SFRN Center Science of Diversity in Clinical Trials/2022. This work is part of the Alzheimer’s Trial Recruitment Innovation Lab (ATRIL), a collaboration between the USC Alzheimer’s Therapeutic Research Institute, the USC Schaeffer Center for Health Policy and Economics, and Howard University; part of Strategically Focused Research Network on the Science of Diversity in Clinical Trials.
Funding: This work was supported by an award from the American Heart Association Grant #946223/ SFRN Center Science of Diversity in Clinical Trials/2022. The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript. Open access funding provided by SCELC, Statewide California Electronic Library Consortium.
Conflict of interest: On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standards: The current systematic rapid review did not have direct access to participants of primary research studies included in the review. However, all reviewed studies reviewed reported that they had obtained written informed consent from their participants.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Andrieu S, Coley N, Gardette V, Subra J, Oustric S, Fournier T, et al. Representations and practices of prevention in elderly populations: investigating acceptance to participate in and adhesion to an intervention study for the prevention of Alzheimer’s disease (ACCEPT study)–the need for a multidisciplinary approach. J Nutr Health Aging. 2012;16(4):352-4. doi: 10.1007/s12603-012-0045-9. PubMed PMID: 22499457.
2. Coley N, Coniasse-Brioude D, Igier V, Fournier T, Poulain JP, Andrieu S. Disparities in the participation and adherence of older adults in lifestyle-based multidomain dementia prevention and the motivational role of perceived disease risk and intervention benefits: an observational ancillary study to a randomised controlled trial. Alzheimers Res Ther. 2021;13(1):157. Epub 20210924. doi: 10.1186/s13195-021-00904-6. PubMed PMID: 34560903; PubMed Central PMCID: PMC8464095.
3. Cocroft S, Welsh-Bohmer KA, Plassman BL, Chanti-Ketterl M, Edmonds H, Gwyther L, et al. Racially diverse participant registries to facilitate the recruitment of African Americans into presymptomatic Alzheimer’s disease studies. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2020;16(8):1107-14. doi: 10.1002/alz.12048. PubMed PMID: 32543781.
4. Brown P, Tan AC, El-Esawi MA, Liehr T, Blanck O, Gladue DP, et al. Large expert-curated database for benchmarking document similarity detection in biomedical literature search. Database-the Journal of Biological Databases and Curation. 2019. doi: 10.1093/database/baz085. PubMed PMID: WOS:000494411700001.
5. Bleakley A, Maloney EK, Harkins K, Nelson MN, Akpek E, Langbaum JB. An Elicitation Study to Understand Black, Hispanic, and Male Older Adults’ Willingness to Participate in Alzheimer’s Disease-Focused Research Registries. Journal of Alzheimers Disease. 2022;88(4):1499-509. doi: 10.3233/jad-220196. PubMed PMID: WOS:000842012100021.
6. Canevelli M, Bruno G, Grande G, Quarata F, Raganato R, Remiddi F, et al. Race reporting and disparities in clinical trials on Alzheimer’s disease: A systematic review. Neuroscience & Biobehavioral Reviews. 2019;101:122-8. doi: https://doi.org/10.1016/j.neubiorev.2019.03.020.
7. Grill JD, Raman R, Ernstrom K, Aisen P, Karlawish J. Effect of study partner on the conduct of Alzheimer disease clinical trials. Neurology. 2013;80(3):282-8. Epub 20121219. doi: 10.1212/WNL.0b013e31827debfe. PubMed PMID: 23255824; PubMed Central PMCID: PMC3589183.
8. Kennedy RE, Cutter GR, Wang G, Schneider LS. Challenging Assumptions About African American Participation in Alzheimer Disease Trials. Am J Geriatr Psychiatry. 2017;25(10):1150-9. Epub 20170425. doi: 10.1016/j.jagp.2017.04.013. PubMed PMID: 28554539; PubMed Central PMCID: PMC5842064.
9. Krogstad JM. Key facts about U.S. Latinos for national Hispanic Heritage Month. In: Passel JS, editor. Noe-Bustamante, L. Pew Research Center2022.
10. Hispanic Population to Reach 111 Million by 2060. 2017 National Population Projections and Vintage 2017 Population Estimates: Census.gov; 2018.
