S. Saeedi1, S. Hetjens2, M.O.W. Grimm3,4, B. Barsties v. Latoszek5
1. Independent Researcher in Laryngology, Voice Pathology, and Speech-Language Pathology, Tehran, Iran; 2. Department for Medical Statistics and Biomathematics, Medical Faculty Mannheim, University of Heidelberg, Mannheim, Germany; 3. Experimental Neurology, Saarland University, 66424 Homburg, Germany; 4. Nutrition Therapy and Counseling, Campus Rheinland, SRH University of Applied Health Sciences, 51377 Leverkusen, Germany; 5. Speech-Language Pathology, SRH University of Applied Health Sciences, Düsseldorf, Germany
Corresponding Author: Prof. Dr. Ben Barsties v. Latoszek, Graf-Adolf-Straße 67, 40210 Düsseldorf, Tel: +49 211 2807390, E-mail: benjamin.barstiesvonlatoszek@srh.de
J Prev Alz Dis 2024;6(11):1789-1797
Published online August 13, 2024, http://dx.doi.org/10.14283/jpad.2024.132
Abstract
BACKGROUND: The potential of biomarkers in the detection of Alzheimer’s disease (AD) is prominent. Acoustics may be useful in this context but the evaluation and weighting for specific acoustic parameters on continuous speech is missing. This meta-analysis aimed to explore the significance of acoustic parameters from acoustic speech analysis on continuous speech, as a diagnostic tool for clinical AD.
METHODS: Applying PRISMA protocol, a comprehensive search was done in MEDLINE, Scopus, Web of Science, and CENTRAL, from 1960 to January 2024. Cross-sectional studies comparing the acoustic speech analysis between AD patients and healthy controls (HC), were taken into account. The bias risk of the included studies were examined via JBI checklist. Using Review Manager v.5.4.1, the mean differences of acoustic speech parameters among AD and HC were weighted, and the pooled analysis and the heterogeneity statistics were conducted.
RESULTS: In total, 1112 records (without duplicates) were obtained, and 11 papers with 7 acoustic parameters were included for this study, and 8 from 11 studies were identified with a low level of bias. Five from 7 acoustic parameters revealed significant differences among the two groups (p-values ≤ 0.01), in which for all rate-related and interruption-related acoustic parameters were the most prominent and less in temporal-related acoustic parameters.
CONCLUSIONS: Although a small number of acoustic parameters on continuous speech could be evaluated in the detection of clinical AD, the greatest potential of acoustic biomarkers for AD appeared to exist in two of three categories. Further contributions of high-quality studies are needed to support evidence for acoustics as biomarkers for AD.
Key words: Alzheimer’s disease, diagnosis, acoustic, signal processing, speech and voice analysis.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that significantly impacts cognitive function, leading to severe impairment in daily living activities. It is the most common cause of dementia among older adults, characterized by a gradual decline in memory, language, problem-solving abilities, and other critical thinking skills. The exact cause of AD remains largely unknown, although it is believed to result from a combination of genetic, environmental, and lifestyle factors. The early detection of AD poses a significant challenge yet is crucial for managing symptoms and planning care.
Over the last few decades, significant progressions have been achieved in the field of biomarker development regarding AD diagnosis. Prominent examples include the identification of biomarkers such as analyzing proteins, the utilization of neuroimaging techniques, and the implementation of neuropsychological tests (1-3). Beside neurofilament light chain, both the amyloid-b (Ab) peptide as well as hyperphosphorylated forms of the microtubule-associated protein tau play an important role as biomarkers, as senile plaques consisting of aggregated Ab-peptides and neurofibrillary tangles which consist of hyperphosphorylated tau proteins represent the main histopathological hallmarks of AD (4, 5). Notably, these proteins can be detected in cerebrospinal fluid as well as blood of AD patients and can be detected in vivo by PET (positron emission tomography) scans (6, 7). Nevertheless, these diagnostic methods are constrained by their substantial financial burden on the healthcare systems of countries and also invasive characteristics (8). Therefore, AD is often diagnosed based on clinical criteria, such as the National Institute on Aging-Alzheimer’s Association (NIA-AA) criteria, which rely on the presence of characteristic cognitive and functional impairments. Thus, it is important to note that there is a distinction between the biological and clinical diagnoses of AD. While the biological diagnosis is based on the presence of AD neuropathology, as evidenced by biomarkers, the clinical diagnosis is based on the presence of characteristic symptoms and signs of the disease. Some studies have shown that a significant proportion of individuals with a clinical diagnosis of AD may not have evidence of AD neuropathology on biomarker testing (9, 10). Conversely, some individuals with biomarker evidence of AD neuropathology may not meet clinical criteria for AD due to the absence of significant cognitive or functional impairments.
Due to the noted speech and language impairments observed in the initial phases of AD (11, 12), a question arises as to whether or not acoustic speech analysis can be used to assess and screen for AD. In recent years, the importance of speech signal processing in medical practice has gained considerable attention, as indicated by the numerous studies conducted on the topic (13). Advancements in technology and the availability of relevant user-friendly software packages have led to an increase in the clinical use of these measurements (14). Speech signal processing can provide valuable information regarding different aspects of speech signal such as speech rate, pitch and loudness variability, quality, resonance, articulation precision, and regularity (15-17). Acoustic measurements can be performed with easily available recording devices, making it a cost-effective and easily accessible tool. In addition, acoustics offers an objective analysis of quantitative measurements that have great potential for future developments in the field of diagnostics research.
The purpose of this meta-analysis is to assess acoustic speech parameters on continuous speech material in the detection of AD. The aim of the study was to comprehensively analyze the existing literature on this topic and identify the strengths and limitations of current research. By synthesizing the available evidence, this paper aimed to provide a comprehensive understanding of the potential benefits of speech signal processing in the identification of AD. Furthermore, this study seeks to bridge the gap between traditional biomarker research and the emerging field of acoustic analysis, highlighting the potential for non-invasive, cost-effective screening methods. The integration of acoustic biomarkers could significantly enhance early detection strategies, ultimately contributing to improved patient care and management. The exploration of acoustic parameters not only enriches our diagnostic toolbox but also underscores the multidisciplinary approach necessary for tackling complex diseases like AD. Through meticulous analysis and evaluation, this meta-analysis endeavors to elevate the discourse surrounding acoustic biomarkers and lay the groundwork for future investigations that could redefine diagnostic paradigms for AD.