11. S C. Projections of the Size and Composition of the U.S :2014-2060. In: J O, editor. United States Census Bureau.2015.
12. Williams MM, Scharff DP, Mathews KJ, Hoffsuemmer JS, Jackson P, Morris JC, et al. Barriers and facilitators of African American participation in Alzheimer disease biomarker research. Alzheimer Disease and Associated Disorders. 2010;24(SUPPL. 1):S24-S9. doi: 10.1097/WAD.0b013e3181f14a14.
13. Grill JD, Karlawish J. Addressing the challenges to successful recruitment and retention in Alzheimer’s disease clinical trials. Alzheimers Res Ther. 2010;2(6):34. Epub 20101221. doi: 10.1186/alzrt58. PubMed PMID: 21172069; PubMed Central PMCID: PMC3031880.
14. Gallagher-Thompson D, Singer LS, Depp C, Mausbach BT, Cardenas V, Coon DW. Effective recruitment strategies for Latino and Caucasian dementia family caregivers in intervention research. Am J Geriatr Psychiatry. 2004;12(5):484-90. doi: 10.1176/appi.ajgp.12.5.484. PubMed PMID: 15353386.
15. Raman R, Quiroz YT, Langford O, Choi J, Ritchie M, Baumgartner M, et al. Disparities by Race and Ethnicity Among Adults Recruited for a Preclinical Alzheimer Disease Trial. JAMA Netw Open. 2021;4(7):e2114364. Epub 20210701. doi: 10.1001/jamanetworkopen.2021.14364. PubMed PMID: 34228129; PubMed Central PMCID: PMC8261604.
16. Deters KD, Napolioni V, Sperling RA, Greicius MD, Mayeux R, Hohman T, et al. Amyloid PET Imaging in Self-Identified Non-Hispanic Black Participants of the Anti-Amyloid in Asymptomatic Alzheimer’s Disease (A4) Study. Neurology. 2021;96(11):e1491-e500. Epub 20210210. doi: 10.1212/wnl.0000000000011599. PubMed PMID: 33568538; PubMed Central PMCID: PMC8032379.
17. Galvin JE, Meuser TM, Boise L, Connell CM. Predictors of physician referral for patient recruitment to Alzheimer disease clinical trials. Alzheimer Dis Assoc Disord. 2009;23(4):352-6. doi: 10.1097/WAD.0b013e31819e0cac. PubMed PMID: 19561438; PubMed Central PMCID: PMC2787738.
18. Watson JL, Ryan L, Silverberg N, Cahan V, Bernard MA. Obstacles and opportunities in Alzheimer’s clinical trial recruitment. Health affairs (Project Hope). 2014;33(4):574-9. doi: https://dx.doi.org/10.1377/hlthaff.2013.1314.
19. Cox CG, Davis MA, Grill JD, Roberts JS. US Adults’ Likelihood to Participate in Dementia Prevention Drug Trials: Results from the National Poll on Healthy Aging. J Prev Alzheimers Dis. 2023;10(1):34-40. doi: 10.14283/jpad.2022.86. PubMed PMID: 36641608; PubMed Central PMCID: PMC9579667.
20. Karlawish J, Cary MS, Rubright J, Tenhave T. How redesigning AD clinical trials might increase study partners’ willingness to participate. Neurology. 2008;71(23):1883-8. doi: 10.1212/01.wnl.0000336652.05779.ea. PubMed PMID: 19047560; PubMed Central PMCID: PMC2649726.
21. Karlawish JH, Casarett D, Klocinski J, Sankar P. How do AD patients and their caregivers decide whether to enroll in a clinical trial? Neurology. 2001;56(6):789-92. doi: 10.1212/wnl.56.6.789. PubMed PMID: 11274319.
22. Grill JD, Karlawish J, Elashoff D, Vickrey BG. Risk disclosure and preclinical Alzheimer’s disease clinical trial enrollment. Alzheimers Dement. 2013;9(3):356-9.e1. Epub 20121108. doi: 10.1016/j.jalz.2012.03.001. PubMed PMID: 23141383; PubMed Central PMCID: PMC3572336.
23. Roberts JS, Connell CM, Cisewski D, Hipps YG, Demissie S, Green RC. Differences between African Americans and whites in their perceptions of Alzheimer disease. Alzheimer Dis Assoc Disord. 2003;17(1):19-26. doi: 10.1097/00002093-200301000-00003. PubMed PMID: 12621316.
24. Alzheimer’s Disease Facts And Figures. Association As: Alzheimers Dement.2021; 2021. p. 237-406.
25. George S, Duran N, Norris K. A Systematic Review of Barriers and Facilitators to Minority Research Participation Among African Americans, Latinos, Asian Americans, and Pacific Islanders. American Journal of Public Health. 2014;104(2):e16-e31. doi: 10.2105/ajph.2013.301706. PubMed PMID: 24328648.
26. Ford ME, Siminoff LA, Pickelsimer E, Mainous AG, Smith DW, Diaz VA, et al. Unequal Burden of Disease, Unequal Participation in Clinical Trials: Solutions from African American and Latino Community Members. Health & Social Work. 2013;38(1):29-38. doi: 10.1093/hsw/hlt001.
27. Grill JD, Galvin JE. Facilitating Alzheimer Disease Research Recruitment. Alzheimer Disease & Associated Disorders. 2014;28(1):1-8. doi: 10.1097/wad.0000000000000016. PubMed PMID: WOS:000332086700001.
28. Otado J, Kwagyan J, Edwards D, Ukaegbu A, Rockcliffe F, Osafo N. Culturally Competent Strategies for Recruitment and Retention of African American Populations into Clinical Trials. Clin Transl Sci. 2015;8(5):460-6. Epub 20150514. doi: 10.1111/cts.12285. PubMed PMID: 25974328; PubMed Central PMCID: PMC4626379.
29. Tremblay M-C, Martin DH, McComber AM, McGregor A, Macaulay AC. Understanding community-based participatory research through a social movement framework: a case study of the Kahnawake Schools Diabetes Prevention Project. BMC Public Health. 2018;18(1):487. doi: 10.1186/s12889-018-5412-y.
30. Brewer LC, Jenkins S, Hayes SN, Kumbamu A, Jones C, Burke LE, et al. Community-based, cluster-randomized pilot trial of a cardiovascular mHealth intervention: Rationale, design, and baseline findings of the FAITH! Trial. American Heart Journal. 2022;247:1-14. doi: https://doi.org/10.1016/j.ahj.2022.01.009.
31. Greiner KA, Friedman DB, Adams SA, Gwede CK, Cupertino P, Engelman KK, et al. Effective Recruitment Strategies and Community-Based Participatory Research: Community Networks Program Centers’ Recruitment in Cancer Prevention Studies. Cancer Epidemiology, Biomarkers & Prevention. 2014;23(3):416-23. doi: 10.1158/1055-9965.epi-13-0760.
32. Ford ME, Havstad SL, Tilley BC. Recruiting Older African American Men to a Cancer Screening Trial (The AAMEN Project). The Gerontologist. 2003;43(1):27-35. doi: 10.1093/geront/43.1.27.
33. Services UDoHaH. Principles of Community Engagement. 2nd ed. Washington, DC: US Department of Health and Human Services.; 2011.
34. National Institutes of Health. NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research –amended, October, 2001. Available from: https://grants.nih.gov/grants/funding/women_min/guidelines_amended_10_2001.htm.
35. Wong R, Amano T, Lin SY, Zhou Y, Morrow-Howell N. Strategies for the Recruitment and Retention of Racial/Ethnic Minorities in Alzheimer Disease and Dementia Clinical Research. Curr Alzheimer Res. 2019;16(5):458-71. doi: 10.2174/1567205016666190321161901. PubMed PMID: 30907319.
36. Gilmore-Bykovskyi AL, Jin YY, Gleason C, Flowers-Benton S, Block LM, Dilworth-Anderson P, et al. Recruitment and retention of underrepresented populations in Alzheimer’s disease research: A systematic review. Alzheimers & Dementia-Translational Research & Clinical Interventions. 2019;5(1):751-70. doi: 10.1016/j.trci.2019.09.018. PubMed PMID: WOS:000737692800079.
37. JP H. Cochrane handbook for systematic reviews of interventions. In: Green S E, editor. London: The Cochrane Collaboration; 2011.