Materials and methods
Data sources and searches
The conduction of this review followed the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (18). Relevant studies were identified by a systematic search electronic databases from MEDLINE (PubMed), Scopus, Web of Science, and Cochrane Central Register of Controlled Trials (CENTRAL), starting from 1960 until January 2024. Different key words were combined relating to acoustics measurements of speech analysis on continuous speech between Alzheimer’s disease and healthy controls (HC). Appendix 1 contains a comprehensive list of selected keywords with Boolean operators used in each database.
We identified articles through the review of their titles and abstracts. A systematic search was then undertaken for scientific reports published in English, and only those located within the databases were incorporated into the meta-analysis.
Study Selection
This study comprised cross-sectional investigations that examined differences in the outcomes of acoustic speech measures on continuous speech material between AD and HC. The features of the quantitative acoustic measures for inclusion represent gender-independence and provide information on prosodic (alterations in rhythm, timing, stress, and pitch) and quality-related aspects of speech. Moreover, it is important to acknowledge that the included studies might rely mostly on clinical diagnoses of AD, and biomarker confirmation of the underlying AD neuropathology would be low. As such, the results of this meta-analysis should be interpreted as distinguishing between individuals with and without dementia, rather than specifically between those with and without biological AD.
Studies were excluded if: [1] a study group of participants with mild cognitive impairment or other types of dementia that are not associated with or do not present with the (clinical) symptoms of Alzheimer’s disease; [2] published studies were written in a language other than English; [3] studies that were not published in a peer-reviewed journal; [4] studies were either traditional narrative, or systematic reviews, case studies, or contained other study designs than cross-sectional studies.
Risk of Bias Assessment
The evaluation of bias in the studies included was conducted using a checklist for cross-sectional studies provided by the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Analytical Cross-Sectional Studies which consists of 8 items (19). The checklist items assess the study sample (inclusion criteria, detailed description of subjects, and the study setting), exposure and outcomes measures (validity and reliability of measuring exposure and outcome, and criteria used for measurement), confounding bias (identification of confounding factors, and strategies to deal with them), and data analysis (appropriateness of statistical analysis). The possible responses for each 8 items are «yes,» «unclear,» «no,» or «not applicable.» Before starting the critical appraisal, all reviewers agreed on the scoring decisions based on the previous studies (19, 20). The studies were then grouped according to the following criteria: (a) studies with a score of above 70% «yes» were considered to have a low risk of bias, (b) studies with «yes» scores between 50% and 69% were categorized as having a moderate risk of bias, and (c) studies with «yes» scores below 49% were determined to have a high risk of bias.
Data Extraction