38. Types of Human Subjects Research | National Institute of Dental and Craniofacial Research. www.nidcr.nih.gov. Accessed June 14, 2024. https://www.nidcr.nih.gov/research/human-subjects-research/types-of-human-subjects-research#:~:text=Back%20to%20top-
39. Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract. 2012;18(1):12-8. Epub 20100804. doi: 10.1111/j.1365-2753.2010.01516.x. PubMed PMID: 20698919.
40. C. L. Qualitative research synthesis: methodological guidance for systematic reviewers utilizing meta-aggregation. In: Z. M, editor.: Int J Evid Based Healthc; 2015. p. 179-87.
41. Bardach SH, Benton B, Walker C, Alfred DL, Ighodaro E, Caban-Holt A, et al. Perspectives of African American Older Adults on Brain Health “Brains Get Tired Too”. Alzheimer Disease & Associated Disorders. 2019;33(4):354-8. doi: 10.1097/wad.0000000000000335. PubMed PMID: WOS:000500026600010.
42. Cocroft S, Welsh-Bohmer KA, Plassman BL, Chanti-Ketterl M, Edmonds H, Gwyther L, et al. Racially diverse participant registries to facilitate the recruitment of African Americans into presymptomatic Alzheimer’s disease studies. Alzheimers & Dementia. 2020;16(8):1107-14. doi: 10.1002/alz.12048. PubMed PMID: WOS:000577872500001.
43. Epps F, Skemp L, Specht J. Using Culturally Informed Strategies to Enhance Recruitment of African Americans in Dementia Research: A Nurse Researcher’s Experience. Journal of Research Practice. 2015;11(1):19. PubMed PMID: WOS:000389583200003.
44. Marquez DX, Perez A, Johnson JK, Jaldin M, Pinto J, Keiser S, et al. Increasing engagement of Hispanics/Latinos in clinical trials on Alzheimer’s disease and related dementias. Alzheimers & Dementia-Translational Research & Clinical Interventions. 2022;8(1):11. doi: 10.1002/trc2.12331. PubMed PMID: WOS:000830120200001.
45. Neugroschl J, Sewell MC, Umpierre M, Rodriguez R, Meyers L, Kranes S, et al. Elderly Latino community members make an educational video: an academic-community collaboration to promote memory evaluations. International Psychogeriatrics. 2019;31(7):989-95. doi: 10.1017/s1041610218001448. PubMed PMID: WOS:000514156700010.
46. Parveen S, Barker S, Kaur R, Kerry F, Mitchell W, Happs A, et al. Involving minority ethnic communities and diverse experts by experience in dementia research: The Caregiving HOPE Study. Dementia (London, England). 2018;17(8):990-1000. doi: https://dx.doi.org/10.1177/1471301218789558.
47. Portacolone E, Palmer NR, Lichtenberg P, Waters CM, Hill CV, Keiser S, et al. Earning the trust of african american communities to increase representation in dementia research. Ethnicity & Disease. 2020;30:719-34. doi: 10.18865/ed.30.S2.719. PubMed PMID: WOS:000603397700003.
48. Sharma RK, Teng A, Asirot MG, Taylor JO, Borson S, Turner AM. Challenges and opportunities in conducting research with older adults with dementia during COVID-19 and beyond. Journal of the American Geriatrics Society. 2022;70(5):1306-13. doi: 10.1111/jgs.17750. PubMed PMID: WOS:000770997500001.
49. Souder E, Terry TL. Use of lay educators to overcome barriers to research with Black older adults: a case study using Alzheimer’s Disease Center. Research in gerontological nursing. 2009;2(4):235-42. doi: https://dx.doi.org/10.3928/19404921-20090731-04.
50. McDougall GJ, Becker H, Pituch K, Acee TW, Vaughan PW, Delville CL. Differential Benefits of Memory Training for Minority Older Adults in the SeniorWISE Study. Gerontologist. 2010;50(5):632-45. doi: 10.1093/geront/gnq017. PubMed PMID: WOS:000281962200008.
51. Ajrouch KJ, Vega IE, Antonucci TC, Tarraf W, Webster NJ, Zahodne LB. Partnering with Middle Eastern/Arab American and Latino Immigrant Communities to Increase Participation in Alzheimer’s Disease Research. Ethn Dis. 2020;30(Suppl 2):765-74. Epub 20201119. doi: 10.18865/ed.30.S2.765. PubMed PMID: 33250623; PubMed Central PMCID: PMC7683026.
52. Ashford MT, Camacho MR, Jin CS, Eichenbaum J, Ulbricht A, Alaniz R, et al. Digital culturally tailored marketing for enrolling Latino participants in a web-based registry: Baseline metrics from the Brain Health Registry. Alzheimers & Dementia.15. doi: 10.1002/alz.12805. PubMed PMID: WOS:000863622900001.
53. Bardach SH, Barber JM, Schmitt FA, Van Eldik LJ, Boggess MB, Yarbrough M, et al. The Effectiveness of Community-based Outreach Events for the Promotion of African American Research Participation. Alzheimer Dis Assoc Disord. 2020;34(4):344-9. doi: 10.1097/wad.0000000000000404. PubMed PMID: 32809985; PubMed Central PMCID: PMC7677178.
54. Barnes LL, Shah RC, Aggarwal NT, Bennett DA, Schneider JA. The Minority Aging Research Study: Ongoing Efforts to Obtain Brain Donation in African Americans without Dementia. Current Alzheimer Research. 2012;9(6):734-45. doi: 10.2174/156720512801322627. PubMed PMID: WOS:000308864500012.
55. Boyd AD, Railey AF, Kirkpatrick AW, Hsu YC, Muller C, Buchwald D. Communication about Alzheimer’s disease and research among American Indians and Alaska Natives. Alzheimers & Dementia-Translational Research & Clinical Interventions. 2022;8(1):8. doi: 10.1002/trc2.12302. PubMed PMID: WOS:000793258400001.
56. Chao SZ, Lai NB, Tse MM, Ho RJ, Kong JP, Matthews BR, et al. Recruitment of Chinese American Elders into Dementia Research: The UCSF ADRC Experience. Gerontologist. 2011;51:S125-S33. doi: 10.1093/geront/gnr033. PubMed PMID: WOS:000290611300013.
57. DeCaro R, O’Connor MK, DiTerlizzi C, Sekyi-Appiah N, Polk J, Budson AE. Educating students while recruiting underrepresented populations for Alzheimer’s disease research: the Student Ambassador Program. Bmc Medical Education. 2022;22(1). doi: 10.1186/s12909-022-03749-1. PubMed PMID: WOS:000864118600001.
58. Etkin CD, Farran CJ, Barnes LL, Shah RC. Recruitment and enrollment of caregivers for a lifestyle physical activity clinical trial. Research in Nursing & Health. 2012;35(1):70-81. doi: 10.1002/nur.20466. PubMed PMID: WOS:000298672200007.
59. Fritsch T, Adams KB, Redd D, Sias T, Herrup K. Use of live theater to increase minority participation in Alzheimer disease research. Alzheimer Dis Assoc Disord. 2006;20(2):105-11. doi: 10.1097/01.wad.0000213806.66811.ea. PubMed PMID: 16772746.
60. Gallagher-Thompson D, Rabinowitz Y, Tang PCY, Tse C, Kwo E, Hsu S, et al. Recruiting Chinese Americans for dementia caregiver intervention research: Suggestions for success. American Journal of Geriatric Psychiatry. 2006;14(8):676-83. doi: 10.1097/01.JGP.0000221234.65585.f9. PubMed PMID: WOS:000239205500006.
61. Han HR, Choi S, Wang J, Lee HB. Pilot testing of a dementia literacy intervention for Korean American elders with dementia and their caregivers. J Clin Transl Res. 2021;7(6):712-6. Epub 20211030. PubMed PMID: 34901516; PubMed Central PMCID: PMC8654362.
62. Hinton L, Carter K, Reed BR, Beckett L, Lara E, DeCarli C, et al. Recruitment of a Community-based Cohort for Research on Diversity and Risk of Dementia. Alzheimer Disease & Associated Disorders. 2010;24(3):234-41. doi: 10.1097/WAD.0b013e3181c1ee01. PubMed PMID: WOS:000281310200004.