Two reviewers (SS and BBvL) were responsible for extracting the data. Both reviewers independently evaluated the titles and abstracts of the retrieved studies. In the subsequent screening phase, a comprehensive analysis and evaluative assessment were conducted on the full text of the chosen studies. This meticulous process aims to ascertain the suitability of including these studies in the meta-analysis. The collected information from the chosen studies encompassed various aspects, including article attributes (such as authors, publication year, journal, and paper title), study characteristics (such as research design, sample size, participants with AD in comparison with cognitively normal individuals, speech task, software, and acoustic data), patient demographics (age and gender), and outcomes of the various acoustic measures of speech analyses. Any disagreements between the reviewers were resolved through discussion. Articles that did not meet the inclusion criteria were excluded.
Statistics
The program Review Manager (RevMan), version 5.4.1 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, 2020) was used for the statistical analyses of the meta-analysis on the final selected results of acoustic measures from the systematic search listed in Table 1, which were assessed more than once. First, the difference between HC and AD was calculated of the outcomes of the specific acoustic measures. This difference was weighted according to the DerSimonian & Laird method (21). Second, the results of the pooled analysis and the heterogeneity statistics were presented in a forest plot. The heterogeneity of studies was calculated using the I² index. An I² value of 0 – 25 % represents insignificant heterogeneity; > 25 % – 50 % low heterogeneity; > 50 % – 75 % moderate heterogeneity; and > 75 % high heterogeneity (22). Analyses with insignificant heterogeneity were calculated using a fixed-effects model, otherwise with random effects model. A p-value of less than 0.05 was considered as statistically significant.
Results
Study Characteristics
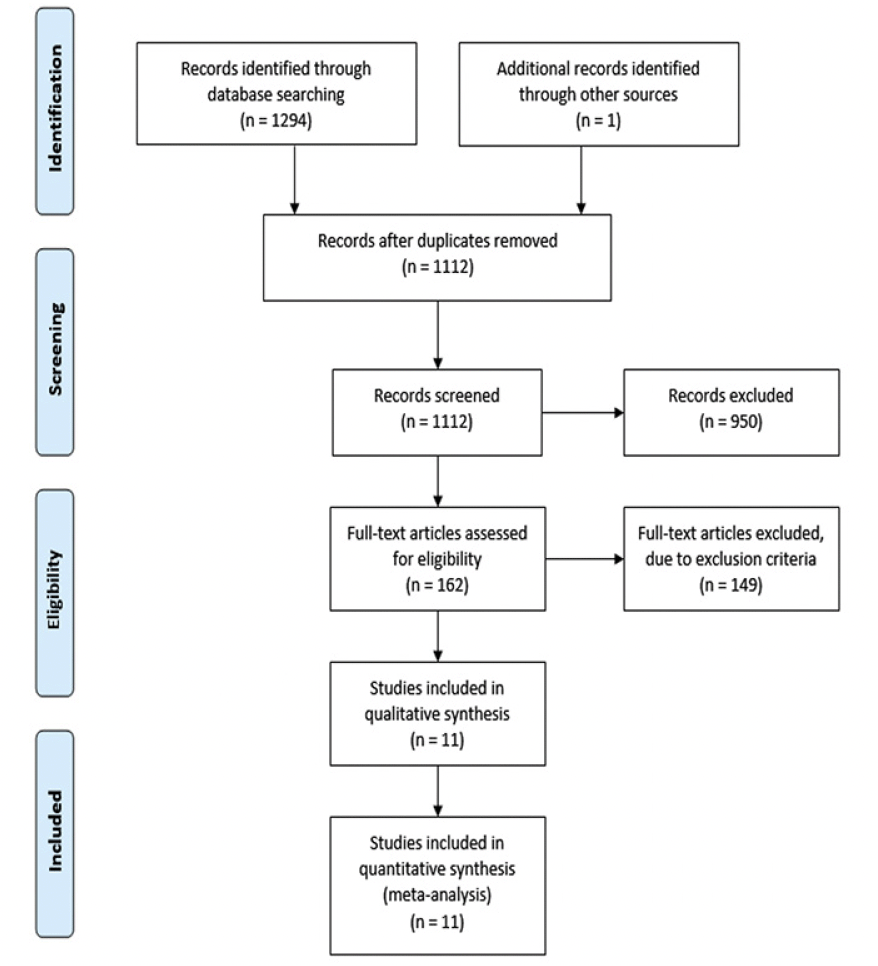
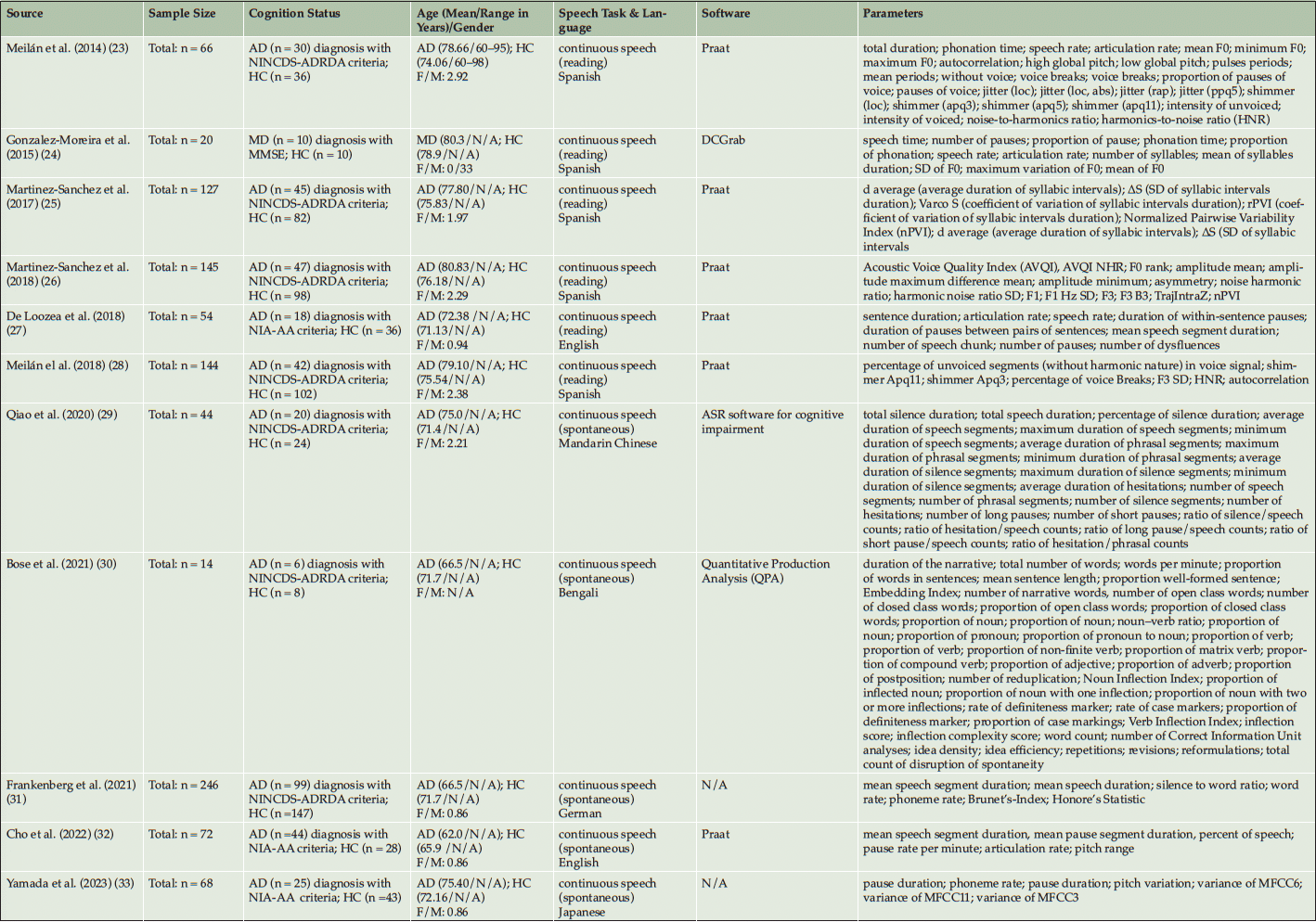
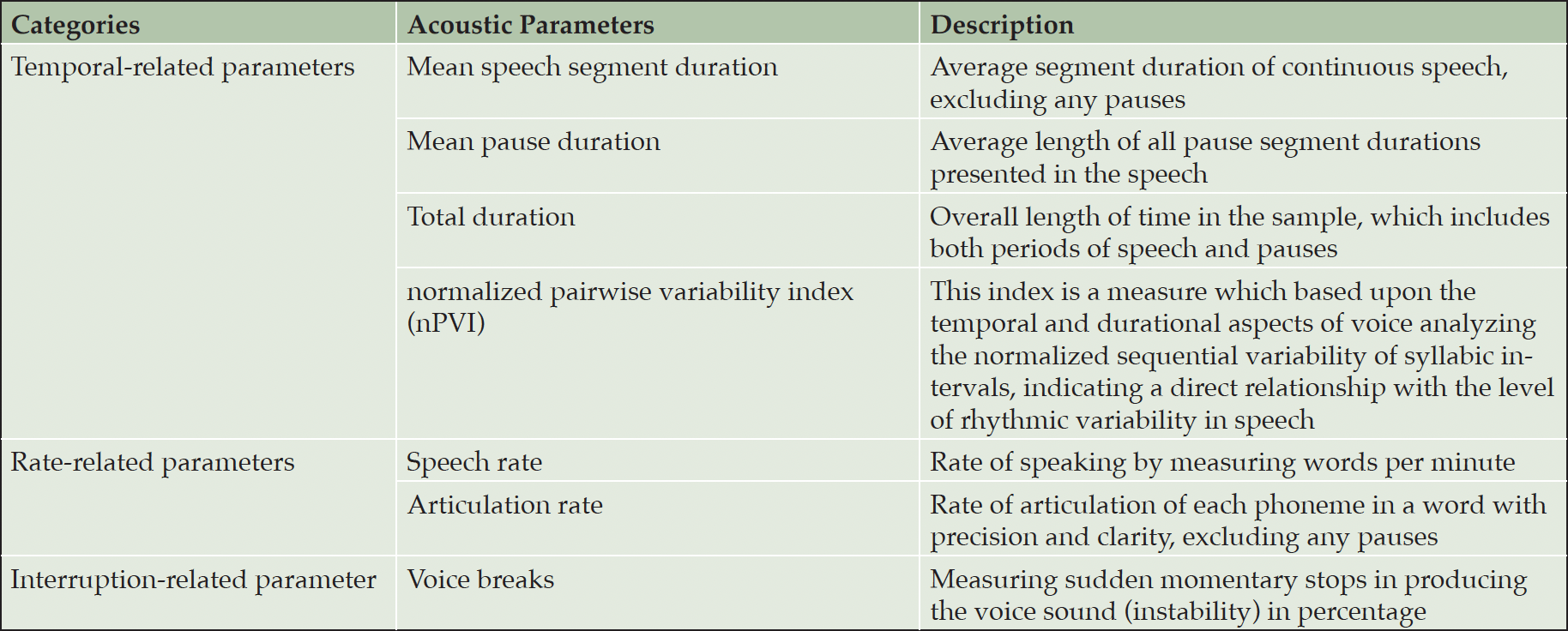
The PRISMA flowchart in Figure 1 provides a concise overview of the systematic search process. A comprehensive search yielded a total of 1,294 studies, of which 182 duplicates were identified and subsequently removed. After a thorough screening based on title and abstract, 162 studies were deemed suitable for a full-text review. However, after applying the specified exclusion criteria, 149 studies were excluded, resulting in 11 articles being included (see Table 1). Each study included a control group of healthy individuals and at least one experimental group of patients with AD. The acoustic measurements that were shared among the included studies provide quantitative information about various aspects of speech signals. For the present meta-analysis just 7 acoustic parameters could be analyzed from Table 1 as these parameters have been evaluated more than once in the same study context. These 7 acoustic parameters can be categorized into three groups: time (mean speech segment duration, mean pause duration, total duration, and normalized pairwise variability index (nPVI)), rate (speech rate, and articulation rate), and interruption (voice breaks) parameters (see Table 2).
Abbreviations: n, number; AD, Alzheimer’s disease; NINCDS-ADRDA, National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; HC, healthy controls; F, female; M, male; MMSE, Mini-Mental State Examination; MD, mild dementia; N/A, not applicable; SD, standard deviation; NIA-AA, National Institute on Aging and Alzheimer’s Association; MFCC, mel frequency cepstrum coefficient.