63. Howell JC, Soyinka O, Parker M, Jarrett TL, Roberts DL, Dorbin CD, et al. Knowledge and Attitudes in Alzheimer’s Disease in a Cohort of Older African Americans and Caucasians. American Journal of Alzheimers Disease and Other Dementias. 2016;31(4):361-7. doi: 10.1177/1533317515619037. PubMed PMID: WOS:000376305200008.
64. Li C, Neugroschl J, Umpierre M, Martin J, Huang QY, Zeng XY, et al. Recruiting US Chinese Elders Into Clinical Research for Dementia. Alzheimer Disease & Associated Disorders. 2016;30(4):345-7. doi: 10.1097/wad.0000000000000162. PubMed PMID: WOS:000389258200009.
65. Lingler JH, Ren D, Tamres LK, Knox ML, Mbawuike U, Williams IC, et al. Mechanisms by which Cultural-Centric Narrative Influences Interest in ADRD Research Among African American Adults. The Gerontologist. 2022. doi: 10.1093/geront/gnac179.
66. Meyer OL, Sun MX, Do T, Ho JN, Dinh BT, Nguyen S, et al. Community-Engaged Research with Vietnamese Americans to Pilot-Test a Dementia Caregiver Intervention. Journal of Cross-Cultural Gerontology. 2020;35(4):479-92. doi: 10.1007/s10823-020-09410-y. PubMed PMID: WOS:000561512000001.
67. Milani SA, Swain M, Otufowora A, Cottler LB, Striley CW. Willingness to Participate in Health Research Among Community-Dwelling Middle-Aged and Older Adults: Does Race/Ethnicity Matter? Journal of Racial and Ethnic Health Disparities. 2021;8(3):773-82. doi: 10.1007/s40615-020-00839-y. PubMed PMID: WOS:000560312500004.
68. Overman AA, Robbins RE. Game-Based Community Cognitive Health Intervention for Minority and Lower Socioeconomic Status Older Adults: A Feasibility Pilot Study. Games for Health Journal. 2014;3(5):303-10. doi: 10.1089/g4h.2014.0052. PubMed PMID: WOS:000363384100006.
69. Parker LJ, Gaugler JE, Gitlin LN. Use of Critical Race Theory to Inform the Recruitment of Black/African American Alzheimer’s Disease Caregivers into Community-Based Research. The Gerontologist. 2022;62(5):742-50. doi: https://dx.doi.org/10.1093/geront/gnac001.
70. Perales-Puchalt J, Shaw A, McGee JL, Moore WT, Hinton L, Resendez J, et al. Preliminary Efficacy of a Recruitment Educational Strategy on Alzheimer’s Disease Knowledge, Research Participation Attitudes, and Enrollment Among Hispanics. Hisp Health Care Int. 2020;18(3):144-9. Epub 20191215. doi: 10.1177/1540415319893238. PubMed PMID: 31840539; PubMed Central PMCID: PMC7293919.
71. Romero HR, Welsh-Bohmer KA, Gwyther LP, Edmonds HL, Plassman BL, Germain CM, et al. Community engagement in diverse populations for Alzheimer disease prevention trials. Alzheimer Dis Assoc Disord. 2014;28(3):269-74. doi: 10.1097/wad.0000000000000029. PubMed PMID: 24614272; PubMed Central PMCID: PMC4139415.
72. Samus QM, Amjad H, Johnston D, Black BS, Bartels SJ, Lyketsos CG. A Multipronged, Adaptive Approach for the Recruitment of Diverse Community-Residing Elders with Memory Impairment: The MIND at Home Experience. American Journal of Geriatric Psychiatry. 2015;23(7):698-708. doi: 10.1016/j.jagp.2015.01.005. PubMed PMID: WOS:000355926200006.
73. Shaw AR, Perales-Puchalt J, Moore T, Weatherspoon P, Robinson M, Hill CV, et al. Recruitment of Older African Americans in Alzheimer’s Disease Clinical Trials Using a Community Education Approach. Jpad-Journal of Prevention of Alzheimers Disease. 2022;9(4):672-8. doi: 10.14283/jpad.2022.82. PubMed PMID: WOS:000860368400002.