In total 1000 voice samples of participants were evaluated, in which 386 volunteers were examined in studies involving AD and 614 healthy controls. From the 386 participants of the experimental group 289 had an AD diagnosis based NINCDS-ADRDA criteria, 87 with NIA-AA criteria, and 10 with clinical presentations based on clinical ratings such as Mini Mental State Examination. The number of participants ranging from 8 to 147 for HC and 6 to 99 for AD. The bias risk assessment is presented in Appendix 2 in which a majority of the included studies demonstrated a low risk of bias (n=8), and rest of them showed a moderate risk of bias (n=3).
Meta-analysis
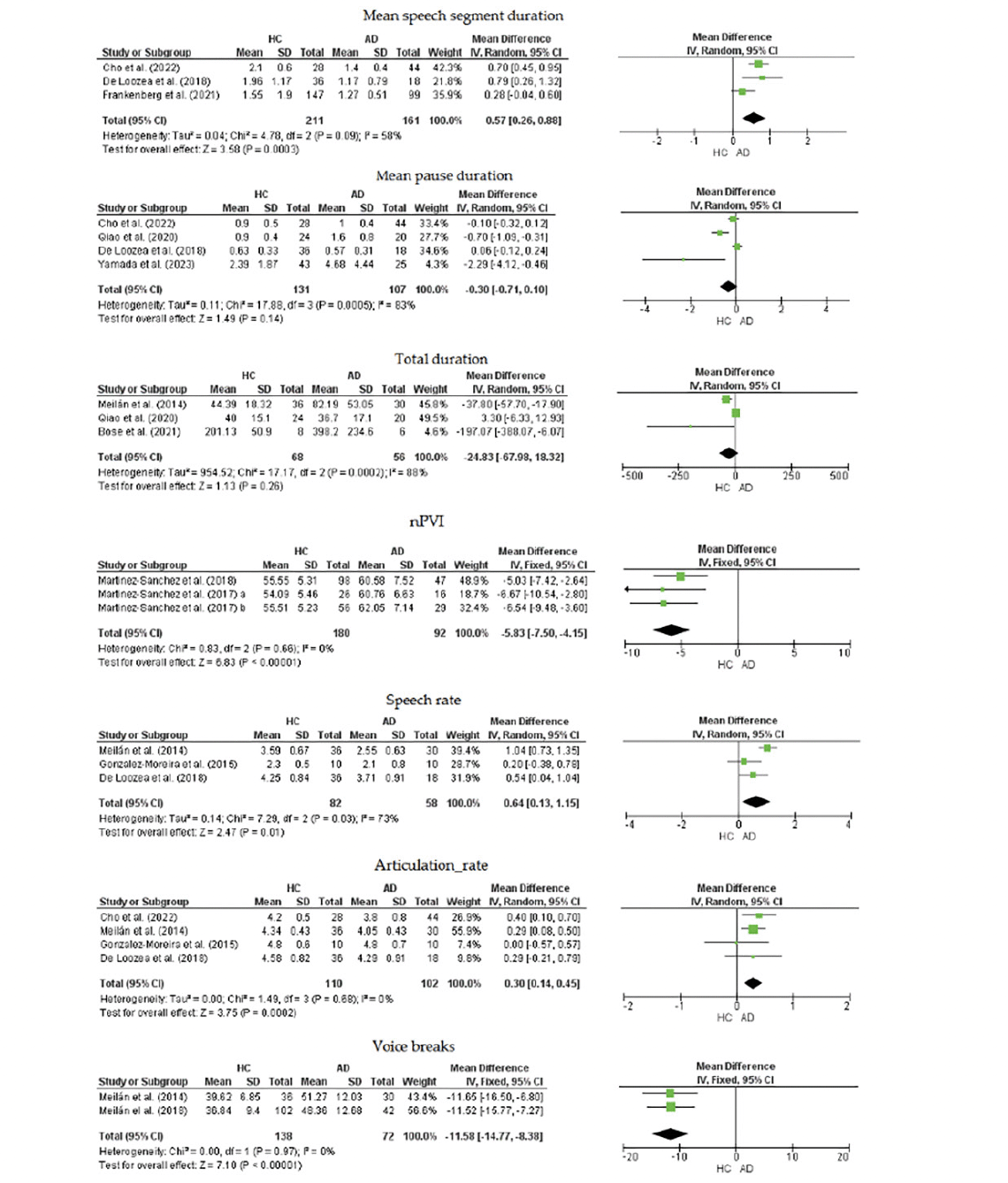
Figure 2 shows the results for the seven acoustic parameters: Mean speech segment duration, mean pause duration, speech rate, articulation rate, total duration, nPVI and voice breaks. The mean difference (MD) of nPVI between healthy controls and AD was significantly slower in the healthy controls: MD = -5.83 (95% CI: -7.50 to -4.15, p < 0.0001). The voice breaks was significantly less in healthy controls than in AD: MD = -11.58 % (95% CI: -14.77 % to, -8.38 %, p < 0.0001). The following acoustic parameters were significantly faster in healthy controls than in AD: articulation rate: MD = 0.30 (95% CI: 0.14 to 0.45, p = 0.0002), mean speech segment duration: MD = 0.57 seconds (95% CI: 0.26 seconds to 0.88 seconds, p = 0.0003), and speech rate: MD = 0.64 (95% CI: 0.13 to 1.15, p = 0.0100). Mean pause duration and total duration were not significantly different between the two groups: MD = -0.30 seconds (95% CI: -0.71 seconds to 0.10 seconds, p = 0.1400), and MD = -24.83 seconds (95% CI: -67.98 seconds to 18.32 seconds, p = 0.2600), respectively. No heterogeneity was present in articulation rate, nPVI, and voice breaks. However, there was moderate heterogeneity in mean speech segment duration and high heterogeneity in mean pause duration, speech rate, and total duration.
Discussion
The present study assessed the detection of Alzheimer’s disease in comparison to healthy controls by summarizing statistically the outcomes of seven speech acoustic parameters of the categories time, rate, and interruption. The neurodegenerative disease Alzheimer’s primarily affects memory and cognitive functions, but also various regions and centers in the brain that are responsible for language deficits (e.g. naming and word retrieval) such as bilateral inferior frontal gyrus (IFG), right superior frontal gyrus (SFG), the posterior aspect of the left middle temporal gyrus (MTG), left fusiform gyrus (FFG), and left inferior temporal gyrus (ITG) (34). In addition, the acoustic signal of speech and the expressive language could be influenced by mild cognitive impairment as well. For example, magnetic resonance imaging (MRI) measures of the volume of the segmented gray matter of the frontal lobe were associated with acoustic measurements, with the result that an acoustic and MRI-based model can sufficiently predict the diagnosis of mild cognitive impairment (35). Although mild cognitive impairment was not investigated in the present study, these findings highlight a potential reason that comparable acoustic speech changes as described in this meta-analysis may also be present in earlier stages of Alzheimer’s disease. Further structural MRI scans of Alzheimer’s disease and mild cognitive impairment patients revealed that the left inferior parietal lobe (IPL), the right ITG, and the right FFG all contributed to lowering the pitch level (36). There was also a significant relationship between the right FFG, the left FFG and the loudness level, with the consequence that a reduction in the volume of both regions leads to a decreased loudness level (36). Finally, significant relationships between the right IPL and left FFG and speech rate were also investigated, with atrophy of these brain regions leading to a decrease in speech rate (36).