74. Ta Park VM, Meyer OL, Tsoh JY, Kanaya AM, Tzuang M, Nam B, et al. The Collaborative Approach for Asian Americans and Pacific Islanders Research and Education (CARE): A recruitment registry for Alzheimer’s disease and related dementias, aging, and caregiver-related research. Alzheimers Dement. 2023;19(2):433-43. Epub 20220414. doi: 10.1002/alz.12667. PubMed PMID: 35420258; PubMed Central PMCID: PMC9562598.
75. Weiner MW, Veitch DP, Miller MJ, Aisen PS, Albala B, Beckett LA, et al. Increasing participant diversity in AD research: Plans for digital screening, blood testing, and a community-engaged approach in the Alzheimer’s Disease Neuroimaging Initiative 4. Alzheimers Dement. 2023;19(1):307-17. Epub 20221009. doi: 10.1002/alz.12797. PubMed PMID: 36209495; PubMed Central PMCID: PMC10042173.
76. Wiese LK, Williams IC, Schoenberg NE, Galvin JE, Lingler J. Overcoming the COVID-19 Pandemic for Dementia Research: Engaging Rural, Older, Racially and Ethnically Diverse Church Attendees in Remote Recruitment, Intervention and Assessment. Gerontology and Geriatric Medicine. 2021;7. doi: 10.1177/23337214211058919. PubMed PMID: WOS:000721360800001.
77. Zhou Y, Elashoff D, Kremen S, Teng E, Karlawish J, Grill JD. African Americans are less likely to enroll in preclinical Alzheimer’s disease clinical trials. Alzheimer’s & dementia (New York, N Y). 2016;3(1):57-64. doi: 10.1016/j.trci.2016.09.004. PubMed PMID: 29067319.
78. O’Bryant SE, Johnson L, Balldin V, Edwards M, Barber R, Williams B, et al. Characterization of Mexican Americans with mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis. 2013;33(2):373-9. doi: 10.3233/jad-2012-121420. PubMed PMID: 22976076; PubMed Central PMCID: PMC3524411.
79. Bachman DL, Stuckey M, Ebeling M, Wagner MT, Evans WJ, Hirth V, et al. Establishment of a predominantly African-American cohort for the study of Alzheimer’s disease: the South Carolina Alzheimer’s disease clinical core. Dementia and geriatric cognitive disorders. 2009;27(4):329-36. doi: https://dx.doi.org/10.1159/000207446.
80. Ballard EL, Nash F, Raiford K, Harrell LE. Recruitment of black elderly for clinical research studies of dementia – the cerad experience. Gerontologist. 1993;33(4):561-5. doi: 10.1093/geront/33.4.561. PubMed PMID: WOS:A1993LQ87400021.
81. Gauthier MA, Clarke WP. Gaining and sustaining minority participation in longitudinal research projects. Alzheimer Disease & Associated Disorders. 1999;13:S29-S33. doi: 10.1097/00002093-199904001-00008. PubMed PMID: WOS:000084471700008.
82. Rexroth DF, Friedland RP. Lessons learned regarding recruitment to the national African American Alzheimer disease health literacy program. Alzheimer Disease and Associated Disorders. 2010;24(SUPPL. 1):S54-S7. doi: 10.1097/WAD.0b013e3181f14b22.
83. Williams MM, Meisel MM, Williams J, Morris JC. An Interdisciplinary Outreach Model of African American Recruitment for Alzheimer’s Disease Research. Gerontologist. 2011;51:S134-S41. doi: 10.1093/geront/gnq098. PubMed PMID: WOS:000290611300014.
84. Nkimbeng M, Rosebush CE, Akosah KO, Yam H, Russell WN, Bustamante G, et al. The Immigrant Memory Collaborative: A Community-University Partnership to Assess African Immigrant Families’ Experiences with Dementia. International Journal of Environmental Research and Public Health. 2022;19(7):19. doi: 10.3390/ijerph19074075. PubMed PMID: WOS:000783113200001.
85. Sun F, Gao X, Shen H, Burnette D. Levels and correlates of knowledge about Alzheimer’s disease among older Chinese Americans. J Cross Cult Gerontol. 2014;29(2):173-83. doi: 10.1007/s10823-014-9229-6. PubMed PMID: 24728621.