From the included acoustic parameters in this meta-analysis, rate-related parameters and the interruption-related parameters had the highest potential in the acoustic detection of AD, with an overall significant difference confirmed between healthy controls (p ≤ 0.01). Temporal-related measures were only evident in two of four acoustic parameters for significant detection of AD: mean speech segment duration and nPVI (p < 0.01). No significant effects were assessed by the total duration and mean pause duration between AD and HC, and can be excluded as potential acoustic detection parameters for AD.
The publications included generally exhibited a low risk of bias, with eight out of the eleven studies indicating a low risk. Heterogeneity varied from none to high, with three out of seven analyses showing no heterogeneity. Only one parameter demonstrated moderate heterogeneity, mean speech segment duration of temporal-related category. Mean pause duration and total duration of temporal-related, and speech rate of rate-related categories presented a high heterogeneity. On the one hand, this heterogeneity between studies underscores the complexity of speech dynamics in AD and requires a nuanced understanding of how different acoustic parameters reflect underlying neurological changes. On the other hand, duration measurements can vary greatly depending on how the author groups in the studies defined the minimum length of «speech segments» and «silent pauses». For example, the minimum length of silent pauses was set at 150 milliseconds (ms) (29) or even 100 ms (27), which is mostly longer than the onset time of voiceless stop consonants in English. Depending on the definition of silent pauses, the duration and number of mean speech segments may also vary and might explain moderate to large heterogeneity in these measurements.
This meta-analysis confirmed that individuals with AD revealed significant lower results in the performance of the two rate-related parameters of speech rate and articulation rate. Furthermore, the significance of these findings lies in their potential to inform non-invasive and cost-effective screening processes for AD, offering an alternative to the more traditional and often expensive diagnostic methods. Speech rate refers to the speed at which an individual speaks, measured in words per minute (37). Furthermore, speech rate also includes the presence of pauses (38). Another measurement of speech rate is the articulation rate which describe the rate at which someone can articulate each phoneme in a word with precision and clarity, without taking into account any pauses (39). In individuals with AD, the degeneration of brain cells can lead to a decline in the co-ordination and regulation of the muscles movements resulting in apraxia, which can be evident in speech and orofacial muscles (40). This apraxia further complicates the speech production process, making acoustic analysis an effective tool in capturing these subtle yet clinically relevant changes. This can cause a slowdown in speech as individuals struggle to express their thoughts (23, 24, 27). Additionally, the precision of articulation may decrease as individuals find it challenging to coordinate the movements of their lips, tongue, and jaw to produce each sound in a word (23, 24, 27, 32). Rate-related parameters are both closely related to temporal-related parameters as patients who have lower speech rate produce a shorter speech segment and vice versa (41). Therefore, the results from rate-related parameters are compatible with temporal-related parameters.