86. Withers M, Sayegh P, Rodriguez-Agudelo Y, Ernstrom K, Raman R, Montoya L, et al. A mixed-methods study of cultural beliefs about dementia and genetic testing among Mexicans and Mexican-Americans at-risk for autosomal dominant Alzheimer’s disease. Journal of Genetic Counseling. 2019;28(5):921-32. doi: 10.1002/jgc4.1133. PubMed PMID: WOS:000489240200001.
87. Ashford MT, Zhu D, Bride J, McLean E, Aaronson A, Conti C, et al. Understanding Online Registry Facilitators and Barriers Experienced by Black Brain Health Registry Participants: The Community Engaged Digital Alzheimer’s Research (CEDAR) Study. Jpad-Journal of Prevention of Alzheimers Disease. doi: 10.14283/jpad.2023.25. PubMed PMID: WOS:000946893400001.
88. Israel BA, Eng E, Schulz AJ, Parker EA. Introduction to methods in community-based participatory research for health. Methods in community-based participatory research for health. 2005;3:26.
89. Bartsch DAP, Rodgers VKM, Strong DELMS. Outcomes of Senior Reach Gatekeeper Referrals: Comparison of the Spokane Gatekeeper Program, Colorado Senior Reach, and Mid-Kansas Senior Outreach. Care Management Journals. 2013;14(1):11-20. doi: https://doi.org/10.1891/1521-0987.14.1.11. PubMed PMID: 1346648143; 23721039.
90. Fishbein M, Ajzen I. Predicting and Changing Behavior: The Reasoned Action Approach: Psychology Press; 2009.
91. Kleinman A, Benson P. Anthropology in the Clinic: The Problem of Cultural Competency and How to Fix It. PLOS Medicine. 2006;3(10):e294. doi: 10.1371/journal.pmed.0030294.
92. Leventhal H, Brissette I. The common sense model of self-regulation of health and illness In: Cameron LD, Leventhal H, eds. The self-regulation of health and illness behavior. London: Routledge; 2003.
93. Arnstein SR. A ladder of citizen participation: Journal of the American Institute of Planners; 1969.
94. Morrow E, Ross F, Grocott P, Bennett J. A model and measure for quality service user involvement in health research. International Journal of Consumer Studies. 2010;34(5):532-9. doi: https://doi.org/10.1111/j.1470-6431.2010.00901.x.
95. McDougall GJ, Jr., Simpson G, Friend ML. Strategies for research recruitment and retention of older adults of racial and ethnic minorities. Journal of gerontological nursing. 2015;41(5):14-5. doi: https://dx.doi.org/10.3928/00989134-20150325-01.
96. Wiese LK, Williams CL, Tappen R, Newman D, Rosselli M. Assessment of Basic Knowledge About Alzheimer’s Disease Among Older Rural Residents: A Pilot Test of a New Measure. J Nurs Meas. 2017 Dec 1;25(3):519-548. doi: 10.1891/1061-3749.25.3.519. PMID: 29268833.
97. Carpenter BD, Balsis S, Otilingam PG, Hanson PK, Gatz M. The Alzheimer’s Disease Knowledge Scale: development and psychometric properties. Gerontologist. 2009 Apr;49(2):236-47. doi: 10.1093/geront/gnp023. Epub 2009 Mar 25. PMID: 19363018; PMCID: PMC2667675.
98. Roberts JS, Connell CM. Illness representations among first-degree relatives of people with Alzheimer disease. Alzheimer Dis Assoc Disord. 2000 Jul-Sep;14(3):129-136,Discussion 127-8. doi: 10.1097/00002093-200007000-00003. PMID: 10994653.
99. Annear MJ, Toye CM, Eccleston CE, McInerney FJ, Elliott KE, Tranter BK, Hartley T, Robinson AL. Dementia Knowledge Assessment Scale: Development and Preliminary Psychometric Properties. J Am Geriatr Soc. 2015 Nov;63(11):2375-81. doi: 10.1111/jgs.13707. Epub 2015 Oct 27. PMID: 26503020.
100. Quiñones AR, Mitchell SL, Jackson JD, Aranda MP, Dilworth-Anderson P, McCarthy EP, et al. Achieving Health Equity in Embedded Pragmatic Trials for People Living with Dementia and Their Family Caregivers. Journal of the American Geriatrics Society (JAGS). 2020;68(2):S8-S13. doi: 10.1111/jgs.16614.
© The Authors 2024