Also the mean speech segment duration revealed significant lower results in the AD groups as the healthy controls. This parameter refers to the average length of uninterrupted speech segments. The reduction in mean speech segment duration among individuals with AD could reflect difficulties in sustaining speech flow, possibly due to impaired cognitive planning and execution of language. The significant difference in mean speech segment duration between the two groups suggests that individuals with AD may exhibit shorter speech timing compared to control group (27, 31, 32). In individuals with AD, this specific aspect might be affected due to difficulties in maintaining coherent and fluent speech. This impairment in fluency suggests a disruption in the lexical-semantic network, critical for the organized retrieval of conceptual knowledge necessary for coherent speech production. This decline has been well correlated with a decrease in metabolic activity within certain regions of the brain, specifically the IFG located in the frontal lobe, as well as various regions within the temporal lobe (34, 42).
In two other parameters patients with AD performed significant higher results as the HC (voice breaks and nPVI). The nPVI which is based upon the temporal and durational aspects of voice is a measure of the normalized sequential variability index of syllabic intervals (25). It is a robust measurement for the purpose of quantifying the level of variability present in speech rhythm, one key element of prosody of speech (43). According to research findings, stress-timed languages such as English and German exhibit more significant variations in duration between consecutive vocalic intervals (section of speech between vowel onset and offset). This is attributed to the presence of both complex and simple syllabic structures in these languages. As a result, stress-timed languages tend to have higher nPVI values compared to syllable-timed languages like Spanish and French. Conversely, syllable-timed languages are anticipated to demonstrate less deviation in vocalic interval duration and consequently lower nPVI values (44). Based on the present meta-analysis, the rhythm of speech can be negatively affected by AD, resulting in impaired timing and coordination. Consequently, individuals with AD may exhibit increased variability in their speech rhythm, as indicated by higher values of nPVI (25, 26). Voice breaks, also known as one of the symptoms of voice disorders, are characterized by abrupt alterations in the sound of the voice, for example the melodic pitch curve, in which characteristic noises such as bubbling or tremor in the voice also occur (23). According to outcomes, the significant difference in voice breaks between the two groups suggests that individuals with AD may experience more frequent instabilities in pitch compared to HC individuals (23, 28). The increased incident of voice breaks in AD patients may suggest the presence of underlying physiological alterations in the vocal folds or the neural pathways implicated in vocal production process. The increased variability in speech rhythm and the presence of voice breaks in individuals with AD could potentially serve as acoustic markers of disease severity and progression, offering insights into the impact of AD on motor control and speech planning.
The present results demonstrated opportunities of the disease management to use acoustic measures for the detection of AD but also to monitor the progression, stability, or process of the disease. Furthermore, these results not only highlight the potential of acoustic measures for the detection of AD but also underscore the importance of further refining these parameters to enhance their diagnostic utility.
The limitations of this meta-analysis not only concern the relevance of the results, but also offer valuable perspectives for future research. First, the inclusion of studies published only in written English limits the diversity and scope of the data analyzed, potentially overlooking valuable research in other published languages. Second, each parameter was only evaluated in four studies at top, and this issue highlights the paucity of research in this area. Third, the bias of risk was observed in three studies as moderate. Another drawback relates to comparability and reproducibility between the used software in the studies, and equally important, some of the papers used software that was not known (see Table 1). Fourth, although acoustic rate measures were promising in the present meta-analysis between AD and HC, a slowed speech rate can also occur in other neurological diseases based on motor impairment and needs to be further investigated with regard to a differential diagnosis in neurodegenerative diseases (e.g., Parkinson’s or Amyotrophic Lateral Sclerosis). Fifth, some studies used a small number of sample sizes such as Gonzalez-Moreira et al. (24) and Bose et al. (30). Sixth, two parameters (i.e., nPVI and voice breaks) included in this meta-analysis were analyzed by the same groups of authors but in different publications. In such situations, it becomes more challenging to determine whether the observed effects and low heterogeneity (e.g., potential overlap of participants between the included studies) are truly generalizable across all cohorts, languages, and so on. More comprehensive research is necessary to confirm the present results of these two parameters. Seventh, the most included studies relied on clinical diagnoses of Alzheimer’s disease (AD) and did not provide biomarker confirmation of the underlying AD neuropathology. As a result, the findings of this meta-analysis should be interpreted as demonstrating a distinction between individuals with and without dementia, rather than specifically between those with and without biological AD. This distinction is important, as it highlights the potential limitations of using purely clinical diagnostic criteria in research studies. Despite these limitations, the current meta-analysis provides valuable insights into the potential utility of acoustic markers in distinguishing between individuals with and without dementia. Future studies that incorporate biomarker data using more stringent diagnostic criteria will be important to further refine our understanding of the relationship between acoustic markers and AD neuropathology. Eighth, the present meta-analysis used two different types of continuous speech tasks (reading and spontaneous). There might be evidence that there are significant differences in the outcomes of acoustic parameters between these two speech tasks, for example, pauses might be fewer in reading than in continuous speech (45). However, there also other studies which found no significant differences in acoustic speech parameters (46, 47). Thus, we would like to emphasize that a possible influence of the two different speech tasks in the same category of continuous speech cannot be excluded and should be taken into account in future studies. Ninth, just a few of acoustic parameters have been investigated in these studies, but there are much more which could be of interest such as the cepstral analysis. This analysis is being vastly utilized in the assessment and treatment of patients with voice disorders, motor-speech disorder, other neurodegenerative diseases, and hypokinetic disorders (48-53). Furthermore, the exploration of additional acoustic parameters such as cepstral analysis could enrich our understanding of speech alterations in AD, paving the way for more sophisticated diagnostic models.
Conclusion
The analysis of the gathered evidence through meta-analysis illuminated the susceptibility of specific speech signal components to alteration in the context of AD. These findings not only contribute to our understanding of the acoustic manifestations of AD but also highlight the broader implications for early detection and monitoring of the disease. Individuals with AD, as opposed to those who have normal cognitive function, tend to have shorter utterances, speak at a slower pace, and show more variability in voice breaks, which impacts their speech rhythm. Moreover, the potential integration of acoustic analysis into routine diagnostic and monitoring practices represents a significant advancement in our approach to managing AD, emphasizing the need for interdisciplinary collaboration to harness the full potential of this technology. As a result, these discoveries, in conjunction with other accessible resources, can aid healthcare professionals in attaining a more accurate diagnosis of AD in a manner that is relatively economical and straightforward to implement.
Funding: This research received no external funding. Open Access funding enabled and organized by Projekt DEAL.
Conflicts of Interest: The authors declare no conflict of interest.
Author Contributions: Conceptualization, B.B.v.L. and M.G.; methodology, B.B.v.L. and S.S.; software, S.H.; formal analysis, B.B.v.L., S.S and S.H.; writing—original draft preparation, S.S., M.G. and S.H.; writing—review and editing, B.B.v.L. All authors have read and agreed to the published version of the manuscript.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Kim KY, Shin KY, Chang KA. GFAP as a potential biomarker for Alzheimer’s disease: A systematic review and meta-analysis. Cells. 2023;12(9):1309. doi:10.3390/cells12091309
2. Modir A, Shamekhi S, Ghaderyan P. A systematic review and methodological analysis of EEG-based biomarkers of Alzheimer’s disease. Measurement. 2023; 220:113274. doi:10.1016/j.measurement.2023.113274
3. Torrealba E, Aguilar-Zerpa N, Garcia-Morales P, Díaz M. Compensatory mechanisms in early Alzheimer’s disease and clinical setting: The need for novel neuropsychological strategies. J Alzheimers Dis Rep. 2023;7(1):513-525. doi:10.3233/ADR-220116
4. Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. 1984. Biochem Biophys Res Commun. 2012;425(3):534-539. doi:10.1016/j.bbrc.2012.08.020
5. Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83(13):4913-4917. doi:10.1073/pnas.83.13.4913
6. Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood-based biomarkers for Alzheimer’s disease: towards clinical implementation. Lancet Neurol. 2022;21(1):66-77. doi:10.1016/S1474-4422(21)00361-6
7. Blennow K, Zetterberg H. Biomarkers for Alzheimer’s disease: current status and prospects for the future. J Intern Med. 2018;284(6):643-663. doi:10.1111/joim.12816
8. Martínez-Nicolás I, Llorente TE, Martínez-Sánchez F, Meilán JJG. Ten years of research on automatic voice and speech analysis of people with Alzheimer’s disease and mild cognitive impairment: A systematic review article. Front Psychol. 2021;12:620251. doi:10.3389/fpsyg.2021.620251
9. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. doi:10.1016/j.jalz.2011.03.005
10. Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71(4):266-273. doi:10.1097/NEN.0b013e31824b211b
11. Ahmed S, Haigh AM, de Jager CA, Garrard P. Connected speech as a marker of disease progression in autopsy-proven Alzheimer’s disease. Brain. 2013;136(Pt 12):3727-3737. doi:10.1093/brain/awt269
12. Beltrami D, Gagliardi G, Rossini Favretti R, Ghidoni E, Tamburini F, Calzà L. Speech analysis by natural language processing techniques: A possible tool for very early detection of cognitive decline?. Front Aging Neurosci. 2018;10:369. doi:10.3389/fnagi.2018.00369
13. Schultz BG, Vogel AP. A tutorial review on clinical acoustic markers in speech science. J Speech Lang Hear Res. 2022;65(9):3239-3263. doi:10.1044/2022_JSLHR-21-00647
14. Vogel AP, Morgan AT. Factors affecting the quality of sound recording for speech and voice analysis. Int J Speech Lang Pathol. 2009;11(6):431-437. doi:10.3109/17549500902822189
15. Duffy JR. Motor Speech disorders-E-Book: Substrates, differential diagnosis, and management. 2012. Elsevier Health Sciences.
16. Stemple JC, Roy N, Klaben BK. Clinical voice pathology theory and management. 2020. Plural Publishing, San Diego.
17. Buder EH. Acoustic analysis of voice quality: A tabulation of algorithms 1902–1990. In: Kent RD, Ball MJ (ed) Voice Quality Measurement, 1st edn. 2000. Singular, San Diego, pp 119-244.
18. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. doi:10.7326/0003-4819-151-4-200908180-00135
19. Moola S, Munn Z, Tufanaru C, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Lockwood C, Porritt K, Pilla B, Jordan Z (ed) Joanna briggs institute reviewer’s manual. 2017. The Joanna Briggs Institute, pp. 217-269.
20. Melo G, Dutra KL, Rodrigues Filho R, et al. Association between psychotropic medications and presence of sleep bruxism: A systematic review. J Oral Rehabil. 2018;45(7):545-554. doi:10.1111/joor.12633
21. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. doi:10.1016/0197-2456(86)90046-2
22. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi:10.1136/bmj.327.7414.557
23. Meilán JJ, Martínez-Sánchez F, Carro J, López DE, Millian-Morell L, Arana JM. Speech in Alzheimer’s disease: Can temporal and acoustic parameters discriminate dementia?. Dement Geriatr Cogn Disord. 2014;37(5-6):327-334. doi:10.1159/000356726
24. Gonzalez-Moreira E, Torres-Boza D, Kairuz HA, et al. Automatic prosodic analysis to identify mild dementia. Biomed Res Int. 2015;2015:916356. doi:10.1155/2015/916356
25. Martínez-Sánchez F, Meilán JJG, Vera-Ferrandiz JA, et al. Speech rhythm alterations in Spanish-speaking individuals with Alzheimer’s disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2017;24(4):418-434. doi:10.1080/13825585.2016.1220487
26. Martínez-Sánchez F, Meilán JJG, Carro J, Ivanova O. A prototype for the voice analysis diagnosis of Alzheimer’s disease. J Alzheimers Dis. 2018;64(2):473-481. doi:10.3233/JAD-180037
27. De Looze C, Kelly F, Crosby L, et al. Changes in speech chunking in reading aloud is a marker of mild cognitive impairment and mild-to-moderate Alzheimer’s disease. Curr Alzheimer Res. 2018;15(9):828-847. doi:10.2174/1567205015666180404165017
28. Meilan JJG, Martinez-Sanchez F, Carro J, Carcavilla N, Ivanova O. Voice markers of lexical access in mild cognitive impairment and Alzheimer’s disease. Curr Alzheimer Res. 2018;15(2):111-119. doi:10.2174/1567205014666170829112439
29. Qiao Y, Xie XY, Lin GZ, et al. Computer-assisted speech analysis in mild cognitive impairment and Alzheimer’s disease: A pilot Study from Shanghai, China. J Alzheimers Dis. 2020;75(1):211-221. doi:10.3233/JAD-191056
30. Bose A, Dash NS, Ahmed S, et al. Connected speech characteristics of Bengali speakers with Alzheimer’s disease: Evidence for language-specific diagnostic markers. Front Aging Neurosci. 2021;13:707628. doi:10.3389/fnagi.2021.707628
31. Frankenberg C, Weiner J, Knebel M, et al. Verbal fluency in normal aging and cognitive decline: Results of a longitudinal study. Comput Speech Lang. 2021;68: 101195. doi:10.1016/j.csl.2021.101195
32. Cho S, Cousins KAQ, Shellikeri S, et al. Lexical and acoustic speech features relating to Alzheimer disease pathology. Neurology. 2022;99(4):e313-e322. doi:10.1212/WNL.0000000000200581
33. Yamada Y, Shinkawa K, Nemoto M, Nemoto, K, Arai T. A mobile application using automatic speech analysis for classifying Alzheimer’s disease and mild cognitive impairment. Comput Speech Lang. 2023;81:101514. doi: 10.1016/j.csl.2023.101514
34. Melrose RJ, Campa OM, Harwood DG, Osato S, Mandelkern MA, Sultzer DL. The neural correlates of naming and fluency deficits in Alzheimer’s disease: an FDG-PET study. Int J Geriatr Psychiatry. 2009;24(8):885-893. doi:10.1002/gps.2229
35. Ding H, Hamel A, Karjadi C, Ang TFA, Lu S, Thomas R, Au R, Lin H. Association between acoustic features and brain volumes: the Framingham Heart Study. Front Dement. 2023;2:10. doi:10.3389/frdem.2023.1214940
36. Bae M, Ham H, Lee D, Kim K, Y, Lee JY. Structural correlates of speech biomarkers in Alzheimer’s Disease and Mild Cognitive Impairment. Alzheimer’s & Dementia. 2023;19(S16): e075538. doi:10.1002/alz.075538.
37. Boschi V, Catricalà E, Consonni M, Chesi C, Moro A, Cappa SF. Connected speech in neurodegenerative language disorders: A review. Front Psychol. 2017;8:269. Published 2017 Mar 6. doi:10.3389/fpsyg.2017.00269
38. Flipsen P Jr. Articulation rate and speech-sound normalization failure. J Speech Lang Hear Res. 2003;46(3):724-737. doi:10.1044/1092-4388(2003/058)
39. Jacewicz E, Fox RA, O’Neill C, Salmons J. Articulation rate across dialect, age, and gender. Lang Var Change. 2009;21(2):233-256. doi:10.1017/S0954394509990093
40. Cera ML, Ortiz KZ, Bertolucci PH, Minett TS. Speech and orofacial apraxias in Alzheimer’s disease [published correction appears in Int Psychogeriatr. 2013 Oct;25(10):1686]. Int Psychogeriatr. 2013;25(10):1679-1685. doi:10.1017/S1041610213000781
41. Fletcher AR, McAuliffe MJ, Lansford KL, Liss JM. The relationship between speech segment duration and vowel centralization in a group of older speakers. J Acoust Soc Am. 2015;138(4):2132-2139. doi:10.1121/1.4930563
42. Biesbroek JM, van Zandvoort MJ, Kappelle LJ, Velthuis BK, Biessels GJ, Postma A. Shared and distinct anatomical correlates of semantic and phonemic fluency revealed by lesion-symptom mapping in patients with ischemic stroke. Brain Struct Funct. 2016;221(4):2123-2134. doi:10.1007/s00429-015-1033-8
43. Gharsellaoui S, Selouani SA, Cichocki W, Alotaibi Y, Dahmane AO. Application of the pairwise variability index of speech rhythm with particle swarm optimization to the classification of native and non-native accents. Comput Speech Lang. 2018;48(2):67-79. doi:10.1016/j.csl.2017.10.006
44. Grabe, E., & Low, E. L. (2002). Durational variability in speech and the rhythm class hypothesis. In C. Gussenhoven & N. Warner (Eds.), Papers in Laboratory Phonology 7 (pp. 515–546). Berlin: Mouton de Gruyter
45. Howell P, Kadi-Hanifi K. Comparison of prosodic properties between read and spontaneous speech material. Speech Communication. 1991;10(2), 163–169. doi:10.1016/0167-6393(91)90039-V
46. Lee Y, Kreiman J. Acoustic voice variation in spontaneous speech. J Acoust Soc Am. 2022;151(5):3462. doi:10.1121/10.0011471
47. Venkatagiri HS. Clinical measurement of rate of reading and discourse in young adults. J Fluency Disord. 1999;24(3):209-226. doi:10.1016/S0094-730X(99)00010-8
48. Aghajanzadeh M, Saeedi S. Efficacy of cepstral measures in voice disorder diagnosis: A literature review. JMR. 2022;16(2):120-129. doi:10.18502/jmr.v16i2.9298.
49. Smith KM, Demers-Peel M, Manxhari C, Stepp CE. Voice acoustic instability during spontaneous speech in Parkinson’s disease. J Voice. Published online July 25, 2023. doi:10.1016/j.jvoice.2023.06.004
50. Jannetts S, Lowit A. Cepstral analysis of hypokinetic and ataxic voices: correlations with perceptual and other acoustic measures. J Voice. 2014;28(6):673-680. doi:10.1016/j.jvoice.2014.01.013
51. Carson C, Ryalls J, Hardin-Hollingsworth K, Le Normand MT, Ruddy B. Acoustic analyses of prolonged vowels in young adults with Friedreich ataxia. J Voice. 2016;30(3):272-280. doi:10.1016/j.jvoice.2015.05.008
52. Brown KA, Spencer KA. The relationship between speech characteristics and motor subtypes of Parkinson’s disease. Am J Speech Lang Pathol. 2020;29(4):2145-2154. doi:10.1044/2020_AJSLP-20-00058
53. Maffei MF, Green JR, Murton O, et al. Acoustic measures of dysphonia in Amyotrophic Lateral Sclerosis. J Speech Lang Hear Res. 2023;66(3):872-887. doi:10.1044/2022_JSLHR-22-00363
© The Authors 2024